Abstract
Aim: To evaluate the efficacy of periocular triamcinolone acetonide for the treatment of thyroid associated ophthalmopathy (TAO), and the presence of ocular or systemic adverse effects.
Methods: A multicentre prospective pilot study was performed on patients diagnosed with Graves’ ophthalmopathy less than 6 months before entry to the study. Patients were admitted to the study and were randomised into two groups: treatment and control. The treatment group received four doses of 20 mg of triamcinolone acetate 40 mg/ml in a peribulbar injection to the inferolateral orbital quadrant. Both groups were evaluated by measuring the area of binocular vision without diplopia on a Goldmann perimeter and the size of the extraocular muscles on computed tomography (CT) scans. Ophthalmological and systemic examinations were done to rule out ocular and systemic adverse effects. Follow up was 6 months for both groups.
Results: 50 patients were eligible for the study. 41 patients completed the study. There was an increase in the area of binocular vision without diplopia in the treatment group (Σ initial: mean 231.1 (SD 99.9) and final absolute change, mean 107.1 (SD 129.0)) compared to the control group (Σ initial: mean 350.7 (SD 86.5) and final absolute change, mean −4.5 (SD 67.6)). The sizes of the extraocular muscles were reduced in the treatment group (mean (inferior rectus initial values): 1.3 (0.7), final percentage change: −13.2 (25.7), medial rectus initial values: 1.2 (0.6), final percentage change: −8.2 (20.7), superior rectus-levator palpebrae initial values: 1.2 (0.6), final percentage change: −9.5 (29.1), lateral rectus initial values: 1.0 (0.4), final percentage change: −11.5 (20.6)) compared to the control group (inferior rectus initial values: 0.9 (0.3), final percentage change: −4.0 (21.5), medial rectus initial values: 0.9 (0.3), final percentage change: 0.6 (22.4), superior rectus-levator palpebrae initial values: 0.9 (0.3), final percentage change: 12.5 (37.5), lateral rectus initial values: 0.9 (0.4), final percentage change: −0.5 (31.6)). Both measurements (degree of diplopia and muscle thickness) were statistically significant between groups (initial − final). No systemic or ocular adverse effects were found.
Conclusions: Triamcinolone administered as a periocular injection is effective in reducing diplopia and the sizes of extraocular muscles in TAO ophthalmopathy of recent onset. This form of treatment is not associated with systemic or ocular side effects.
Keywords: thyroid associated ophthalmopathy, periocular injections, triamcinolone
There is no gold standard of treatment for the thyroid associated ophthalmopathy (TAO) in the early (inflammatory) stages of the disease. Corticosteroids reduce the transitory manifestations of TAO but their multiple adverse effects make the risk/benefit relation unsatisfactory.1–16
The beneficial effects of steroids used locally (subconjunctival or retrobulbar injections) in the treatment of TAO have been reported in the literature.17–26
We are aware of no study designed to demonstrate the advantages of steroids used locally (periocular injections) improving TAO in the early stages. We also analysed the impact of secondary effects associated with local steroid administration. This multicentre, prospective pilot study was designed to evaluate this treatment.
METHODS
Fifty patients with TAO diagnosed between April 1998 and April 1999 were admitted to the study under the following inclusion criteria: TAO of 6 months’ or less duration, with diplopia noticed either in primary position or at any position of regard. Exclusion criteria were previous treatment for TAO with steroids or radiation, compressive optic neuropathy, absence of diplopia, and contraindications to steroids (diabetes, systemic hypertension, gastritis, psychosis and pregnancy). Patients were included regardless their endocrine status.
They were randomised simply into two groups: group 1 (treatment group) received treatment with triamcinolone and group 2 (non-treatment group) received no treatment and acted as control group.
Patients were initially examined (week 0) for best corrected visual acuity (BCVA), measured in a decimal scale on a universal Snellen chart, intraocular pressure (IOP), measured in mm Hg with applanation tonometry, exophthalmometry (Ex), measured in mm with a Hertel exophthalmometer, optic nerve head examination (ON), graded as normal, papilloedema or optic nerve head atrophy. Total body weight (BW) were measured in kg and systemic systolic and diastolic arterial blood pressures (SBP, DBP) was measured in mm Hg. Ocular motility was measured with a Goldmann perimeter by a masked technician, according to Feibel and Roper-Hall methods.27 The patient was positioned at the perimeter with both eyes uncovered. A 2-IV size light was used. It was moved along eight radial lines from the centre to the periphery and the patient was asked to say when double vision first appeared. A line was obtained encircling the area without diplopia. The summation of angular points was used for comparison (Σ).
