Abstract
Background/aims: To determine outcomes of transplants of cultivated autologous oral epithelial cells in patients with severe ocular surface disorders.
Methods: The eyes (n = 6) of four patients with Stevens-Johnson syndrome (three eyes) or chemical burns (three eyes) were studied. Autologous oral epithelial cells, grown for 2–3 weeks on a denuded amniotic membrane carrier in the presence of 3T3 fibroblasts, were air lifted. The resultant sheet was transplanted onto the damaged eye, and acceptance of the sheet by the corneal surface was confirmed 48 hours after surgery. The success of ocular surface reconstruction, graft survival, changes in visual acuity, and postoperative complications were assessed and the quality of the cultivated oral epithelial sheet was evaluated histologically.
Results: At 48 hours after transplant, the entire corneal surface of all six eyes was free of epithelial defects indicating complete survival of the transplanted oral epithelium. Visual acuity was improved in all eyes. During follow up (mean 13.8 (SD 2.9) months), the corneal surface remained stable, although all eyes manifested mild peripheral neovascularisation.
Conclusions: Autologous oral epithelial cells grown on denuded amniotic membrane can be transplanted to treat severe ocular surface disorders.
Keywords: amniotic membrane, cellular surgery, corneal epithelium, ocular surface reconstruction, oral mucosa
The normal ocular surface is covered with highly specialised corneal, limbal, and conjunctival epithelial cells (EC) that, together with the tear film, maintain surface integrity.1–3 Severe ocular surface damage (OSD) caused by thermal and chemical burns or Stevens-Johnson syndrome (SJS) represents a serious clinical challenge. In such cases, the corneal epithelial stem cells in the corneal limbus are destroyed and coverage of the corneal surface by invading neighbouring conjunctival EC results in neovascularisation, chronic inflammation, ingrowth of fibrous tissue, and stromal scarring.4,5 Conventional management is generally unsatisfactory, and the long term consequences tend to be devastating.
Attempts have been made to establish a surgical treatment for severe OSD.6–12 Although corneal epithelial transplantation (limbal transplantation or keratoepithelioplasty) and cultivated corneal epithelial stem cell transplantation have been developed to improve the outcome of ocular surface reconstruction, significant problems remain. Transplantation from allogeneic donors carries the risk of rejection and the intensive, prolonged postoperative immunosuppressant therapy necessary to prevent inflammation and rejection markedly reduces the patients’ quality of life, especially in younger patients. In cases with unilateral, but not bilateral damage, reconstruction of the affected ocular surface can be attempted by transplanting cultivated autologous corneal EC from the contralateral eye.13–15
We investigated the possibility of reconstructing the human corneal surface using autologous mucosal epithelium of non-ocular surface origin. In rabbits, we have already established a surgical method for transplanting cultivated autologous oral epithelial cells using amniotic membrane (AM) as a carrier.16 We successfully applied this method in six eyes of four patients with severe OSD and our study represents a first step toward assessing the feasibility of transplanting autologous cultivated epithelial transplants of non-ocular surface origin.
MATERIALS AND METHODS
Subjects
The study included six eyes from four patients who underwent autologous cultivated oral epithelial transplantation at our university hospital between May and December 2002. We deliberately selected patients with severe bilateral ocular surface diseases because their treatment necessitates autologous rather than allogeneic transplantation. There were three males and one female; their mean age was 24.5 (standard deviation 7.9) years. All had been diagnosed as totally stem cell deficient on the basis of complete disappearance of the palisade of Vogt. Three eyes each had chemical injuries and SJS. Cases 1 and 3 had previously undergone amniotic membrane transplantation and allogeneic cultivated corneal epithelial transplantation, respectively. All six eyes manifested severe epithelial damage and were resistant to conventional therapy. Preoperative tear function significantly affects the surgical outcome.17,18 Slit lamp examination and fluorescein staining showed that in all patients reported here, the preoperative tear production and tear meniscus were within the normal range and relatively stable. All patients were followed for more than 11 months after transplantation (table 1).
Table 1.
