Abstract
The nonreceptor tyrosine kinase FAK (“focal adhesion kinase”) is a key mediator of integrin signaling events controlling cellular responses to the extracellular matrix, including spreading, migration, proliferation, and survival. Integrin-ligand interactions stimulate FAK tyrosine phosphorylation and activation of FAK signaling functions. Here evidence is presented that the FAK autophosphorylation site Tyr-397 mediates a direct interaction with the C-terminal Src homology 2 domain of phospholipase C (PLC)-γ1 and that this is required for both adhesion-dependent association of the two molecules and increased inositol phosphate production in mouse embryo fibroblasts. Overexpression of FAK and PLC-γ1 in COS-7 cells increases PLC-γ1 enzymatic activity and tyrosine phosphorylation, also dependent on FAK Tyr-397. However, FAK appears incapable of directly phosphorylating PLC-γ1. These observations suggest a role for FAK in recruiting PLC-γ1 to the plasma membrane at sites of cell-matrix adhesion and there promoting its enzymatic activity, possibly by releasing the repression caused by intramolecular interactions of the PLC-γ1 Src homology domains and/or by positioning it for phosphorylation by associated Src-family kinases. These findings expand the known signaling functions of FAK and provide mechanistic insight into integrin-stimulation of PLC-γ1.
The interactions of integrins with their ligands, including components of the extracellular matrix, influence diverse cellular traits and activities including survival, proliferation, differentiation, and migration (1, 2). Focal adhesion kinase (FAK) has emerged as a primary mediator of integrin signaling (3). FAK is a nonreceptor tyrosine kinase that colocalizes with integrins at sites at which cells make close adhesive contact with the extracellular matrix substratum (4, 5). Integrin-mediated cell adhesion promotes rapid FAK tyrosine phosphorylation (5), which, like receptor tyrosine kinases, results in the activation of signaling functions.
Six FAK tyrosines (397, 407, 576, 577, 861, and 925) have been identified as sites of adhesion-dependent phosphorylation (3). Of these, Tyr-397 is the only apparent site of autophosphorylation (6). Phosphorylation of Tyr-397 creates a high-affinity binding site for the Src homology 2 (SH2) domains of Src-family tyrosine kinases, including c-Src and Fyn (6, 7), which could promote their activation by C-terminal tail displacement. Phosphotyrosine (pTyr) 397 appears to have other signaling roles, however, as this site also mediates interactions with SH2 domains of the p85 subunit of phosphatidylinositol 3-kinase (PI3K) (8). Unlike Tyr-397, the other FAK phosphoacceptor tyrosines are not autophosphorylation sites. Rather, they appear to be phosphorylated by Src-family kinases that associate with FAK (3). Phosphorylation of Tyr-576 and Tyr-577, which lie in the FAK kinase domain activation loop, appears to elevate FAK catalytic activity (9, 10). Phosphorylation of Tyr-925 creates a binding site for the Grb2 SH2 domain (11). Signaling functions have yet to be assigned to FAK Tyr-407 and Tyr-861, although the conservation of these sites in diverse vertebrates suggests a possible SH2 binding function.
Here we report that FAK can directly interact with the γ1 isoform of phospholipase C (PLC-γ1)—an enzyme that catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate to generate the second messengers diacylglycerol, which activates protein kinase C, and inositol 1,4,5-trisphosphate, which mobilizes intracellular Ca2+. The FAK/PLC-γ1 interaction is mediated by the C-terminal SH2 domain of PLC-γ1 (PLC-γ1-SH2C) binding to FAK pTyr-397 and appears to promote PLC-γ1 activation in response to cell adhesion.
MATERIALS AND METHODS
Cells, Cell Culture, and Fibronectin-Replating.
BALB/c 3T3 mouse fibroblasts and COS-7 cells and culture conditions have been described (9). “TetFAK(WT)” and “TetFAK(F397)” (10) are mouse embryo fibroblasts initially derived from FAK-null E8.0 embryos and engineered to inducibly express either WT-FAK or F397-FAK, respectively, under control of the tetracycline repression system. Tet-FAK cells were maintained in DMEM supplemented with 4,500 mg/liter d-glucose, 584 mg/liter glutamine, 1 mM sodium pyruvate, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml Amphotericin B, 1 mM nonessential amino acids (all from GIBCO/BRL), and 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA). Tetracycline (Calbiochem) at 1.0 μg/ml was included to maintain Tet-FAK cells in the uninduced state, and FAK expression was induced by culturing 48 hr in the absence of tetracycline. Cell detachment and fibronectin-replating were carried out as described (5), except cells were starved before detachment by incubating 20 hr in DMEM containing 0.5% fetal bovine serum.