The sizes of the four recti muscles were measured on computed tomography (CT) scans, coronal views, using a caliper. The scans selected for measurements were taken at the medial third of the orbit.
The size (diameter) of the optic nerve was used as a unit, dividing the measured size of each muscle by the size of the optic nerve, the ratio obtained was used for comparison. This method was used to avoid bias when using CT scans printed with different magnifications. Measures were made by an unmasked physician.
Blood tests were done for glycaemia (Gl), calcaemia (Ca), plasma cortisol (Cpl), and urinary cortisol (Cur). Normal values were adopted from those used at the Massachusetts General Hospital Laboratory.
Patients in the treatment group were treated with four injections of triamcinolone acetonide (Kenacort A, 40 mg/ml, Bristol-Meyers-Squibb) of 20 mg in each orbit administered weekly during 4 consecutive weeks (weeks 1, 2, 3, and 4). The injected compound represents a deposit formulation of triamcinolone.
The injection was placed in the inferior lateral quadrant of the orbit using a 27 gauge half inch disposable needle.
Before each injection, IOP, SBP, DBP, and BW were recorded.
Both groups were followed at week 10, measuring SBP, DBP, BW, BCVA, IOP, Ex, and NO. Ocular motility and blood tests were also recorded (Gl, Ca, Cpl, and Cur). At week 24 both groups were examined for BCVA, IOP, Ex, ON, ocular motility, and muscle sizes on a new CT scan.
Results were compared for both groups using Student’s t test. Blood tests were defined as normal or abnormal, calculating the median for each value. Additional statistical analysis was performed (Dunnet T test, test of comparison of treatment versus control and analysis of log normal distribution).
RESULTS
From the 50 enrolled patients, five were excluded from the analysis as a result of violations to the protocol or were withdrawn. Therefore, 45 patients were available for safety analyses (25 in the treatment group and 20 in the non-treatment group). From them, 41 patients were available for efficacy analyses (24 in the treatment group and 17 in the non-treatment group).
From the efficacy population of 41, 37 patients were available for ocular motility evaluation (20 in the treatment group and 17 in the non-treatment group) and other 37 (23 in the treatment group and 14 the non-treatment group) were available for muscle size evaluation. Table 1 depicts the distribution of the safety population according to age, weight, and sex.
Table 1.
Major demographic features—safety population
| Demographic feature | Treatment group (n = 25) | Non-treatment group * (n = 20) | Difference between groups |
| Age (years) | p = 0.0017 | ||
| Mean (SD) | 50.3 (13.3) | 36.1 (15.2) | |
| Range | (22–78) | (11–62) | |
| Sex | p = 0.4283† | ||
| Number (%) | |||
| Male | 9 (36.0%) | 5 (25.0%) | |
| Female | 16 (64.0%) | 15 (75.0%) | |
| Body weight (kg) | p = 0.4353† | ||
| Mean (SD) | 67.5 (14.2) | 63.3 (20.9) | |
| Range | (47–111) | (40–132) |
*n = 19 for body weight; †not significant.
The analysis of motility showed that the treatment group had a mean Σ of 231.1 (SD 99.9) and the non-treatment group a mean Σ of 350.7 (86.5) at baseline. At week 10, the treatment group mean absolute change was 93.6 (SD 129.4), showing an improvement of 91.56%, and the non-treatment group mean absolute change was 1.18 (39.1), a change of 2%. At week 24, the treatment group mean absolute change was 107.1 (SD 129.0) showing an improvement of 105.93%, the non-treatment group mean absolute change was −4.5 (67.6), a change of 1.30% (see fig 1 and table 2).
Figure 1.
