Abstract
Aim: To study the anatomical and visual performance following implantation of a model of artificial cornea and to evaluate the postoperative long term complications.
Methods: 11 eyes of 11 patients with bilateral corneal blindness considered as potentially having high risk of failure of penetrating corneal keratoplasty were implanted with biocolonisable Kpro keratoprosthesis (BIOKOP I, FCI, Rantigny, France) in the period between January 1996 and May 1998. Only one eye was implanted in all patients and followed up for a period of 60 months. The visual outcome, anatomical and functional stability, complications, and the general performance of the keratoprosthesis were evaluated.
Results: The keratoprosthesis (BIOKOP I) only 36.3% remained in position to date. In the patients’ last visit five eyes (45.4%) were blind and one (9.0%) showed a slight improvement in the best corrected visual acuity (BCVA) in comparison to preoperative tests. Six eyes (54.5%) showed improved BCVA before having postoperative complications. Four eyes underwent replacement of a BIOKOP I Kpro with a BIOKOP II as a result of extrusion. The keratoprostheses remained anatomically in situ for a mean of 25.5 months and their functional performance period was limited to a mean of 22 months.
Conclusion: Corneal keratoprosthesis (BIOKOP I, II) does not provide a stable anatomical relation with the surrounding ocular structures. Its ability to restore vision is limited to a short postoperative period in eyes implanted with severe ocular surface disease.
Keywords: corneal graft failure, penetrating keratoplasty, keratoprosthesis
Major causes of blindness in developing countries include infectious corneal diseases such trachoma and xerophthalmia, whereas in industrialised countries it is mainly caused by herpetic disease, acid or alkaline burns, rheumatoid arthritis, pemphigoid, and Stevens- Johnson syndrome.1 Many patients who have visual impairment as a result of corneal opacity can be successfully treated by corneal transplant. A number of these patients are considered as having a high risk of failure of corneal graft procedures because of potential risk of rejection or severe ocular surface disorders despite the advances in immunosuppression on ocular reconstruction.2–4 Moreover, in certain countries, donor corneas are unavailable, as a result of custom or religious practice.
In such cases, keratoprosthesis (Kpro) provides the only alternative to avoid corneal graft failure in an attempt to achieve visual rehabilitation. Kpro has the goal of replacing the central cornea with a clear optical cylinder of adequate refractive power in the visual axis.4,5,6,7,8,9,10,11,12,13 However, postoperative complications are still frequent and severe enough to limit its use. Recent advances in Kpro aim at preventing and managing complications after implantation to improve prognosis and general outcomes.11,14–18 Emerging biocompatible materials are opening the way for visual rehabilitation in cases of severe and intractable corneal blindness. The concept of biocompatibility has been recently implemented with the development of new biomaterials that have the potential of being populated by cells from the surrounding tissues, in this case, the cornea. Such materials, which are called “biocolonisable,” might further improve the integration of the Kpro and are a new way to increase the biocompatibility of new designs of Kpro.
In this study we present the long term outcome of patients, considered as having a high risk of corneal graft failure as a result of severe ocular surface disease, when implanted with one of these new models of the biocolonisable Kpro (BIOKOP I, (FCI, Rantigny, France).15,16
MATERIALS AND METHODS
In this prospective, non-randomised, observational case series 11 eyes (11 patients, six females and five males) were included. The patients were followed for up to 5 years. Ethics board committee approval was obtained for the study. Adequate informed consent was obtained from each patient following the tenets of Helsinki Declaration (Washington, 2002).
Prosthesis
BIOKOP I prosthesis is composed of a biointegrable synthetic hydrogel “core and skirt” with a central transparent optic surrounded by a white porous skirt that allows biointegration to ensure fixation and prevents epithelial downgrowth. The optical and physical characteristics were published in a previous report.15 There are two models, 43 dioptres (D) for phakic eyes and 58 D for aphakic eyes (fig 1).