Clinical data for six eyes that underwent cultivated oral epithelium transplantation
| Case | Age | Sex | Diagnosis | Eye | Previous surgery | Graft survival | ED | PNV | Conjunctivalisation | Visual acuity | Follow up | |
| Preoperative | Postoperative | |||||||||||
| 1 | 33 | M | Chemical burn (A) | R | AMT | + | − | + | − | HM | 4/200 | 17 |
| L | AMT | + | − | + | − | HM | 20/30 | 17 | ||||
| 2 | 27 | M | Chemical burn (C) | L | + | − | + | − | HM | 4/200 | 15 | |
| 3 | 24 | M | SJS (C) | R | CCET | + | + | + | − | HM | 20/600 | 12 |
| L | CCET | + | + | + | − | HM | 4/200 | 11 | ||||
| 4 | 14 | F | SJS (C) | L | + | − | + | − | HM | 20/200 | 11 | |
SJS, Stevens Johnson syndrome; AMT, amniotic membrane transplantation; CCET, cultivated corneal epithelial transplantation; ED, epithelial defect; PNV, peripheral neovascularisation; HM, hand motion; M, male; F, female; A, acute phase; C, chronic phase.
The Human Studies Committee of Kyoto Prefectural University of Medicine approved the transplantation of autologous cultivated oral epithelial cells for these patients; we obtained prior oral and written informed consent from all patients.
Preparation of human amniotic membrane
With proper informed consent in accordance with the tenets of the Declaration of Helsinki for research involving human subjects, and with approval by the Institutional Review Board of Kyoto Prefectural University of Medicine, human amniotic membrane (AM) was obtained at the time of elective caesarean section. Under sterile conditions, the membranes were cryopreserved at −80°C using our previously reported process.16 For the oral epithelial cultures, the AM were deprived of their amniotic EC by incubation with 0.02% ethylene diamine tetra-acetic acid (EDTA) at 37°C for 2 hours to loosen cell adhesion; this was followed by gentle scraping with a cell scraper.
Oral management
The presence of healthy oral mucosa was confirmed by a dentist before biopsy. All patients were followed up regarding their adherence to the requirements for tooth decay treatment, no alcohol or tobacco use, and regular brushing and iodine gargle.
Cultivation of oral mucosal epithelial cells
To culture the oral epithelial cells (EC) we used a modified culture system for rabbit oral EC.16 Briefly, confluent 3T3 fibroblasts, incubated for 2 hours with 4 μg/ml mitomycin C (MMC) to inactivate their proliferative activity, were rinsed with phosphate buffered saline to remove MMC, trypsinised, and plated onto plastic dishes at a density of 2×104 cells/cm2. Denuded AM were spread, epithelial basement membrane side up, on the bottom of culture plate inserts which were placed in dishes containing treated 3T3 fibroblasts.
Oral mucosal biopsy specimens, each measuring approximately 2–3 mm2, were taken from each patient under local anaesthesia 2–3 weeks before transplantation. Submucosal connective tissues were removed with scissors to the extent possible; the resulting samples were cut into small explants that were immersed three times (10 minutes at room temperature) in phosphate buffered saline solution containing antibiotics, (50 IU/ml penicillin-streptomycin and 5 µg/ml amphotericin B). The explants were then incubated at 37°C for 1 hour with 1.2 IU dispase as previously described19 and treated with 0.25% trypsin-EDTA solution for 10 minutes at room temperature to separate the cells. Enzyme activity was stopped by washing with Dulbecco’s modified Eagle’s medium and Ham’s F12 medium (1:1) containing 10% fetal bovine serum, insulin (5 μg/ml), cholera toxin (0.1 nmol/l), human recombinant epidermal growth factor (10 ng/ml), and penicillin-streptomycin (50 IU/ml). The oral EC (1×105/ml) were then seeded onto denuded AM spread on the bottom of culture inserts, and co-cultured with MMC inactivated 3T3 fibroblasts. The culture was submersed in medium for 1–2 weeks and then exposed to air by lowering the level of the medium (air lifting)20 over the course of 1 week. Cultures were incubated at 37°C in a 5% CO2 95% air incubator; the medium was changed every day.
Surgical procedures
After removing both conjunctivalised tissues on the corneal surface and subconjunctival tissues using surgical scissors, subconjunctival fibroblasts were treated for 5 minutes with 0.04% mitomycin C followed by vigorous repeated washing with saline.21 The cultivated oral epithelium on AM was transplanted onto the corneal surface of the damaged eye and secured with 10–0 nylon sutures at the limbus. We used fluorescein staining of the cultivated oral epithelial sheet to confirm its quality and then covered it with a therapeutic soft contact lens.