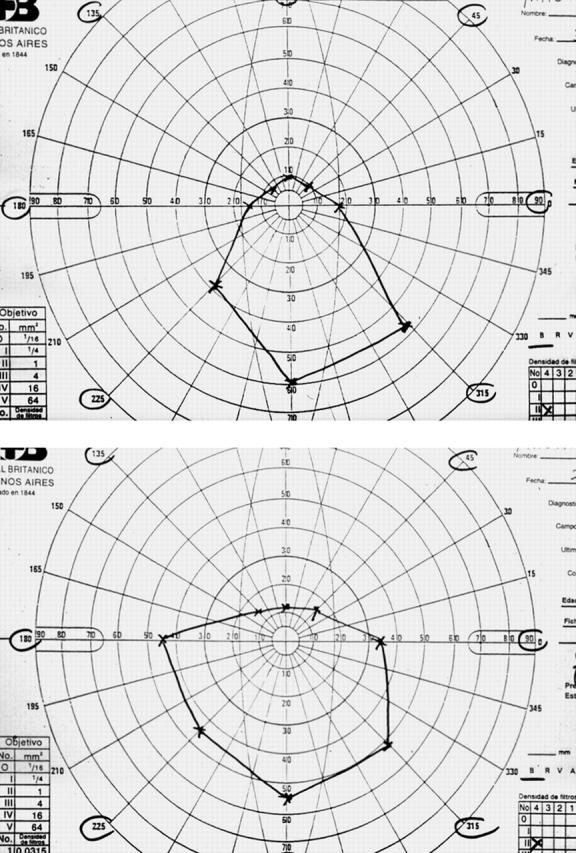
(A) Area of non-diplopia acquired with Goldmann perimeter, the treatment group patient before treatment. (B) Improvement in the area of non-diplopia of same the treatment group patient at week 24.
Table 2.
Ocular motility area of no diplopia (Σ°)—efficacy population
| Ocular motility | Treatment group | Non-treatment group | Difference between groups |
| Week 0 | p = 0.0005 | ||
| Mean (SD) | 231.1 (99.9) | 350.7 (86.5) | |
| Range | (40–398) | (185–485) | |
| Number | 20 | 17 | |
| Week 10, absolute change | p = 0.0072 | ||
| Mean (SD) | 93.6 (129.4) | 1.18 (39.1) | |
| Range | (−60.0–517.0) | (−77.0–82.0) | |
| Number | 20 | 17 | |
| Week 24, absolute change | p = 0.0048 | ||
| Mean (SD) | 107.1 (129.0) | −4.5 (67.6) | |
| Range | (−20.0–497.0) | (−181.0–118.0) | |
| Number | 19 | 15 |
In this pilot study, the treatment group and the non-treatment group differed initially in motility (p = 0.0005); therefore, we conducted a second exploratory analysis of the data of: A, the entire efficacy population; and B, a population were the motility disturbances were not observed in the primary position (or permanent diplopia), or manifest only in the extremes positions of regard. A covariance analysis was applied. The results obtained in A showed no significant statistical differences between groups but statistical significant differences were observed between weeks 0 and 24 (tables 2 and 3). For B, Σ initial mean value (SD) was 304.5 (59.3) for the treatment group (n = 11), and 329.7 (61.0) for the non-treatment group (n = 12); no statistical significant differences between groups were detected (p = 0.3290). At week 10, the treatment group absolute change mean (SD) was 72.3 (63.3), a change of 30.03%, and the non-treatment group absolute change mean (SD) was −4.17 (39.2), a change of −0.59%. Statistically significant differences were detected between groups (p = 0.0060). At week 24, the treatment group absolute change mean (SD) was 79.2 (69.3) with a change of 31.45%, and the non-treatment group absolute change mean (SD) was −11.1 (75.5) with a change of −1.91%. Statistically significant differences were detected between groups (p = 0.0122). Analysis of covariance for this population showed statistically non-significant differences between groups at baseline and significant differences between weeks 10 and 24 (see tables 4 and 5).
Table 3.
Ocular motility area of no diplopia (Σ°): analysis of covariance—efficacy population
| Ocular motility | Treatment group | Non-treatment group | Difference between groups |
| Week 10, absolute change | p = 0.2972* | ||
| LS mean (SE) | 68.4 (22.1) | 30.7 (24.3) | |
| Number | 20 | 17 | |
| Week 24, absolute change | p = 0.2899* | ||
| LS mean (SE) | 76.4 (23.6) | 34.3 (27.1) | |
| Number | 19 | 15 |
*Not significant; LS, mean least square mean from model.
Week 10 v week 0: p = 0.0081.
Week 24 v week 0: p = 0.0032.
Table 4.