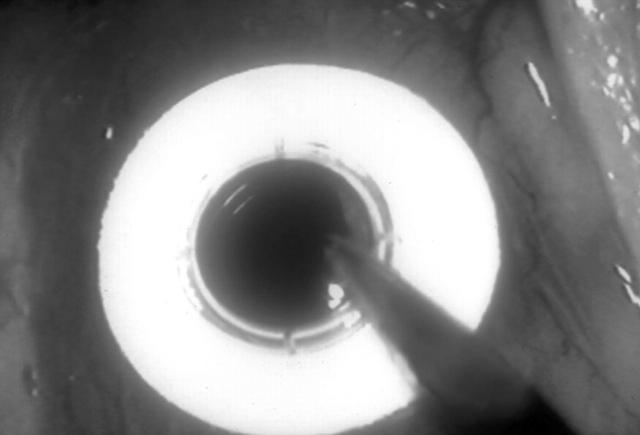
Figure 1.
BIOKOP I before implantation.
In cases where severe peripheral melting and potential extrusion occurred, the BIOKOP I was explanted and replaced with the BIOKOP II (FCI, Rantigny, France). The BIOKOP II has the same haptics design as the previous prosthesis, but the optic is manufactured in silicone and has a diameter of 6 mm.
Patients
Indication for surgery included bilateral corneal blindness not treatable by penetrating or lamellar keratoplasty because of chronic unfavourable ocular surface or lid disease or previous repetitive history of corneal graft failure. The mean age of the patients was 62.9 (range 18–85 years). All patients were diagnosed as having bilateral blindness caused by severe ocular surface disease preventing the success of corneal grafting. The preoperative best corrected visual acuity (BCVA) ranged between ability to count fingers (CF) and light perception (LP) (see table 1).
Table 1.
Pre-existing conditions of trial patients and surgery associated with the Kpro implantation
| Patient | Age (years) | Preoperative BCVA, LPP, and CP | Original ocular disease | Pre-existing associated conditions | Associated surgery |
| 1 | 70 | LP, LPP, CP | Leprosy | Lid and conjunctival surgery | Vitrectomy, ECCE |
| 2 | 66 | LP, LPP, CP | Keratomalacia, graft failure | Rheumatoid arthritis, type 2 diabetes, KP failures, dry eye | Vitrectomy, ECCE |
| 3 | 61 | LP, LPP, CP | Ocular pemphigoid | Diabetes, retinitis pigmentosa, phaco + IOL dry eye | Vitrectomy |
| 4 | 42 | LP, LPP, CP | Corneal alkali burn | 3 KP failures, dry eye | Vitrectomy, ECCE |
| 5 | 18 | LP, LPP, CP | Local radiotherapy (brain tumour), graft failure | Dry eye, 3 KP failures | Vitrectomy, ECCE+IOL |
| 6 | 65 | CF, LPP, CP | Ocular pemphigoid | Diabetes, cataract, dry eye | ECCE+IOL |
| 7 | 76 | LP, LPP, CP | Ocular pemphigoid | Cataract, dry eye | ECCE+IOL |
| 8 | 80 | LP, LPP, CP | Corneal alkali burn | KP + IOL | IOL explanted |
| 9 | 67 | HM, LPP, CP | Trachoma | Cataract, lid surgery, dry eye | ECCE+IOL |
| 10 | 85 | LP, LPP, CP | Ocular pemphigoid | Cataract (phaco + IOL), dry eye | |
| 11 | 62 | LP, LPP, CP | Ocular pemphigoid | Diabetes, cataract, dry eye | ECCE+IOL |
BCVA, best corrected visual acuity; LP, light perception; LPP, light projection; CP, colour perception; KP, keratoplasty; ECCE, extracapsular cataract extraction; iop. intraocular pressure.
All the patients had good light projection (LPP) and good (CP). B-scan ultrasound was performed routinely in the preoperative evaluation and in the follow up to study the intraocular structures. Patients with no light perception or having structural vitreous or retinal abnormalities with the B-scan were excluded from the study. Other exclusion criteria included antecedents with clinical evidence of glaucoma, the inability to self administer medication, or inability to fulfil the postoperative follow up visits.