Postoperative management
Postoperatively, 0.3% ofloxacin and 0.1% dexamethasone were instilled four times a day; the doses were tapered to a maintenance dose at 2–3 months, depending on the severity of inflammation. Betamethasone (1 mg/day) and cyclophosphamide (50 mg/day), administered to prevent postoperative inflammation and conjunctival fibrosis, were stopped 1–2 months after surgery. Both renal and liver function were monitored periodically.
RESULTS
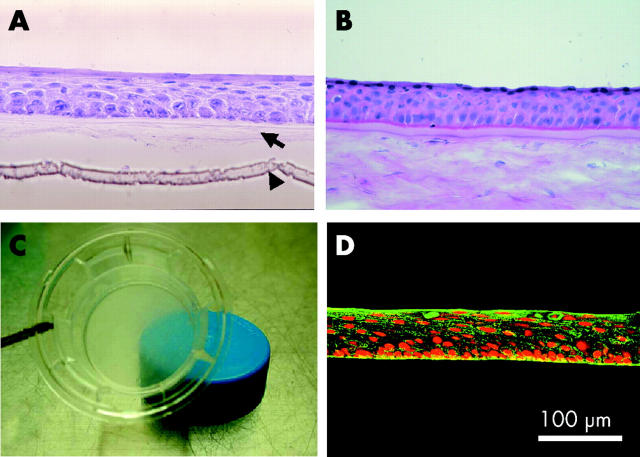
There were no complications during or after excision of the oral mucosa. After 2–3 weeks in culture, the cultivated oral epithelium consisted of 5–6 layers and was very similar to normal in vivo corneal epithelium (fig 1A and B). It retained the transparency of normal cornea (fig 1C) and stained positive for antibody specific for corneal epithelium keratin 3 (fig 1D) and specific for non-keratinised, stratified keratin 4/13 (data not shown).22,23 These results were the same as in our rabbit model.16
Figure 1.
Cultivation of oral epithelium on amniotic membrane. Light micrographs of cross sections of (A) epithelial cells from oral biopsy samples of patient 2 cultivated on amniotic membrane and (B) normal corneal epithelial cells stained with haematoxylin and eosin. The arrow in (A) points to the denuded amniotic membrane; the arrowhead identifies the membrane of the culture insert (original magnification ×400). (C) The culture insert retained its transparency as indicated by the translucence of the blue cap beneath the culture insert. (D) The cultured oral epithelial cells are immunohistochemically stained for keratin 3 (green). Their nuclei are positive for propidium iodide (red).
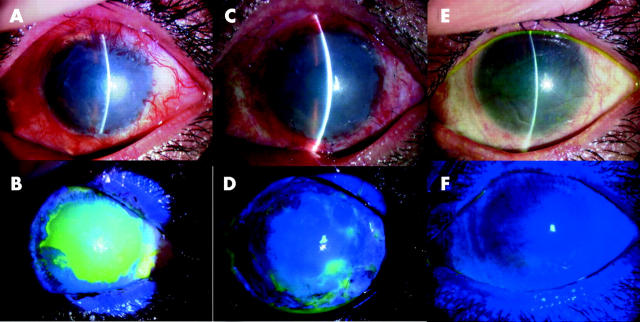
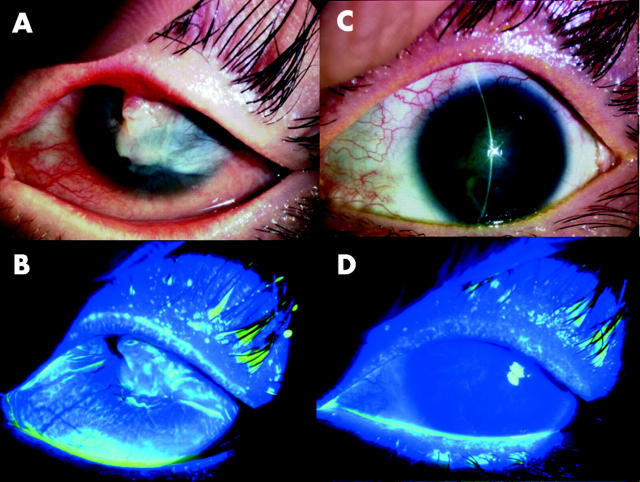
Two days after transplantation, the corneal surface of all treated eyes was clear and smooth; fluorescein staining confirmed that the entire corneal surface was covered by the cultivated autologous oral epithelium (fig 2). The transplanted epithelial sheet was surrounded at 360° by conjunctival epithelial defects, indicating that there was no contamination of the host conjunctival epithelium. Shortly after the transplantation procedure, conjunctival inflammation rapidly subsided in all patients. Slit lamp examination showed that conjunctival fibrosis was successfully suppressed in all patients and there was no conjunctival invasion into the corneal surface at the last follow up visit (figs 2 and 3). By slit lamp examination using fluorescein staining we confirmed that the surviving oral epithelial area manifested the characteristic staining pattern different from both the cornea and the conjunctival epithelium. In case 3 (both eyes), a small epithelial defect with minimal cell infiltration suggested a low toxic bacterial infection which was controlled by the frequent use of ofloxacin and cefmenoxime eye drops.
