Abstract
Background/aim: Alterations of the immune system may have a role in thrombogenesis. Artery sites occluded with thrombi apparently release pro-inflammatory cytokines. Non-arteritic anterior ischaemic optic neuropathy (NAION) results from occlusion of the blood supply to the optic nerve. The aim of this study was to analyse levels of pro-inflammatory cytokines in patients with acute event of NAION.
Methods: Study participants included 10 patients (12 eyes) with NAION and 20 age matched controls with the same risk factors for atherosclerosis disease. Peripheral blood samples were obtained immediately at the acute onset of NAION. Plasma interleukin 8 (IL-8), IL-6, and tumour necrosis factor alpha (TNF-α) levels were measured immediately following diagnosis and during the follow up intervals.
Results: The plasma levels of IL-8 were significantly higher in NAION patients at the time of diagnosis in comparison to the control group (p = 0.002), and decreased during the follow up period (6–12 months) (p = 0.05). There were no differences in plasma levels of IL-6 and TNF-α between NAION patients and controls, either in the acute phase or during the follow up period.
Conclusion: Plasma levels of IL-8 are elevated during the acute phase of NAION, but not IL-6 and TNF-α. These elevated levels are in accordance with other acute vascular thrombosis. The clinical significance of these findings should be further evaluated.
Keywords: interleukins, tumour necrosis factor, anterior ischaemic optic neuropathy
Alterations of the immune system appear to have a crucial role in the pathogenesis of thrombosis.1,2 Thrombosis superimposed on atherosclerotic plaque is a major cause of occlusive vascular disease.3 Researchers believe that arterial sites occluded with thrombi release local proinflammatory secretory polypeptides, termed cytokines, such as tumour necrosis factor alpha (TNF-α), interleukin (IL) 6, and IL-8, via several types of cells (lymphocytes, monocytes, macrophages, vascular endothelial cells, smooth muscle cells and fibroblasts).4,5 A systemic increase in plasma IL-6 and IL-8 levels after ischaemia and reperfusion processes has so far been confirmed clinically in patients with acute coronary arteries occlusion and cerebral vascular accidents (CVA).6,7
Anterior ischaemic optic neuropathy (AION) results from interruption of the blood supply to the anterior part of the optic nerve.8,9,10 It may be arteritic AION (AAION) or non-arteritic AION (NAION). NAION is essentially caused by transient hypoperfusion or non-perfusion of the posterior ciliary artery circulation in the optic nerve head.11 NAION is characterised by small vessel occlusion as a result of atherosclerotic disease, as opposed to AAION in which giant cell vasculitis is pathognomonic. NAION has the same classic risk factors of atherosclerosis—namely, hypertension, diabetes mellitus, hyperlipidaemia, and older age.12
NAION is the leading cause of sudden optic nerve related vision loss in the United States, affecting 2.3–10.2 per 100 000 people over 50 years old.13 Patients typically present with sudden, painless unilateral loss of vision associated with swelling of the optic nerve head in the affected eye.14–16 The diagnosis may be made immediately or weeks to months following the acute event. Unfortunately, 15–40% of patients experience another event in the fellow eye within a short period.12 The mechanism underlying involvement of the fellow eye is not understood.
Identification of the factors that trigger the evolution of NAION associated thrombosis may result in better diagnosis and treatment. The aim of this study was to determine changes in plasma levels of proinflammatory cytokines in patients with acute NAION.
METHODS
Patients
The study group consisted of 10 patients (12 eyes) who presented with acute NAION at the Department of Ophthalmology of Rabin Medical Center between January 1998 and June 2000 and agreed to participate in the study. Arteritic AION patients, diagnosed by clinical and laboratory tests, and patients with either elevated erythrocyte sedimentation rate (ESR), C reactive protein (CRP), or positive temporal biopsy were excluded. Blood for cytokine levels (IL-6, IL-8, and TNF-α) was drawn from all patients immediately at diagnosis, during the acute event, and in majority of the patients during the follow up interval.
Control serum samples were obtained from 20 age matched subjects with similar atherosclerosis risk factors and without visual symptoms. All were candidates for elective cataract surgery with no history of optic neuropathy, unilateral visual loss, or history suggestive for giant cell arthritis.
The study was approved by the institutional review board and was performed in accordance with the Declaration of Helsinki for research involving human subjects.