The surgical procedure was performed by the same surgeon (JLA) using the standard method previously described by Legeais and Renard.16 The haptic was inserted into lamellar pocket delaminated in the stroma and the optic was positioned through a hole trephined in the central cornea. In five of the eyes, extracapsular cataract extraction (ECCE) was accompanied by the implantation of a posterior chamber intraocular lens (IOL) (AcrySof, Alcon, Fort Worth, TX, USA) and we used the aphakic Kpro model. The procedure was associated with an anterior vitrectomy in five of the cases. A buccal mucous membrane was placed in front of the Kpro following implantation and a temporary tarsorrhaphy was performed and opened after 2 weeks (fig 2). At the end of the procedure dexamethasone and gentamicin were injected subconjunctivally and tobramycin ointment was applied. Mucous membrane opening was performed to expose the optic at the end of the second postoperative month using radiofrequency probe (Ellman International Inc, USA). During the follow up the patients used one drop of tobramycin (Tobrex, Alcon Cusi, Barcelona, Spain) twice a day and artificial tears (Liquifilm, Alcon-Cusi, Barcelona, Spain) at least four times daily. The patients were followed up weekly for the first 2 months, then every 15 days until the fourth month, then every 3 months thereafter. At each visit, the visual performance, the stability of the prosthesis and the mucous membrane, leak from Kpro, and the condition of the ocular surface, were evaluated.
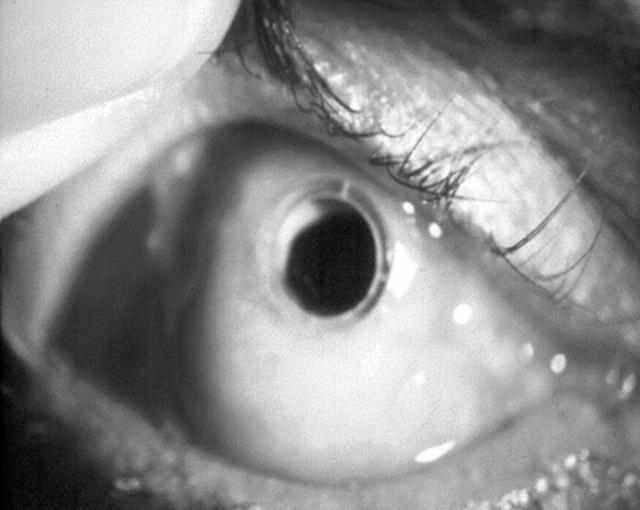
Figure 2.
An implanted BIOKOP I opening the mucous membrane.
RESULTS
The preoperative ocular diagnosis of the patients, their clinical condition leading to corneal blindness, and the surgical procedures are presented in table 1. The follow up after Kpro implantation was 60 months.
Preoperative BCVA was LP in 81.8% (nine) of the eyes, CF in 9.1% (one) of eyes, and HM in another 9.1% (one) of eyes. Other existing preoperative ocular conditions included ocular pemphigoid in five (45.5%) eyes, corneal alkali burn two (18.2%) eyes, trachoma in one (9.1%) eye, ocular leprosy in one (9.1%) eye, and keratomalacia in one (9.1%) eye. One eye (9.1%) developed total corneal opacity following local radiotherapy followed by graft failure.
Associated general conditions included diabetes in four (36.4%) patients, rheumatoid arthritis in one (9.1%), and retinitis pigmentosa in one (9.1%). Four eyes (36.4%) had single previous unsuccessful penetrating keratoplasty (PKP), and one patient underwent repeated PKP. The visual outcomes at the different postoperative visits, the evolution of the BCVA, and the complications developed during the follow up visits are given in table 2.
Table 2.