Figure 2.
Transplantation to a patient with chemical burns of autologous oral epithelial cells cultivated on amniotic membrane (patient 1). Representative slit lamp photographs taken before transplantation without (A) and with fluorescein (B). The photographs in (C–F) were taken 48 hours after transplantation and are without (C) and with fluorescein (D), (E–F) were obtained at the last follow up visit without (E) and with fluorescein (F). Before transplantation, the eye manifested persistent epithelial defects surrounded by inflammatory subconjunctival fibrosis. At 48 hours post-transplantation, the ocular surface was covered with transplanted cells. At the last follow up visit, the corneal surface was stable without defects.
Figure 3.
Transplantation to a patient with SJS of autologous oral epithelial cells cultivated on amniotic membrane (patient 4). Representative slit lamp photographs taken before transplantation without (A) and with fluorescein (B). The photographs in (C–D) were taken at the last follow up visit without (C) and with fluorescein (D). Before transplantation, the eye manifested inflammatory subconjunctival fibrosis with neovascularisation, conjuntivalisation, and severe symblepharon. At the last follow up visit, the corneal surface was stable without defects.
In all cases, we observed superficial peripheral neovascularisation just under the AM stroma. Most of these vessels involved the peripheral and not the central corneal region, and did not cause any postoperative complications. No clinical complications except small epithelial defects were observed.
Visual acuity improved in all eyes and all were restored to good vision (postoperative visual acuity improved by two or more lines) at more than 11 months after transplantation. The mean (standard deviation) postoperative period is now 13.8 (2.9) months, the ocular surface harbours surviving transplanted epithelium, and all treated eyes are stable without defects.
DISCUSSION
Severe ocular surface disorders such as SJS and chemical burns present an unsolved challenge to the ophthalmologist. The conventional approach is generally unsatisfactory, and the long term clinical results are devastating. We previously performed corneal epithelial transplantation—that is, limbal transplantation and cultivated corneal epithelial transplantation—and succeeded in human ocular surface reconstruction.21 However, as most severe OSD are bilateral, we were forced to use allografts, which subject the recipients to high risk for allogeneic rejection and necessitate prolonged immunosuppression. We propose autologous transplantation as the ideal treatment for ocular surface reconstruction. Allograft rejection was the main reason we decided to investigate the possibility of ocular surface replacement with autografts of stratified, non-keratinised mucosal epithelium of non-ocular surface origin.
There are numerous candidates for mucosal epithelia of non-ocular surface origin—for example, oral, nasal, oesophageal, tracheal, and vaginal mucosa. We previously focused on oral mucosa in our rabbit model16 because EC isolated from the oral mucosa are thought to be at a lower stage of differentiation than epidermal keratinocytes. Their short cell turnover time requires shorter cultures and they can be maintained in culture for prolonged periods without keratinisation.24 Moreover, the oral cavity is an ideal location for tissue biopsy as the resulting scar is inconspicuous. Lastly, keratin 3 is a reliable marker for corneal type differentiation and is positive for EC of normal in vivo oral mucosa.25–27 These characteristics recommend oral EC as an ideal substitute for corneal EC for use in ocular surface reconstruction.