Laboratory tests
Serum samples from patients and controls were kept at −20°C until analysis. Levels of IL-6, IL-8, and TNF-α were measured by enzyme linked immunosorbent assay (ELISA) kits (R & D Systems, MN, USA).
Statistical analysis
The data were analysed statistically with the SPSS-X package. The χ2 test and Fisher’s exact test were used to compare nominal data between the groups, and Mann-Whitney test was used to compare cytokine levels between groups. Analysis of the change in cytokines levels during the follow up period of each patient was performed with the aparametric Wilcoxon test.
RESULTS
Patient characteristics
The study group included 10 patients, three men and seven women, mean age 67.8 years (range 56–85 years, SD 7.5 years), with mean follow up of 2.3 years (range 1–3.2 years). NAION was diagnosed in the right eye in five cases and in the left eye in the other seven cases.
All 10 patients had plasma cytokine levels sample on diagnosis. Repeat plasma cytokines levels were checked at 3, 6, 9, and 12 months for three, six, two, and three patients respectively.
Of 10 patients with NAION, eight had a unilateral event whereas two had bilateral events, with one being evaluated in both acute episodes. The time interval between the two episodes was 12 months in one patient (with blood samples in the two episodes) and 9 months in the other.
All patients had normal ESR values at diagnosis (mean, 31 mm in the first hour) and CRP levels within normal range (mean 0.4 mg/dl). All patients started aspirin treatment (325 mg once a day) on diagnosis.
Underlying systemic diseases in the patient group included hypertension (five patients), ischaemic heart disease (two patients), CVA (one patient), and non-insulin dependent diabetes (NIDDM) (three patients). Two patients were on low dose aspirin at the time of the acute event, and none was treated with coumadin or corticosteroids.
The control group included eight men and 12 women, mean age 70.2 years (range 53–85 years, SD 8.9 years). Underlying systemic diseases included hypertension (eight subjects), ischaemic heart disease (two subjects), and seven had NIDDM. In addition, five of the control group were receiving low dose aspirin. None was treated with coumadin or corticosteroids.
Serum cytokines
IL-8
Plasma levels of IL-8 were examined at the acute onset of NAION in all patients, and another 14 samples during the follow up interval. Elevated plasma levels of IL-8 in NAION patients at the time of diagnosis (84.6 (SD 69.1) pg/ml) were significantly higher in comparison with the control group (20.2 (12.8) pg/ml; p = 0.002) (fig 1A), and decreased during the follow up period (3–12 months) (p = 0.05). Three months after the acute phase, plasma levels of IL-8 were similar to those of controls (p = 0.63) (fig 1B).
Figure 1.
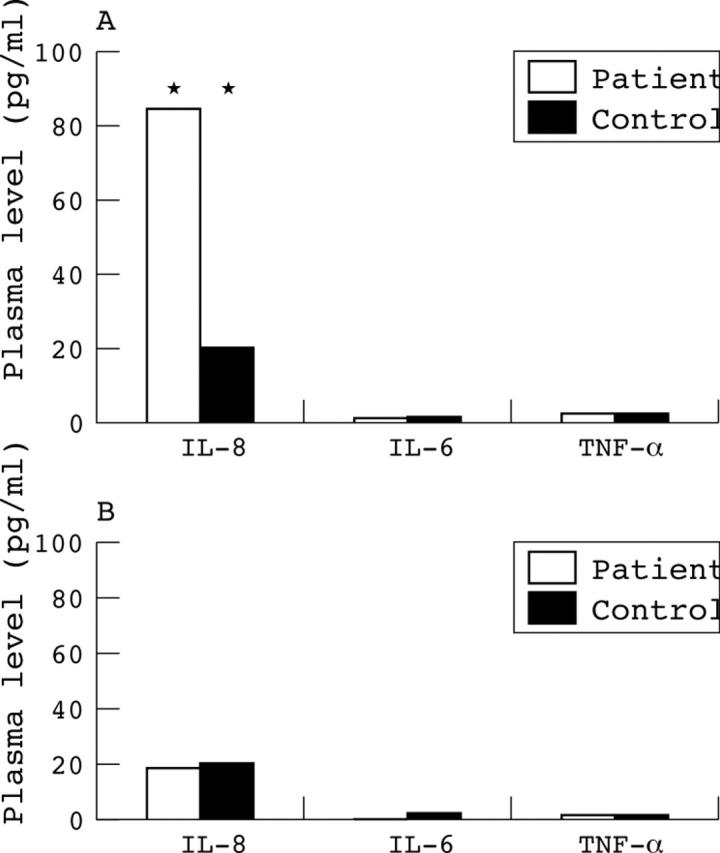
(A) Plasma levels of cytokines (IL-8, IL-6, TNF- α) during acute non-arteritic ischaemic optic neuropathy (NAION). Note the significant elevation of IL-8 in the patient group. (B) Plasma levels of cytokines 3–12 months following NAION event. No differences in plasma levels of cytokines IL-8, IL-6, TNF- α between patients and control groups. Note that IL-8 levels return to normal range.