Ophthalmic complications and visual outcome
| Patients | Follow up (months) | Complications | Preop BCVA | Obtained BCVA during the follow up | Final BCVA |
| 1 | 24 | Endophthalmitis | LP | 0.1 | LP |
| 2 | 11 | None | LP | LP | LP |
| 3 | 8 | Extrusion | LP | LP | LP |
| 4 | 24 | Endophthalmitis, extrusion | LP | HM | NLP |
| 5 | 43 | RD, endophthalmitis | LP | 0.1 | NLP |
| 6 | 33 | RD, phthisis | CF | LP | NLP |
| 7 | 46 | Extrusion | LP | HM | LP |
| 8 | 26 | RD, extrusion | LP | LP | NLP |
| 9 | 57 | Endophthalmitis | HM | 0.2 | NLP |
| 10 | 23 | Endophthalmitis, phthisis | LP | LP | LP |
| 11 | 60 | None | LP | HM | HM |
LP, light perception; NLP, no light perception; HM, hand movement.
Complications
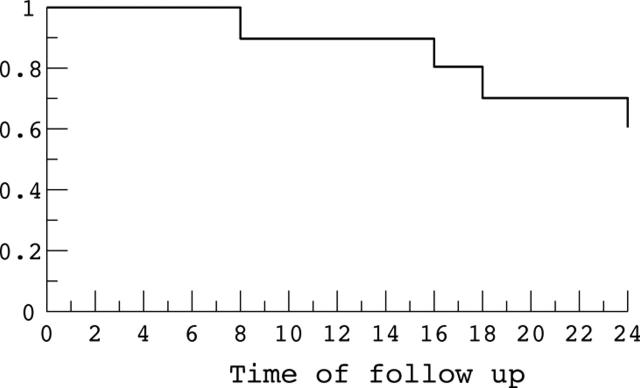
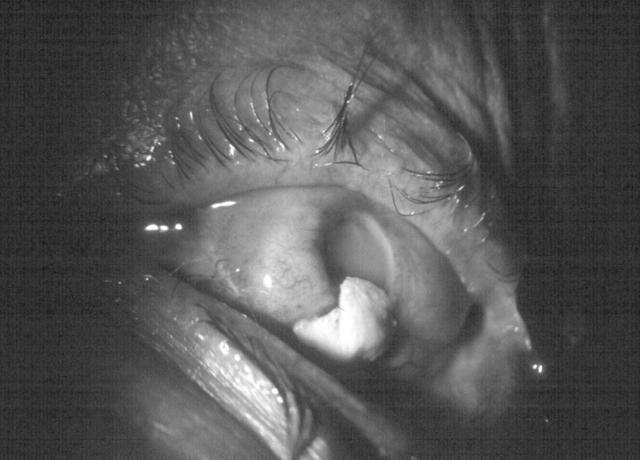
At the end of surgery, the Kpro were anatomically well attached in all eyes and no significant intraoperative complications were detected. Postoperatively and throughout the follow up, 72.7% of the patients showed partial dislocation of the Kpro or melting. 36.4% of the first implanted Kpro were retained to date with a mean duration of anatomical retention of the Kpro being 25.5 months as shown in figures 3 and 4. Endophthalmitis developed in four patients (36.4%) and the isolated pathogens included Gram positive cocci (Streptococcuspneumoniae) in two eyes, Staphylococcus epidermidis in one eye, and Staphylococcusaureus in one eye (fig 5). All these eyes were previously in good condition before endophthalmitis and showed no evidence of leakage in any case.
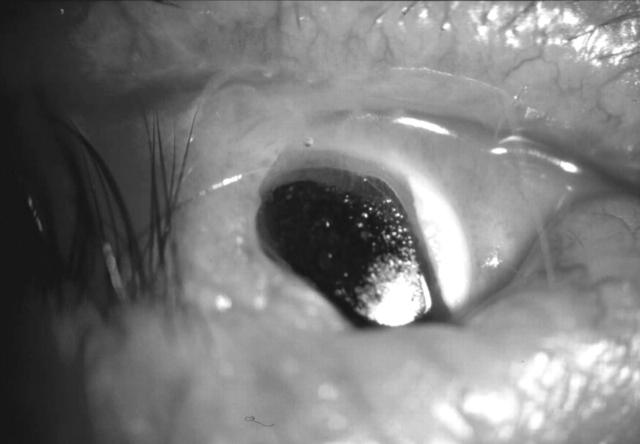
Figure 3.