Ballen, who used oral mucosal membrane grafts that included both epithelium and subepithelial tissues in both human and rabbit eyes, found that they heavily vascularised with early fibrosis.28 Gipson et al transplanted oral epithelia freed by dispase II of underlying connective tissue onto the rabbit ocular surface.29 They reported that it was feasible to transplant in vivo oral mucosal epithelium to corneal-limbal regions, but that it was not maintained in central avascular corneal regions. We previously documented that oral EC cultivated on AM can function as ocular surface epithelium in rabbits.16 Human AM has a thick basement membrane, is devoid of a vascular component, and has an antiscarring effect in ocular surface reconstruction.30 The EC character is thought to depend on the substrate, and AM is a good substrate for cultivating mucosal epithelium such as corneal, conjunctival, and oral epithelium.12–16 Based on these considerations, we suggest that oral EC cultivated on AM may be able to differentiate into cornea like EC under our single cell culture conditions.
There are many difficulties in developing a culture system for human oral EC. Because we found that it was difficult to cultivate human oral EC using our previously reported culture technique for rabbit oral EC16 we modified the culture process using samples from volunteers. We used the experience we gained from transplanting cultivated allogeneic corneal EC in rabbits31 and humans21 to develop our autologous human oral epithelial culture system. Our modified system is unique in that we use denuded AM as the substrate, a 3T3 fibroblast layer to assist epithelial stratification, and air lifting to facilitate the correct formation of epithelial tight junctions. In addition, we modified the culture medium used by others13–15 to encourage EC growth. We succeeded in producing sufficiently stratified, well differentiated epithelium to cover the entire ocular surface of the injured eye from an oral specimen measuring only 2–3 mm2, thereby avoiding extensive scarring of the oral cavity.
Our experience shows that the viability of human oral epithelium depends on donor characteristics and age: cell growth in oral epithelium obtained from aged donors was slower than that of cells from younger donors. This is consistent with the observation that tissue specific stem cells gradually decrease with age.32 It is also important whether the oral epithelium has the characteristics of normal mucosa. For example, as SJS is an acute inflammatory disorder of the skin and mucosal epithelium, it is possible that the oral mucosal epithelium in patients with SJS is damaged. As we were able to cultivate the oral epithelium from SJS patients under our culture conditions, we suggest that, like skin lesions, oral mucosal lesions in SJS patients are self limited.
Successful transplantation of cultivated oral epithelial sheets requires strong attachment of the basal cells to the underlying AM and the development of a normal barrier function by the superficial cells. Using electron microscopy, we observed that, like in vivo corneal EC, human oral EC cultivated on AM exhibit junctional specialisation such as desmosomal and hemidesmosomal junctions (data not shown).33
In all cases reported here, neovascularisation developed postoperatively just under the graft. We noted that postoperative inflammation facilitated neovascularisation and that most of these vessels were limited to the peripheral cornea region. However, 5-FU eye drops and anti-inflammatory therapy controlled these activities. We posit that postoperative neovascularisation occurs because in vivo oral mucosa requires a vascular bed for maintenance. At present, we do not know whether oral epithelium synthesises an angiogenic factor and/or lacks an anti-angiogenic factor. We are currently monitoring the expression of angiogenic and anti-angiogenic factors such as vascular endothelial growth factor, angiostatin, and endostatin in cultured oral epithelium sheets. Although the main purpose of transplanting cultivated oral epithelium is to maintain ocular surface integrity, visual improvement is important. Even if the ocular surface, with transplanted cultivated oral epithelium, were to heal with peripheral neovascularisation, the ocular surface would be intact in patients with severe damage and their visual acuity would be improved, thereby improving their quality of life without requiring intensive immunosuppression.