Ocular motility area of no diplopia (Σ°)—patients with Σ° between 200° and 400° at baseline—efficacy population
| Ocular motility | Treatment group | Non-treatment group | Difference between groups |
| Week 0 | p = 0.3290* | ||
| Mean (SD) | 304.5 (59.3) | 329.7 (61.0) | |
| Range | (216–398) | (205–387) | |
| Number | 11 | 12 | |
| Week 10, absolute change | p = 0.0060 | ||
| Mean (SD) | 72.3 (63.3) | −4.17 (39.2) | |
| Range | (−4.0–225.0) | (−77.0–63.0) | |
| Number | 11 | 12 | |
| Week 24, absolute change | |||
| Mean (SD) | 79.2 (69.3) | −11.1 (75.5) | p = 0.0122 |
| Range | (−10.0–189.0) | (−181.0–118.0) | |
| Number | 10 | 10 |
*Not significant.
Table 5.
Ocular motility area of no diplopia (Σ°): patients with Σ° between 200° and 400° at baseline, analysis of covariance—efficacy population
| Ocular motility | Treatment group | Non-treatment group | Difference between groups |
| Week 10, absolute change | |||
| LS mean (SE) | 66.5 (14.0) | 1.2 (13.4) | p = 0.0033 |
| Number | 11 | 12 | |
| Week 24, absolute change | |||
| LS mean (SE) | 71.5 (23.6) | −3.4 (20.1) | p = 0.0184 |
| Number | 10 | 10 |
LS, mean least square mean from model.
Week 10 v week 0: p = 0.0146.
Week 24 v week 0: p = 0.0176.
Measurements of extraocular muscles showed the following variations between week 0 and 24: for the inferior rectus muscle, in the treatment group percentage change mean (SD) was −13.21 (25.7) and the non-treatment group percentage change mean (SD) was −4.02 (21.5); no statistically significant differences were detected between groups. For the medial rectus muscle, the treatment group percentage change mean (SD) was −8.24 (20.75) and the non-treatment group percentage change mean (SD) was −0.6 (22.39); no statistically significant differences were detected between groups. For the lateral rectus muscle the treatment group percentage change mean (SD) was −11.5 (20.6) and the non-treatment group percentage change mean (SD) was −0.5 (31.6), no statistically differences were detected between groups. For the superior rectus muscle-levator complex, the treatment group percentage change mean (SD) was −9.5 (29.1) and the non-treatment group percentage mean change (SD) was 12.54 (37.5); statistically significant differences were detected between groups (p = 0. 0060) (fig 2 and table 6).
Figure 2.
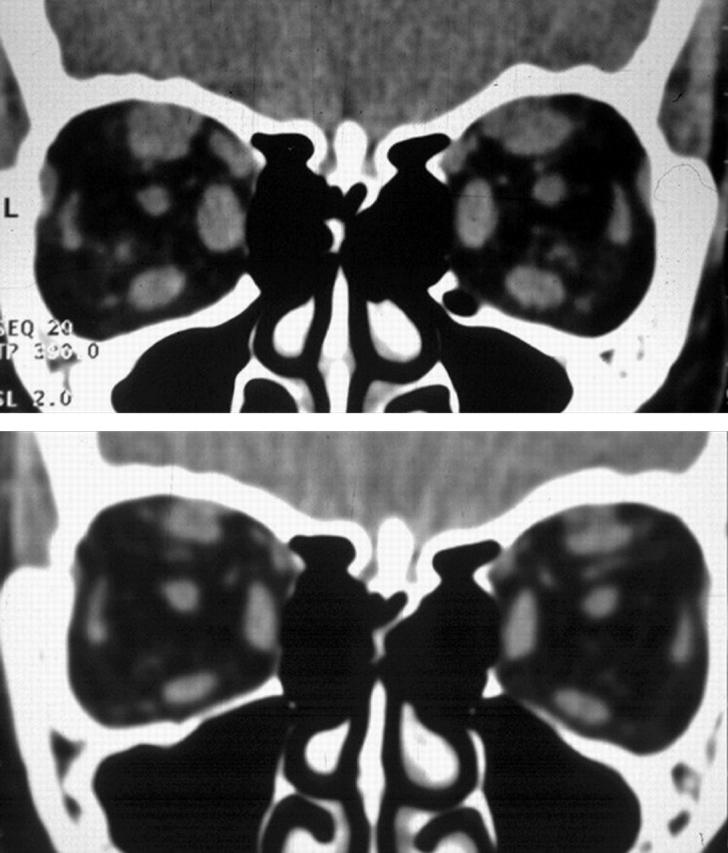
(A) CT scan coronal views of extraocular muscles in the treatment group patient before treatment. (B) CT scan coronal views of extraocular muscles of the same treatment group patient at week 24.