Exposure of Kpro skirt in an extruded implant.
Figure 4.
Kaplan-Meyer curve of keratoprosthesis extrusion.
Figure 5.
Severe endophthalmitis caused by Staphylococcus in patient 4 at 12 months.
Corneal melting was the most frequent complication and occurred in eight cases (table 3). Corneal melting led to the exteriorisation of the Kpro skirt and was treated in all cases by partial mucous membrane grafting, which only solved the problem permanently in one case, multiple mucous regrafts being necessary in the rest.
Table 3.
Kpro retention and its complications
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Retroprosthetic membrane (n) | 1 | 1 | 1 | 1 | 3 | − | 3 | 3 | 3 | 1 | 2 |
| Partial Kpro dislocation or melting | − | + | − | − | + | + | + | + | + | + | + |
| Kpro substitution (at month) | − | − | − | − | − | − | 30 | − | 29 | 15 | 2 |
| Retained at the date of reporting or month of extrusion | R | R | 8 | 18 | R | R | 16 | 24 | R | R | R |
Retroprosthetic membrane
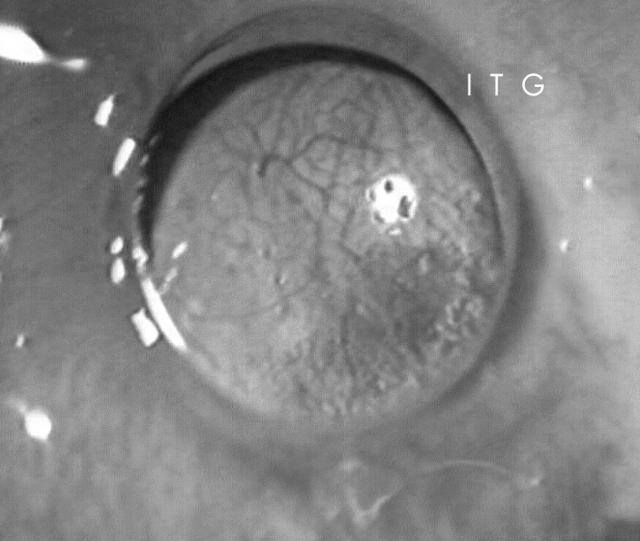
Immediately after removing the mucous graft and opening the median tarsorrhaphy at 2 months, a retroprosthetic membrane was observed in three of the cases. During the follow up period, it was further developed in another seven cases, giving a total incidence of retroprosthetic membrane development of 90.1%. Such membranes were surgically removed in all cases but recurred in 45.5% of the eyes despite the use of systemic corticosteroids (fig 6).
Figure 6.
A dense vascularised retroprosthetic membrane observed 3 months after the surgical procedure.
Retinal detachment (RD) was also a serious complication that affected the postoperative recovery. In table 3, we show the data concerning Kpro retention and the different complications observed during follow up. Retinal detachment developed in three (27.3%) eyes—one eye had a history of repeated multiple corneal grafts and postoperative endophthalmitis, another eye suffered an advanced stage of diabetic retinopathy following the surgery, and one eye had a history of corneal graft and developed recurrent postoperative retroprosthetic membranes resistant to all treatments.
At the time of writing, the postoperative BCVA was LP in five (45.5%), hand movement (HM) in one (9.1%), and no light perception in five (45.5%). Five patients (45.5%) maintained their preoperative BCVA, one eye had a better postoperative BCVA compared to the preoperative value, and the rest of the eyes (five, 45.5%) reported no PL (see table 2).