Although epithelial and subepithelial opacity of the cultivated oral epithelial sheet may develop during a longer follow up period, we contend that it is possible to repeat the transplantation process with new autologous cultivated oral epithelial sheets because there is no risk of postoperative rejection. We previously reported that, in cases where the initially transplanted cultivated corneal epithelium became opaque, the transplantation process could be repeated using new cultivated epithelium; this was true even in patients who had previously received allografts.34
For successful transplantation, it is very important to include stem cells in the cultivated oral epithelial sheet because they affect its longevity. However, it is difficult to determine their number because there are currently no reliable markers specific for oral epithelial stem cells. Based on our observations longer than 11 months, we think that the cultivated oral epithelial sheets contain a sufficient number of stem cells to maintain the graft because slit lamp examination and fluorescein staining identified the area of the surviving oral epithelium. The area exhibited a characteristic staining pattern that was different from both corneal and conjunctival EC. Using impression cytology for immunohistochemistry, we are currently monitoring cultivated autologous oral epithelial sheets in efforts to identify oral mucosa specific markers.
We previously suggested that oral EC cultivated on AM may be able to differentiate into cornea like EC under our culture conditions.16 Here we documented that sufficient epithelium to cover the entire ocular surface can be obtained from oral biopsy specimens measuring 2–3 mm2. We suggest that transplantation of cultivated autologous oral EC represents an effective technique for ocular surface reconstruction in patients with severe OSD, especially those with damage to both eyes. We posit that cultivated epithelial sheets can also be used for conjunctival reconstruction in the fornix, and we are currently adapting our technique to conjunctival reconstruction in patients with OSD with severe symblepharon.
This study is the first demonstration of the survival on severely injured human corneas of transplanted, cultivated autologous EC derived from oral biopsies. Our procedure offers hope to patients with unilateral or bilateral lesions and represents a new therapeutic tool for the treatment of patients with severe OSD. Although the long term survival of these grafts awaits confirmation, we are confident that the method introduced here represents an advance in the treatment of patients with OSD.
Acknowledgments
The authors thank Kenichi Endo, Hisayo Sogabe, and Wakana Ito for assisting with the culture procedure.
Abbreviations
AM, amniotic membrane
EC, epithelial cells
EDTA, ethylene diamine tetra-acetic acid
MMC, mitomycin C
OSD, ocular surface damage
SJS, Stevens-Johnson syndrome
Supported in part by Grants-in-Aid for scientific research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (13557145), a research grant from the Kyoto Foundation for the Promotion of Medical Science, the Intramural Research Fund of Kyoto Prefectural University of Medicine.
REFERENCES
- 1.Thoft RA, Friend J. Biochemical transformation of regenerating ocular surface epithelium. Invest Ophthalmol Vis Sci 1977;16:14–20. [PubMed] [Google Scholar]
- 2.Tsai RJF, Sun TT, Tseng SCG. Comparison of limbal and conjunctival autograft transplantation in corneal surface reconstruction in rabbits. Ophthalmology 1990;97:446–55. [DOI] [PubMed] [Google Scholar]
- 3.Wei ZG, Wu RL, Lavker LM, et al. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia: implications on conjunctival epithelial transdifferentiation and stem cells. Invest Ophthalmol Vis Sci 1993;34:1814–28. [PubMed] [Google Scholar]
- 4.Shapiro MS, Friend J, Thoft RA. Corneal re-epithelialization from the conjunctiva. Invest Ophthalmol Vis Sci 1981;21:135–42. [PubMed] [Google Scholar]
- 5.Dua H, Forrester JV. The corneoscleral limbus in human corneal epithelial wound healing. Am J Ophthalmol 1990;110:646–56. [DOI] [PubMed] [Google Scholar]
- 6.Thoft RA. Conjunctival transplantation. Arch Ophthalmol 1977;95:1425–7. [DOI] [PubMed] [Google Scholar]
- 7.Thoft RA. Keratoepithelioplasty. Am J Ophthalmol 1984;97:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Kenyon KR, Tseng SCG. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 1989;96:709–22. [DOI] [PubMed] [Google Scholar]