Table 6.
Thickness variations of extraocular muscles relative to optic nerve size—efficacy population
| Muscles | Treatment group* (n = 46) | Non-treatment group (n = 28) | Difference between groups |
| Inferior rectus: | |||
| Week 0 | p = 0.0184 | ||
| Mean (SD) | 1.3 (0.7) | 0.9 (0.3) | |
| Range | (0.4–3.2) | (0.3–2.0) | |
| Week 24, percentage change | p = 0.1173† | ||
| Mean (SD) | −13.2 (25.7) | −4.0 (21.5) | |
| Range | (−60.6–69.1) | (−63.6–42.6) | |
| Medial rectus: | |||
| Week 0 | p = 0.0153 | ||
| Mean (SD) | 1.2 (0.6) | 0.9 (0.3) | |
| Range | (0.5–3.1) | (0.5–1.5) | |
| Week 24, percentage change | p = 0.0900† | ||
| Mean (SD) | −8.2 (20.7) | 0.6 (22.4) | |
| Range | (−42.8–42.8) | (−27.8–66.7) | |
| SRLP: | |||
| Week 0 | p = 0.0506† | ||
| Mean (SD) | 1.2 (0.6) | 0.9 (0.3) | |
| Range | (0.5–3.2) | (0.5–1.8) | |
| Week 24, percentage change | p = 0.0060 | ||
| Mean (SD) | −9.5 (29.1) | 12.5 (37.5) | |
| Range | (−63.8–78.6) | (−41.7–139.2) | |
| Lateral rectus: | |||
| Week 0 | p = 0.0662† | ||
| Mean (SD) | 1.0 (0.4) | 0.9 (0.4) | |
| Range | (0.4–2.1) | (0.4–1.8) | |
| Week 24, percentage change | p = 0.0765† | ||
| Mean (SD) | −11.5 (20.6) | −0.5 (31.6) | |
| Range | (−63.1–39.2) | (−51.6–87.5) |
n, number of eyes; *n = 45 for lateral rectus; †not significant.
No variations were detected between groups related to BCVA, IOP, Ex, BW, and BP at weeks 10 and 24.
There were no variations in blood levels of glycaemia, calcaemia, and cortisol (table 7).
Table 7.
Calcaemia, glycaemia, and plasma cortisol variations—safety population
| Laboratory tests | Treatment group | Non-treatment group |
| Calcaemia (mg/dl) | ||
| Week 0 | ||
| Mean (SD) | 9.1 (0.5) | 9.0 (0.8) |
| Range | (8.3–10.1) | (7.8–10.3) |
| Number | 25 | 20 |
| Week 10 | ||
| Mean (SD) | 8.9 (0.3) | 9.1 (0.6) |
| Range | (8.3–9.6) | (8.1–10.0) |
| Number | 23 | 17 |
| Glycaemia (mg/dl) | ||
| Week 0 | ||
| Mean (SD) | 96.2 (15.3) | 92.8 (24.3) |
| Range | (70.0–146.0) | (71.0–186.0) |
| Number | 25 | 20 |
| Week 10 | ||
| Mean (SD) | 90.1 (16.7) | 86.8 (8.3) |
| Range | (62–134) | (70.0–100.0) |
| Number | 22 | 17 |
| Plasma cortisol (μg/dl) | ||
| Week 0 | ||
| Mean (SD) | 17.2 (5.5) | 19.0 (10.5) |
| Range | (6.0–30.7) | (6.0–37.4) |
| Number | 24 | 20 |
| Week 10 | ||
| Mean (SD) | 14.6 (5.0) | 22.9 (16.6) |
| Range | (6.0–25.3) | (6.2–71.1) |
| Number | 22 | 16 |
Urinary cortisol showed a difference in the treatment group between week 0 and week 10. Since these values are not normally distributed, their results were analysed using a logarithmic transformation that showed a geometric mean value of −31.58% (p = 0.114) for the treatment group and −3.75% (p = 0.842) for the non-treatment group in week 10 (table 8).
Table 8.