DISCUSSION
Autologous tooth, bone graft, and cartilage have been used with relative success for Kpro haptics fixation, despite the complexity of the procedures required and the development of complications such as extrusion or glaucoma.19 A combination of PMMA prosthesis and a clear corneal graft in association with anticollagenase were used to prevent tissue ulceration around the PMMA collar.3 Choyce used perforated PMMA discs20 while Cardona used non-stick coatings of the haptic (non-porous Teflon).13 Despite many attempts, adequate cellular colonisation failed because of lack of appropriate porosity.21–23 The Polack Kpro provided considerable visual results with limited ability to remain in place for a long follow up period (20% with BCVA of 0.2 or better at 1 year, but 100% were extruded or removed within the first 2 years).15,22 The Proplast was studied because of its gas and fluid permeability but remained too rigid for the corneal tissue.24
Some studies have approached the development of microporous biocompatible polymers to decrease the mechanical tissue damage, especially the interconnection pore size to improve the biointegration of the implant.9,15,16,23 Designs such as the Seoul-type,9 AlphaCor,25 and the BIOKOP15,16 have followed this principle. Legeais and Renard developed the BIOKOP implant based on the theory of improved biointegration. The implant had biocolonisable micropores of 80 μm that improved the results of biointegration and safety in a follow up period of up to 28 months.15,16 In their series, two (18.9%) eyes developed peri-prosthesis leak from the Kpro skirt, and required additional mucous membrane grafting. In our study, phthisis bulbi was detected in two eyes (18.2%) as a complication of postoperative RD and endophthalmitis, endophthalmitis occurred in five (45.5%) eyes, and Kpro extrusion occurred in four (36.4%) eyes. In four (36.4%) eyes, the Kpro was replaced by BIOKOP II keratoprosthesis to prevent further melting of the ocular tissue. In those eyes, peripheral inflammatory reaction and melting, endophthalmitis, and extrusion followed the surgery, and further treatment was discarded.
Among the difficulties arising during this study were the problems of symblepharon, severe cicatrising conjunctival and adnexal disease, and recurrent inflammatory corneal conditions with a densely opaque, vascularised cornea. We compared the preoperative BCVA of the patients included in this study with those in the study of Legeais et al,15 who implanted the same Kpro. The relatively better results obtained in their study could be explained by the better preoperative conditions of their patients, more specifically the severity of ocular surface disease of the patients reported in both series. Comparing the visual outcome and the complications with those in the study by Legeais et al, in this study 6/11 patients (27.3%) showed improvement in BCVA before having further complications such as endophthalmitis, extrusion or deterioration of vision to preoperative values and only one eye maintained its postoperative BCVA (9.1%). Legeais et al had 70.8% of patients who maintained or improved their preoperative BCVA up to 28 months and only 4.1% of their cases developed endophthalmitis.15 Nouri et al reported endophthalmitis in 12% of the eyes implanted in their animal model study,26 while other authors reported no cases of endophthalmitis,18 but in a series of patients with much better preoperative conditions.
The role of postoperative corticosteroids in Kpro surgery is not clear. It seems that systemic corticosteroids might induce ulceration at the junction between the fluorocarbon and PMMA skirt with extrusion of the Kpro; therefore, in order to prevent the possibility of infection the use of systemic corticosteroids should be kept to a minimum.26
Retroprosthetic membrane developed in 90.1% of the eyes and recurred in 45.5% of the eyes despite the use of systemic corticosteroids which seems to have no role in the prevention of the risk of developing intraocular inflammation and membrane formation. The aetiology remains unknown and might include inflammation, wound exposure, and bleeding during surgery which could be related to the preoperative ocular surface situation.27 The membranes were very thick, vascularised, and required a vitrectomy probe cut for their removal in all the eyes except one (YAG laser). Legeais et al reported intraocular membranes developing in 20.8% of their patients15 and membranes did not develop in other series using a different Kpro.18 Lund hypothesised that the aphakic eyes without vitrectomy were predisposed to retroprosthetic membrane so cataract extraction combined with vitrectomy could prevent further membrane formation and occlusion of the optic of the prosthesis.28 The results of this report do not support this hypothesis. All the eyes underwent cataract extraction, and a group of these patients underwent a combined procedure of cataract removal and anterior vitrectomy (45.5%). Studying the incidence of postoperative membranes (90.9%), this complication therefore contradicts Lund’s theory28; the only significant difference is that retroprosthetic membrane recurred less frequently when the combined procedure was performed, but unfortunately it was not avoided.