- 9.Tsai RJF, Tseng SCG. Human allograft limbal transplantation for corneal surface reconstruction. Cornea 1994;13:389–400. [DOI] [PubMed] [Google Scholar]
- 10.Tsubota K, Satake Y, Ohyama M, et al. Surgical reconstruction of the ocular surface in advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Am J Ophthlmol 1996;122:38–52. [DOI] [PubMed] [Google Scholar]
- 11.Tseng SC, Prabhasawat P, Barton K, et al. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch Ophthalmol 1998;116:431–41. [DOI] [PubMed] [Google Scholar]
- 12.Tsubota K, Satake Y, Kaido M, et al. Treatment of severe ocular surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med 1999;340:1697–703. [DOI] [PubMed] [Google Scholar]
- 13.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 1997;349:990–3. [DOI] [PubMed] [Google Scholar]
- 14.Tsai RJF, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med 2000;343:86–93. [DOI] [PubMed] [Google Scholar]
- 15.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea 2000;19:421–6. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Endo K, Cooper L, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci 2003;44:106–16. [DOI] [PubMed] [Google Scholar]
- 17.Shimazaki J, Yang HY, Tsubota K. Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns. Ophthalmology 1997;104:2068–76. [DOI] [PubMed] [Google Scholar]
- 18.Shimazaki J, Shimmura S, Fujishima H, et al. Association of preoperative tear function with surgical outcome in severe Stevens-Johnson syndrome. Ophthalmology 2000;107:1518–23. [DOI] [PubMed] [Google Scholar]
- 19.Koizumi N, Cooper L, Fullwood NJ, et al. An evaluation of cultivated corneal limbal epithelial cells using cell suspension culture. Invest Ophthalmol Vis Sci 2002;43:2114–21. [PubMed] [Google Scholar]
- 20.Zagorski Z. Experimental studies of the growth in tissue culture of the epithelium and endothelium of rabbit cornea subjected to the effect of low temperature. Klin Oczna 1975;45:733–7. [PubMed] [Google Scholar]
- 21.Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 2001;108:1569–74. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Nishida K, Dota A, et al. Elevated expression of transglutaminase 1 and keratinization-related proteins in conjunctiva in severe ocular surface disease. Invest Ophthalmol Vis Sci 2001;42:549–56. [PubMed] [Google Scholar]
- 23.Nakamura T, Nishida K, Dota A, et al. Changes in conjunctival clustering expression in severe ocular surface disease. Invest Ophthalmol Vis Sci 2002;43:1702–7. [PubMed] [Google Scholar]
- 24.Hata K, Kagami H, Ueda M, et al. The characteristics of cultured mucosal cell sheet as a material for grafting; comparison with cultured epidermal cell sheet. Ann Plast Surg 1995;34:530–8. [DOI] [PubMed] [Google Scholar]
- 25.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64 K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 1986;103:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juhl M, Reibel J, Stoltze K. Immunohistochemical distribution of keratin proteins in clinically healthy human gingival epithelia. Scand J Dent Res 1989;97:159–70. [DOI] [PubMed] [Google Scholar]
- 27.Collin C, Ouhayoun JP, Grund C, et al. Protein suprabasal marker proteins distinguishing keratinizing squamous epithelia: cytokeratin 2 polypeptides of oral masticatory epithelium and epidermis are different. Differentiation 1992;51:137–48. [DOI] [PubMed] [Google Scholar]
- 28.Ballen PH. Mucosal membrane grafts in chemical (lye) burns. Am J Ophthalmol 1963;55:302–12. [DOI] [PubMed] [Google Scholar]
- 29.Gipson IK, Geggel HS, Spurr-Michaud SJ. Transplant of oral mucosal epithelium to rabbit ocular surface wounds in vivo. Arch Ophthalmol 1986;104:1529–33. [DOI] [PubMed] [Google Scholar]
- 30.Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea 1995;14:473–84. [PubMed] [Google Scholar]
- 31.Koizumi N, Inatomi T, Quantock AJ, et al. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea 2000;19:65–71. [DOI] [PubMed] [Google Scholar]
- 32.Liang Y, Van Zant G. Genetic control of stem-cell properties and stem cells in aging. Curr Opin Hematol 2003;10:195–202. [DOI] [PubMed] [Google Scholar]
- 33.Gipson IK, Sugrue SP. Cell biology of the corneal epithelium. In: Albert DM, Jakobiec FA, eds. Principles and practice of ophthalmology. Philadelphia: WB Saunders, 1994:2–16.
- 34.Nakamura T, Koizumi N, Tsuzuki M, et al. Successful regrafting of cultivated corneal epithelium using amniotic membrane as a carrier in severe ocular surface disease. Cornea 2003;22:70–1. [DOI] [PubMed] [Google Scholar]