Glutathione S-Transferase (GST)–SH2 Binding Assays.
pGEX plasmids expressing SH2 domains fused to GST were kindly provided by Peter van der Geer (Univ. of California, San Diego), Tony Pawson (Samuel Lunenfeld Research Institute), and Lan Bo Chen (Dana-Farber Cancer Institute). The cDNA inserts were confirmed by sequencing to encode the following residues: bovine PLC-γ1-SH2C (amino acids 663–759) and -SH2N (amino acids 545–659), bovine p85-SH2C (amino acids 614–724) and -SH2N (amino acids 333–446), human SHC (amino acids 318–473), chicken c-Src (amino acids 148–251), mouse SHP-2 (Syp) -SH2C (amino acids 108–215), and chicken tensin (amino acids 1,417–1,593). Approximately 50 μg of each GST-SH2 protein (or GST alone), expressed in Escherichia coli and adsorbed to glutathione-agarose (12), was added to cell lysates (0.5 mg total protein in 1.0 ml) prepared in Nonidet P-40 buffer [1% Nonidet P-40/50 mM Tris⋅HCl, pH 7.4/150 mM NaCl/50 mM NaF/2 mM Na⋅orthovanadate/1% aprotinin (Sigma)/1× protease inhibitor mixture (Calbiochem)], and samples were incubated 2 hr at 4°C with constant rocking to allow binding of cellular protein. After extensive washing in Nonidet P-40 buffer, bound protein was resolved by SDS/PAGE and was subjected to immunoblot analysis.
Protein Expression in COS-7 Cells.
The mammalian expression vector pRC/CMV (Invitrogen) was engineered to express mouse FAK (5) in which N-terminal residues (MAA) are replaced by the c-Myc epitope MEQKLISEEDLGSP. Plasmids expressing Myc-tagged FAK variants were constructed by replacing relevant small restriction fragments of the resulting pRC/CMV-MycFAK(WT) with those isolated from pRC/CMV-FAKHA plasmids (9). pRC/CMV-Src(F535) expresses a constitutively active mouse c-Src (neuronal form) (13). Rat PLC-γ1 with an N-terminal triple-HA epitope tag was expressed from pcDNA3.1 (Invitrogen). All constructs were confirmed by sequencing. Plasmids were transfected into subconfluent cultures of COS-7 cells by using Lipofectamine (GIBCO/BRL), and lysates were analyzed after 48 hr.
Immunoprecipitation, Immunoblotting, and “Far-Western” Analysis.
For immunoprecipitation, cell lysates were prepared in Nonidet P-40 buffer and were adjusted to a protein concentration of 0.5 mg/ml. FAK was immunoprecipitated from Tet-FAK cells by using 1 μg/ml polyclonal antibody C-20 (Santa Cruz Biotechnology). Myc epitope-tagged FAK and HA epitope-tagged PLC-γ1 were immunoprecipitated by using 3 μg/ml monoclonal antibody 9E10 (Calbiochem) or monoclonal antibody 12CA5 (Boehringer Mannheim), respectively, followed by rabbit anti-mouse IgG polyclonal antibody (10 μg/ml, Cappel). Immunoprecipitates were collected on protein-A Sepharose (Zymed) and were washed extensively in Nonidet P-40 buffer followed by washing buffer (50 mM Tris⋅HCl, pH 7.5/100 mM NaCl/2 mM EGTA/0.1% Nonidet P-40/50 mM NaF/1 mM Na⋅orthovanadate) before further analysis. For immunoblotting, proteins in immunoprecipitates were resolved by SDS/PAGE and were transferred to nylon or nitrocellulose membranes. After blocking, the blots were probed with either rabbit antisera 331 (5) (1:500 dilution) to detect nontagged FAK, monoclonal antibody 9E10 (1 μg/ml) to detect epitope-tagged FAK, monoclonal 12CA5 (1 μg/ml) to detect epitope-tagged PLC-γ1, polyclonal antibody 1249 (Santa Cruz Biotechnology) to detect nontagged PLC-γ1, and monoclonal 4G10 (1 μg/ml, Upstate Biotechnology, Lake Placid, NY) to detect pTyr. Bound antibodies were visualized by either enhanced chemiluminescence or autoradiography by using [125I] protein-A (ICN). To reprobe a blot, antibodies were stripped by incubating in 100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris⋅HCl (pH 6.7) at 50°C for 30 min. For Far-Western analysis (14), blots containing FAK variants were probed with 2 μg/ml GST-SH2 fusion protein, and bound fusion protein was detected by using 1 μg/ml polyclonal antibody against GST (Sigma).
In Vitro Phosphorylation of PLC-γ1.
Kinase reactions were performed in 50 μl kinase assay buffer [50 mM Hepes, pH 7.4/10 mM MnCl2/1 mM DTT/10 μCi [γ-32P] ATP (4,500 Ci/mmol, ICN)] containing 1 μg of baculovirus-expressed PLC-γ1 (15) and either 1 μg of baculovirus-expressed FAK (16) or 15 units of recombinant c-Src (Upstate Biotechnology). In some experiments, 22 μM PD161430 (Src-selective inhibitor “4f” in ref. 17) was included. After incubating 20 min at 25°C, reactions were stopped by adding an equal volume of 2× SDS/PAGE sample buffer, and protein phosphorylation was assessed by SDS/PAGE–autoradiography.
In Vitro Assay of PLC-γ1 Activity.