According to our results the biocompatible inert microporous polymers did not eliminate all the complications associated with this surgery. The patients included in our study are considered a poor prognostic category because of higher risk of significant complications, not only affecting final visual acuity but also reducing the chance of successful long term device retention caused by factors such as severe cicatrising conjunctival disease or recurrent inflammatory corneal conditions.3,10,26,29
In summary, the BIOKOP Kpro evaluated in this clinical study was unsuccessful in restoring visual acuity and was followed by high proportion of significant complications. Long term follow up of eyes implanted with this type of Kpro showed a high percentage of complications resulting in explantation or extrusion. This study shows that for adequate estimation of the potential of new models of Kpro, long term follow up is necessary to prove the anatomical stability and visual improvement.
Abbreviations
BCVA, best corrected visual acuity
BIOKOP, biocolonisable microporous fluorocarbon haptic
CF, count fingers
CP, colour perception
ECCE, extracapsular cataract extraction
Kpro, keratoprosthesis
LP, light perception
LPP, light projection
PKP, penetrating keratoplasty
This study has been financed in part by a grant of the Spanish Ministry of Health, Instituto Carlos III, Red Tematica de Investigation Cooperativa en Oftalmologia, Subproyecto de Cirugía Refractiva, Calidad de Visión C03/13.
REFERENCES
- 1.Whitcher JP. Neonatal ophthalmia: have we advanced in the last 20 years? Int Ophthalmol Clin 1990;30:39–41. [DOI] [PubMed] [Google Scholar]
- 2.Yaghouti F , Nouri M, Abad JC, et al. Keratoprosthesis: preoperative prognostic categories. Cornea 2001;20:19–23. [DOI] [PubMed] [Google Scholar]
- 3.Dholman CH, Doane MG. Some factors influencing outcome after keratoprosthesis surgery. Cornea 1994;13:214–18. [DOI] [PubMed] [Google Scholar]
- 4.Hull CC, Liu CS, Sciscio A, et al. Optical cylinder designs to increase the field of vision in the osteo-odonto-keratoprosthesis. Graefes Arch Clin Exp Opthalmol 2000;238:1002–8. [DOI] [PubMed] [Google Scholar]
- 5.Hiks CR, Lou X, Platten S, et al. Keratoprosthesis results in animals: an update. Aust N Z J Ophthalmol 1997; 1 :S50–2. [DOI] [PubMed]
- 6.Chirila TV. An overview of the development of artificial corneas with porous skirts and the use of phema for such an application. Biomaterials 2001;22:3311–17. [DOI] [PubMed] [Google Scholar]
- 7.Linnola RJ, Happonen RP, Vedel E, et al. Titanium and bioactive glass-ceramic coated titanium as materials for keratoprosthesis. Exp Eye Res 1996;63:474–8. [DOI] [PubMed] [Google Scholar]
- 8.Zhao H , Xie D, Zou H. Current researches in keratoprosthesis. Sheng Wu yi Xue Gong Cheng Xue Za Zhi 2002;19:112–16. [PubMed] [Google Scholar]
- 9.Kim MK, Lee JL, Wee WR, et al. Seoul-type keratoprosthesis: preliminary results of the first 7 human cases. Arch Ophthalmol 2002;120:761–6. [DOI] [PubMed] [Google Scholar]
- 10.Khan B , Dudenhoefer EJ, Dohlman CH. Keratoprosthesis: an update. Curr Opin Ophthalmol 2001;12:282–7. [DOI] [PubMed] [Google Scholar]