Patient 3 was found to have significantly higher levels of IL-8 (100 pg/ml) during the acute NAION event, compared with the average levels of the control group (18 pg/ml). During his follow up interval, the levels of IL-8 decreased gradually (21 pg/ml and 9 pg/ml at 6 and 9 months, respectively). However, significantly increased IL-8 levels reappeared when the patient developed an acute event of NAION in his fellow eye (484 pg/ml) after 12 months of follow up.
Patient 7 also had bilateral NAION, but no blood samples for cytokines were taken during the fellow eye NAION, which appeared after 9 months of follow up.
IL-6
Plasma levels of IL-6 were examined at the acute onset of NAION, and after 3, 6, 9, and 12 months. Plasma levels of IL-6 in acute NAION (3.4 (SD 3.1) pg/ml) were similar to the control group (3.88 (5.17) pg/ml; p = 0.6) (fig 1A). Plasma levels of IL-6 did not change significantly and were similar to those of the control group during the follow up at 3, 6, 9, and 12 months (fig 1B).
TNF-α
Plasma levels of TNF-α were examined at the acute onset of NAION, and after 3, 6, 9, and 12 months. Plasma levels of TNF-α in acute NAION (0.8 (SD 1.2) pg/ml) were similar to the control group (1.4 (1.5) pg/ml; p = 0.93) (fig 1A). Plasma levels of TNF-α did not change significantly and were similar to those of the control group during the follow up at 3, 6, 9 and 12 months (fig 1B).
DISCUSSION
The present clinical study revealed significant elevations of IL-8 during the acute phase of NAION. In accordance with this study, studies in baboons have shown elevations in IL-8 levels after induction of a thrombus, suggesting that the thrombotic event could contribute to the increased IL-8 concentration.17 In addition, thrombotic events in patients with paraneoplastic syndrome were found to be associated with elevated IL-8 levels.18,19
IL-8 is a potent neutrophil chemotactic cytokine (chemokine) known to have a role in inflammation and host defence.20–22 As a product of different types of cells, it may be present in any tissue and be produced during infections, ischaemia, trauma, and other disturbances of tissue homeostasis. It is likely to be the main cause of the local accumulation of neutrophils.21,23 Recently, locally increased levels of IL-8 were detected in the lumen of the coronary arteries following myocardial infarction.7
During acute NAION, no change in plasma levels of TNF-α or IL-6 could be detected. TNF-α is an important induction agent of IL-8. During acute myocardial infarction, increased plasma IL-6 and IL-8 levels were accompanied by increased plasma IL-1β and TNF-α levels. However, the peak of the TNF-α concentration occurs in the late phase.7,24,25 Therefore, TNF-α might serve as a negative control in the early stages of myocardial infarction, as well as in acute NAION. However, IL-8 resistance to inactivation and its slow clearance may prolong the presence of IL-8 in active form beyond that of most other mediators in the immediate environment of the cells from which it is released.20
Recent studies of thrombotic syndromes have shown that inflammation may stimulate coagulation activity and thereby enhance the risk of thrombosis. Atherosclerotic plaques, which are rich in soft extracellular lipids and macrophages, may be more vulnerable to plaque rupture and consequent cytokine secretion. These data suggest that NAION might occur secondary to atherosclerotic plaque rupture and the resulting increase in production of IL-8 by the macrophages.