Urinary cortisol (μg/24 hours)—safety population
| Urinary cortisol (μg/24 hours) | Treatment group (n = 21) | Non-treatment group (n = 15) | Difference between groups |
| Week 0 | p = 0.0582* | ||
| GM | 43.4 | 75.9 | |
| (GM−SD; GM+SD) | (18.2; 103.6) | (34.0; 169.2) | |
| Range | (4–207) | (20–406) | p = 0.0070 |
| Week 10 | |||
| GM | 29.7 | 73.0 | |
| (GM−SD; GM+SD) | (9.4; 93.3) | (46.0; 115.9) | |
| Range | (3–295) | (34–204) | |
| Week 10, percentage change | p = 0.2869* | ||
| GM | −31.6 | −3.8 | |
| (GM−SD; GM+SD) | (−76.1; 95.9) | (−53.6; 99.6) |
GM, geometric mean; *not significant.
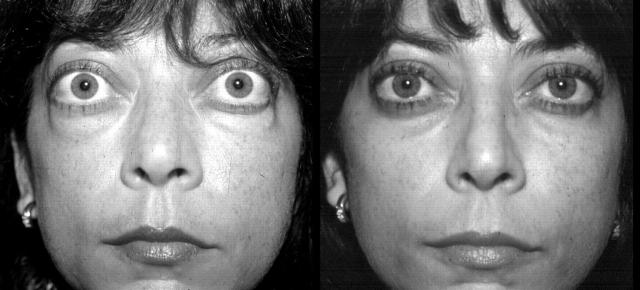
No adverse effects related to the injection were encountered. Figure 3 illustrates the facial aspect of a the treatment group patient at week 0 and 24.
Figure 3.
(A) Treatment group patient, facial aspect before treatment. (B) Same patent at week 24.
DISCUSSION
The use of methyl prednisone and triamcinolone as an intraorbital or subconjunctival injection has already been reported.17–26
Triamcinolone is a synthetic glucocorticosteroid with a potency that equals five times that of cortisol, is metabolised in the liver (tetrahydrocortisol), and excreted as a soluble compound in the urine. It is fluorated in position 9 of the second ring giving it a marked glucocorticoid activity, and a reduced mineralocorticoid activity due to a OH substitution at C16.28,29
The administration by a peribulbar injection in the inferior-lateral quadrant of the orbit allows its diffusion in the retrobulbar fat to the extraocular muscles30,31
Multiple complications have been reported with periocular injections of steroids, including globe perforation,32–36 arterial occlusion,37 toxic optic neuropathy,38 or atrophy of subcutaneous tissue in the face.39,40 We did not encounter any of these problems in our series.
The use of locally administered steroids has been previously reported as beneficial.17–26 Trobe et al have reported unfavourable outcomes in patients with compressive optic neuropathy.24 We have excluded this group of patients from our study.
Sergott and Glaser7 and Lee and Brazis41 warn against their use, based on the lack of studies that demonstrate an improvement in Graves’ ophthalmopathy by local steroids. They are concerned by the increase in volume produced by an injection in a congested orbit.
In this study, we have used triamcinolone injected intraorbitally, and demonstrated an improvement in motility, particularly for the group of patients with non-permanent diplopia (diplopia in eccentric gaze) and a reduction in the extraocular muscle sizes. Best corrected visual acuity has remained unchanged, as well as IOP, exophthalmos, and optic nerve head examination. There were no changes in body weight or blood pressure. Systemic glycaemia, calcaemia, and cortisol remained within normal values for the treatment group and the non-treatment group throughout the study. Urinary cortisol was reduced in 31.58% in the treatment group compared with 3.52% in the non-treatment group. Although these values were not statistically significant, they might suggest a mild depression in endogenous production of cortisol.
We demonstrate in this study the favourable effects of triamcinolone administered as a periocular injection in TAO. Relative to the control group, patients receiving triamcinolone had less diplopia and smaller extraocular muscles. We noted no secondary effects due to the steroid and no local complications caused by the procedure. Owing to the small number of patients entered in this pilot study, a larger series is required to confirm our results.
Acknowledgments
We thank Dr Jonathan Trobe, Kellog Eye Center, for his advice in the early stages of this project; and Dr Ana M Orlandi, Dr Marcela V Moran, Dr Gustavo A Roccatagliata for patient referral; Dr Robert Goldberg for editorial assistance. Statistical consultation: Ricardo Glancszpigel, MSc, Lic Mariangeles Cisneros, Lic María M Callegari.