- 11.Krug A , Kompa S, Schrage NF. The Aachen-keratoprosthesis—a flexible kpro that permits intraocular pressure measurements. Int J Artif Organs 2002;25:238–42. [DOI] [PubMed] [Google Scholar]
- 12.Trinkaus-Randall V , Capecchi J, Newton A, et al. Development of a biopolymeric keratoprosthetic mater evaluation in vitro and in vivo. Invest Ophthalmol Vis Sci 1988;29:393–400. [PubMed] [Google Scholar]
- 13.Cardona H . Keratoprosthesis-acrylic optical cilinder with supporting intralamellar plate. Am J Ophthalmol 1962;54:284–94. [PubMed] [Google Scholar]
- 14.Ray S , Khan BF, Dohlman CH, et al. Management of vitreoretinal complications in eyes with permanent keratoprosthesis. Arch Ophthalmol 2002;120:559–66. [DOI] [PubMed] [Google Scholar]
- 15.Legeais JM, Renard G, Parel JM, et al. Expanded fluorocarbon polymer for keratoprosthesis: cellular ingrowth and transparency. Exp Eye Res 1994;58:41–51. [DOI] [PubMed] [Google Scholar]
- 16.Legeais JM, Renard G, Parel JM, et al. Keratoprosthesis with biocolonizable microporous fluorocarbon haptic. Preliminary results in a 24-patient study. Arch Ophthalmol 1995;113:757–63. [DOI] [PubMed] [Google Scholar]
- 17.Hille K . Keratoprostheses. Clinical aspects. Ophthalmologe 2002;99:523–31. [DOI] [PubMed] [Google Scholar]
- 18.Crawford GJ, Hicks CR, Lou X, et al. The Chirila keratoprosthesis: phase I human clinical trial. Ophthalmology 2002;109:883–9. [DOI] [PubMed] [Google Scholar]
- 19.Marchi V , Ricci R, Pecorella I, et al. Osteo-odontokeratoprosthesis: description of surgical technique with results in 85 patients. Cornea 1994;13:125–30. [PubMed] [Google Scholar]
- 20.Choyce DP. Results of keratoprosthesis in Britain. Am J Ophthalmol 1987;103:331–2.3826241 [Google Scholar]
- 21.Heimke G , Polack FM. Ceramic keratoprosthesis: biomechenics of extrusion in throught the lid implantation. Cornea 1983;2:187–201. [Google Scholar]
- 22.Crawford GJ, Constable IJ, Chirila TV, et al. Tissue interaction with hydrogel sponges implanted in the rabbit cornea. Cornea 1993;12:348–57. [DOI] [PubMed] [Google Scholar]
- 23.Huang YF, Wang LQ, Wang FX. Clinical application of keratoprosthesis for corneal opacity unsuitable for keratoplasty. Zhonghua Yan Ke Za Zhi 2003;39:578–81. [PubMed] [Google Scholar]
- 24.Barber JC, Feaster F, Priour D. The acceptance of vitreous carbon alloplastic material, Proplast, in rabbit eye. Invest Ophthalmol Vis Sci 1980;19:182–91. [PubMed] [Google Scholar]
- 25.Hicks CR, Crawford GJ, Lou X, et al. Corneal replacement using a synthetic hydrogel cornea, AlphaCor: device, preliminary outcomes and complications. Eye 2003;17:385–92. [DOI] [PubMed] [Google Scholar]
- 26.Nouri M , Terada H, Alfonso EC, et al. Endophthalmitis after keratoprosthesis. Arch Ophthalmol 2001;119:484–9. [DOI] [PubMed] [Google Scholar]
- 27.Dudenhoefer EJ, Nouri M, Gipson IK, et al. Histopatology of explanted collar button keratoprostheses: a clinicopathologic correlation. Cornea 2003;22:425–8. [DOI] [PubMed] [Google Scholar]
- 28.Lund OE. Keratoprosthesis: 25 years of experience. Refract Corneal Surg 1993;9:186–7. [Google Scholar]
- 29.Dohlman CH, Terada H. Keratoprosthesis in pemphigoid and Stevens-Johnson syndrome. Adv Exp Med Biol 1998;438:1021–5. [DOI] [PubMed] [Google Scholar]