Cytokines are non-antibody proteins, secreted by inflammatory leucocytes and some non-leucocytic cells that act as intercellular mediators. Treatment of NAION traditionally consists of aspirin, a non-steroidal anti-inflammatory drug, mainly because of its anticoagulant effect. However, a better understanding of the cytokine response may lead to new therapeutic possibilities.26 Experimental studies in a cerebral perfusion injury model has found that rabbits given a neutralising anti-IL-8 antibody had significantly reduced brain oedema and infarct size compared to rabbits receiving a control antibody.27
Although many types of cells, including lymphocytes,28 fibroblasts,29 monocytes,30 macrophages,31 smooth muscle cells, and endothelial cells4,5 are capable of producing IL-6 and IL-8 in cell culture, the cell source of production of IL-8 in NAION remains to be established. So far, endothelial cells are the most likely.4,5
Our findings may also have important diagnostic implications. The most common presenting symptom in AION is sudden painless visual loss. In some cases, the acute event goes unnoticed, and the patient appears later when he or she suddenly discovers the visual loss. To date, the only diagnostic index for discriminating acute from long standing visual loss was the optic nerve head appearance. Disc pallor cannot be detected before 6 weeks from the event. On the basis of this study, we propose measuring IL-8 plasma levels as a possible laboratory tool to discriminate acute onset from long standing or slowly progressive visual loss.
Although human studies revealed plasma IL-8 elevations following brain ischaemia, rabbit models of ischaemia reperfusion showed only a local increase in the brain tissue.27 Other models of coronary artery occlusion demonstrated both local and systemic elevations in IL-8 levels.3,7,32 In this study we evaluated the systemic levels of IL-8 only, as ocular fluid is not routinely obtained in patients with acute visual loss secondary to NAION.
In summary, this study demonstrated remarkable elevation of IL-8 plasma levels in optic nerve related infarct (NAION). In the absence of histological confirmation of the small vessel occlusion, these findings support the pathophysiology of microvascular thrombosis causing NAION. The clinical significance of these findings should be further evaluated.
Abbreviations
AAION, arteritic AION
AION, anterior ischaemic optic neuropathy
CRP, C reactive protein
CVA, cerebral vascular accidents
ELISA, enzyme linked immunosorbent assay
ESR, erythrocyte sedimentation rate
IL-8, interleukin 8
NAION, non-arteritic AION
NIDDM, non-insulin dependent diabetes
TNF-α, tumour necrosis factor alpha
Supported by the Gothalf Fund, Tel Aviv University, Israel.
REFERENCES
- 1.Esmon CT. Inflammation and thrombosis. J Thromb Haemost 2003;1:1343–8. [DOI] [PubMed] [Google Scholar]
- 2.Tousoulis D , Davies G, Stefanadis C, et al. Inflammatory and thrombotic mechanisms in coronary atherosclerosis. Heart 2003;89:993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miya Y , Kanda T, Tamura J, et al. A new murine model of coronary artery thrombosis and role of interleukin-8 in the development of coronary thrombosis. Res Commun Mol Pathol Pharmacol 2000;108:108–15. [PubMed] [Google Scholar]
- 4.Hoch RC, Schraufstatter IU, Cochrane CG. In vivo, in vitro, and molecular aspects of interleukin-8 and the interleukin-8 receptors. J Lab Clin Med 1996;128:134–45. [DOI] [PubMed] [Google Scholar]
- 5.Luster AD. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med 1998;338:436–45. [DOI] [PubMed] [Google Scholar]
- 6.Kanda T , Hirao Y, Oshima S, et al. Interleukin-8 as a sensitive marker of unstable coronary artery disease. Am J Cardiol 1996;77:304–7. [DOI] [PubMed] [Google Scholar]
- 7.Kato K , Matsubara T, Iida K, et al. Elevated levels of pro-inflammatory cytokines in coronary artery thrombi. Int J Cardiol 1999;70:267–73. [DOI] [PubMed] [Google Scholar]
- 8.Foulds WS, Chisholm IA, Stewart JB, et al. The optic neuropathy of pernicious anemia. Arch Ophthalmol 1969b;82:427–32. [DOI] [PubMed] [Google Scholar]
- 9.Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol 1969;53:721–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLeod D , Marshall J, Kohner EM. Role of axoplasmic transport in the pathophysiology of ischaemic disc swelling. Br J Ophthalmol 1980;64:247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayreh SS. Posterior ciliary artery circulation in health and disease: the Weisenfeld lecture. Invest Ophthalmol Vis Sci 2004;45:749–57, 748. [DOI] [PubMed] [Google Scholar]
- 12.Newman NJ, Scherer R, Langenberg P, et al. The fellow eye in NAION: report from the ischemic optic neuropathy decompression trial follow-up study. Am J Ophthalmol 2002;134:317–28. [DOI] [PubMed] [Google Scholar]
- 13.Miller NR, Newman NJ, eds. Walsh & Hoyt’s clinical neuro-ophthalmology, the essentials. 5th ed. Baltimore: Williams & Wilkins, 1999.