Abbreviations
BCVA, best corrected visual acuity
BW, body weight
DBP, diastolic blood pressure
IOP, intraocular pressure
ON, optic nerve
SBP, systolic blood pressure
TAO, thyroid associated ophthalmopathy
REFERENCES
- 1.Brain R . Cortisone in exophtalmos, report on a therapeutic trial of cortisone and corticotrophin (ACTH) in exophthalmos and exophthalmic ophthalmoplegia by a panel appointed by the Medical Research Council. Lancet 1955;1:6–9. [PubMed] [Google Scholar]
- 2.Brown J , Coburn JW, Wigod RA, et al. Adrenal steroid therapy of severe infiltrative ophthalmopathy of Graves’ disease. Am J Med 1963;34:786–95. [DOI] [PubMed] [Google Scholar]
- 3.Werner SC. Predniosne in emergency treatment of malignant exophthalmos. Lancet 1966;1:1004–7. [PubMed] [Google Scholar]
- 4.Day MR, Carroll FD. Corticosteroids in the tratment of optic nerve involvement, associated with thyroid dysfunction. Arch Ophthalmol 1968;79:279–82. [DOI] [PubMed] [Google Scholar]
- 5.Fiebel RM, Roper-Hall G. Evaluation of the field of binocular single vision in incomitant strabismus. Am J Ophthalmol 1974;78:800–5. [DOI] [PubMed] [Google Scholar]
- 6.Apers RCL, Oosterhuis JA, Goslings BM, et al. Prednisone treatment in endocrine opthalmopathy. Mod Probl Ophthalmol 1975;14:414–20. [PubMed] [Google Scholar]
- 7.Sergott RC, Glaser JS. Graves’ophthalmopathy. A clinical and immunologic review. Surv Ophthalmol 1981;26:1–21. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson DH, Gorman CA. Endocrine ophthalmopathy: current ideas concerning etiology, pathogenesis and treatment. Endocr Rev 1984;5:200–20. [DOI] [PubMed] [Google Scholar]
- 9.Leone C h. The management of ophthalmic Graves’ disease. Ophthalmology 1984;91:770–9. [DOI] [PubMed] [Google Scholar]
- 10.McConahey WM. Medical therapy. In: Gorman CA, Waller RR, Dyer JA, eds. In: The eye and orbit in thyroid disease. New York: Raven Press, 1984:317–24.
- 11.Nagayama Y , Izumi M, Kiriyama T, et al. Treatment of Graves’ ophthalmopathy with high-dose intravenous methylprednisone pulse therapy. Acta Endocrinol 1987;116:513–18. [DOI] [PubMed] [Google Scholar]
- 12.Kendall-Taylor P , Combie AL, Stephenson AM, et al. Intraveous methylprednisone in the tratment of Graves’ opthalmopathy. BMJ 1988;297:1547–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartalena L , Marcocci C, Bogazzi F, et al. Use of corticosteroids to prevent progression of Graves’ ophthalmopathy after radioiodine therapy for hyperthyroidism. N Engl J Med 1989;321:1349–52. [DOI] [PubMed] [Google Scholar]
- 14.Kazim M , Trokel S, Moore S. Treatment of acute Graves’ orbitopathy. Ophthalmology 1991;98:1443–8. [DOI] [PubMed] [Google Scholar]
- 15.Hiromatsu Y , Tanaka K, Sato M, et al. Intravenous methylprednisone pulse therapy for Graves’ ophthalmopathy. Endocr J 1993;40:63–72. [DOI] [PubMed] [Google Scholar]
- 16.Singer PA, Cooper DS, Levy EG, et al. Treatment guidelines for patients with hyperthyroidism and hypothyroidism. JAMA 1995;273:808–12. [PubMed] [Google Scholar]
- 17.Gebertt S . Depot-methylprednisolone for subconjuntival and retrobulbar injections. Lancet 1961;2:344–5. [DOI] [PubMed] [Google Scholar]
- 18.Garber MI. Methylprednisolone in the treatment of exophthalmos. Lancet 1966;1:958–60. [DOI] [PubMed] [Google Scholar]
- 19.Della Casa F . Zur Therapie des malignen Exophthlmus. Ophthalmologica 1970;161:145–51. [DOI] [PubMed] [Google Scholar]
- 20.Cant JS. The assessment and treatment of endocrine exophthalmos. Proc Roy Soc Med 1970;63:783–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Ivy HK. Medical aproach to ophthalmopathy of Graves’ disease. Mayo Clin Proc 1972;47:980–5. [PubMed] [Google Scholar]
- 22.Thomas ID, Hart JK. Retrobulbar repository corticosteroid therapy in thyroid ophthalmopathy. Med J Aust 1974;2:484–7. [DOI] [PubMed] [Google Scholar]
- 23.Kramar P . Manegement of eye changes of Graves’ disease. Surv Ophthalmol 1974;18:369–82. [Google Scholar]
- 24.Trobe JD, Glaser JS, Laflamme P. Dysthyroid optic neuropathy. Clinical profile and rationale of management. Arch Ophthalmol 1978;96:1199–209. [DOI] [PubMed] [Google Scholar]
- 25.Marcocci C , Bartalena L, Panicucci M, et al. Orbital cobalt irradiation combined with retrobulbar or systemic corticosteroids for Graves’ ophthalmopathy: a comparative study. Clin Endocrinol 1987;27:33–42. [DOI] [PubMed] [Google Scholar]
- 26.Huber A . Ocular motility in Graves’ disease. Neuroophthalmology 1984;4:227–36. [Google Scholar]
- 27.Feibel RM, Roper-Hall G. Evaluation of the field of binocular single vision in incomitant strabismus. Am J Ophthalmol 1974;78:800–5. [DOI] [PubMed] [Google Scholar]
- 28.Jordan DC, Flood JG, Lapostata M, et al. Normal reference laboratory values. N Eng J Med 1992;327:718–24. [DOI] [PubMed] [Google Scholar]
- 29.Goodman AG, Gilman LS. Adrenocortical steroids and their synthetic analogs. In: The pharmacological basis of therapeutics. 9th ed. Chapter 59. New York: McGraw-Hill, 1996:1465–76.
- 30.Koorneef L . New insights in the human orbital connective tissue. Arch Ophthalmol 1977;95:1269–73. [DOI] [PubMed] [Google Scholar]
- 31.Koorneef L . Orbital septa: anatomy and function. Ophthalmology 1979;86:876–80. [DOI] [PubMed] [Google Scholar]
- 32.Waller SG, Taboada J, O’Connor P. Retrobulbar anesthesia risk. Ophthalmology 1993;100:506–10. [PubMed] [Google Scholar]
- 33.Wearne MJ, Flaxel C h J, Gray P, et al. Vitreoretinal surgery after inadvertent globe penetration during local ocular anesthesia. Ophthalmology 1998;105:371–6. [DOI] [PubMed] [Google Scholar]
- 34.Gomez-Ulla F , Gonzales F, y Ruiz-Fraga C, Unintentional intraocular injection of corticosteroids. Acta Ophthalmol 1993;71:419–21. [DOI] [PubMed] [Google Scholar]
- 35.Bullock JD, Warwar RE, Green R. Ocular explosions from periocular anesthetic injections. Ophthalmology 1999;106:2341–53. [DOI] [PubMed] [Google Scholar]
- 36.Lam DSC, Law RWK, Leung ATS, et al. Intraorbital needle fragment: a rare complication of retrobulbar injection. Arch Ophthalmol 1999;117:1089–90. [PubMed] [Google Scholar]
- 37.Egbert JE, Schwartz S, Walsh AW. Diagnosis and treatment of an ophthalmic artery occlusion during an intralesional injection of corticosteroid into an eyelid capillary hemangioma. Am J Ophthalmol 1996;121:638–42. [DOI] [PubMed] [Google Scholar]
- 38.Teus MA, Teruel JL, Pascual J, et al. Corticosteroid-induced toxic optic neuropathy. Am J Ophthalmol 1991;112:605–6. [DOI] [PubMed] [Google Scholar]
- 39.Droste PJ, Ellis FD, Sondhi N, et al. Linear subcutaneous fat atrophy after corticosteroid injection of periocular hemangiomas. Am J Ophthalmol 1988;105:65–9. [DOI] [PubMed] [Google Scholar]
- 40.Fraunfelder FT, Grove JA. In: Drug-induced ocular side effects. 4th ed. Baltimore: Willams & Wilkins, 1996:323–8.
- 41.Lee AG, Brazis PW. Thyroid eye disease, Graves’ ophthalmopathy. In: Clinical pathways in neuro-ophthalmology. New York: Thieme Medical, 1998:270–1.