- 14.Barron KD, Dentinger MP, Krohel G, et al. Qualitative and quantitative ultrastructural observations on retinal ganglion cell layer of rat after intraorbital optic nerve crush. J Neurocytol 1986;15:345–62. [DOI] [PubMed] [Google Scholar]
- 15.Berkelaar M , Clarke DB, Wang YC, et al. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci 1994;14:4368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida K , Behrens A, Le-Niculescu H, et al. Amino-terminal phosphorylation of c-Jun regulates apoptosis in the retinal ganglion cells by optic nerve transection. Invest Ophthalmol Vis Sci 2002;43:1631–5. [PubMed] [Google Scholar]
- 17.Wakefield TW, Greenfield LJ, Rolfe MW, et al. Inflammatory and procoagulant mediator interactions in an experimental baboon model of venous thrombosis. Thromb Haemost 1993;69:164–72. [PubMed] [Google Scholar]
- 18.Monreal M , Fernandez-Llamazares J, Perandreu J, et al. Occult cancer in patients with venous thromboembolism: which patients, which cancers. Thromb Haemost 1997;78:1316–18. [PubMed] [Google Scholar]
- 19.de Maistre E , Regnault V, Lecompte T, et al. Antibodies to interleukin-8 and paraneoplastic catastrophic recurrent thromboses. Am J Med 2001;111:580–1. [DOI] [PubMed] [Google Scholar]
- 20.Baggiolini M , Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol 1995;17:103–8. [DOI] [PubMed] [Google Scholar]
- 21.Mukaida N . Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol 2000;72:391–8. [PubMed] [Google Scholar]
- 22.Tarlowe MH, Kannan KB, Itagaki K, et al. Inflammatory chemoreceptor cross-talk suppresses leukotriene B4 receptor 1-mediated neutrophil calcium mobilization and chemotaxis after trauma. J Immunol 2003;171:2066–73. [DOI] [PubMed] [Google Scholar]
- 23.Harada A , Sekido N, Akahoshi T, et al. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 1994;56:559–64. [PubMed] [Google Scholar]
- 24.Maury CP, Teppo AM. Circulating tumour necrosis factor-alpha (cachectin) in myocardial infarction. J Intern Med 1989;225:333–6. [DOI] [PubMed] [Google Scholar]
- 25.Guillen I , Blanes M, Gomez-Lechon MJ, et al. Cytokine signaling during myocardial infarction: sequential appearance of IL-1 beta and IL-6. Am J Physiol 1995;269:R229–35. [DOI] [PubMed] [Google Scholar]
- 26.Entman ML, Michael L, Rossen RD, et al. Inflammation in the course of early myocardial ischemia. Faseb J 1991;5:2529–37. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto T , Ikeda K, Mukaida N, et al. Prevention of cerebral edema and infarct in cerebral reperfusion injury by an antibody to interleukin-8. Lab Invest 1997;77:119–25. [PubMed] [Google Scholar]
- 28.Hirano T , Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature 1986;324:73–6. [DOI] [PubMed] [Google Scholar]
- 29.Kohase M , May LT, Tamm I, et al. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol 1987;7:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horii Y , Muraguchi A, Suematsu S, et al. Regulation of BSF-2/IL-6 production by human mononuclear cells. Macrophage-dependent synthesis of BSF-2/IL-6 by T cells. J Immunol 1988;141:1529–35. [PubMed] [Google Scholar]
- 31.Wang JM, Sica A, Peri G, et al. Expression of monocyte chemotactic protein and interleukin-8 by cytokine-activated human vascular smooth muscle cells. Arterioscler Thromb 1991;11:1166–74. [DOI] [PubMed] [Google Scholar]
- 32.Romuk E , Skrzep-Poloczek B, et al. Selectin-P and interleukin-8 plasma levels in coronary heart disease patients. Eur J Clin Invest 2002;32:657–61. [DOI] [PubMed] [Google Scholar]