HA-tagged PLC-γ1, coexpressed with Myc-tagged FAK variants in COS-7 cells, was immunoprecipitated by using 12CA5 monoclonal antibody. After a final wash in 50 mM NaH2PO4/100 mM KCl, the immunoprecipitates were assayed for PLC-γ1 activity by measuring [3H]inositol 1,4,5-trisphosphate produced by hydrolysis of [3H]phosphatidylinositol 4,5-bisphosphate (18).
In Vivo Assay of PLC Activity.
Subconfluent Tet-FAK cells were incubated 48 hr in serum-free DMEM, followed by 8 hr in serum-free inositol-free DMEM (GIBCO/BRL) and 16 hr in serum-free inositol-free DMEM containing 1 μCi/ml myo-[2-3H] inositol (25 mCi/mmol, NEN). After an additional 1 hr incubation in the same media lacking the isotope followed by 20 min in the same media containing 20 mM LiCl, cells were detached by trypsin and were held in suspension in serum-free inositol-free DMEM containing 20 mM LiCl. Half of the cell suspension (≈105 cells) was replated in the presence of 20 mM LiCl onto a fibronectin-coated tissue culture well (6-well plate; Becton Dickinson) and was allowed to attach and spread for 30 min at 37°C whereas the other half was held in suspension at 37°C. The fibronectin-adherent and suspended cells then were washed separately in PBS and were treated with 750 μl of 20 mM formic acid for 30 min on ice to extract inositol phosphates. The extracts were neutralized by adding 250 μl of 50 mM NH4OH, insoluble material pelleted by centrifugation, and supernatants applied to AG1-X8 ion-exchange columns to separate inositol phosphates (19). The formic acid pellets were dissolved in 50 mM Tris⋅HCl (pH 8.0), 1% SDS, and 0.8% Nonidet P-40 and were measured for protein concentration by using the bicinchoninic acid assay (Pierce). Total inositol phosphates were measured by scintillation counting of 3H in appropriate column fractions and were expressed as cpm/mg protein.
RESULTS
FAK pTyr-397 Is Recognized by the C-Terminal SH2 Domain of PLC-γ1.
To explore possible signaling events associated with FAK tyrosine phosphorylation, a variety of SH2 domains, expressed as GST fusions and coupled to agarose beads, were tested for their ability to form a stable complex with FAK from lysates of adherent BALB/c-3T3 fibroblasts (Fig. 1A). Of those tested, the SH2 domain from c-Src most efficiently recovered FAK from the lysate whereas significant FAK recovery also was obtained by using PLC-γ1-SH2C and the two SH2 domains of the p85 subunit of PI3K. Little or no FAK was recovered by other SH2 domains, including PLC-γ1-SH2N, SHC, SHP2-SH2C, and tensin. Because the potential of a direct signaling link between FAK and PLC-γ1 has not been extensively examined, we decided to study the interaction between these two proteins and to investigate the possibility that FAK plays a role in activating PLC-γ1 signaling in response to integrin-mediated cell adhesion.
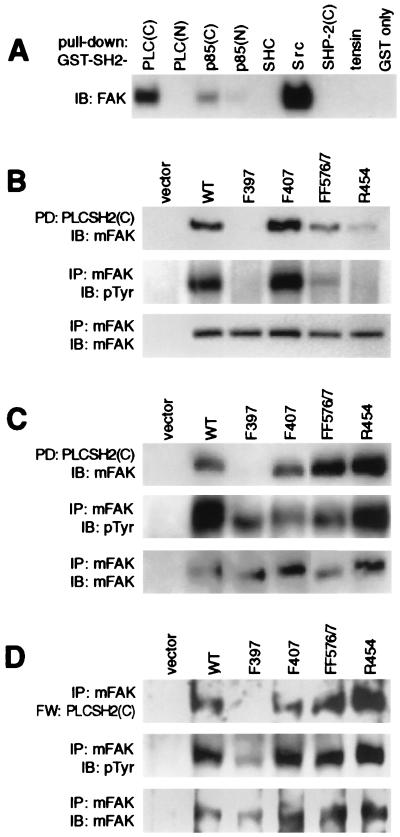
Figure 1.
The C-terminal SH2 domain of PLC-γ1 interacts with FAK pTyr397. (A) Lysates of adherent BALB/c-3T3 cells were incubated with various GST-SH2 proteins (or GST only) immobilized on glutathione agarose beads. Proteins “pulled down” by the beads were separated by SDS/PAGE and were immunoblotted (IB) with anti-FAK polyclonal antibody 331. (B) Lysates of COS-7 cells expressing Myc-tagged FAK (mFAK) variants (WT, F397, F407, FF576/7, or R454) were subjected to GST-PLC-γ1-SH2C pull-down [PD: PLCSH2(C)], and the presence of mFAK variants was detected by immunoblotting with anti-Myc antibody 9E10 (Top). Relative mFAK protein and pTyr levels in the lysates were determined by immunoprecipitation with 9E10 followed by immunoblotting with either 9E10 (Bottom) or anti-pTyr antibody 4G10 (Middle), respectively. (C) mFAK variants were coexpressed with activated Src in COS-7 cells, and lysates were examined for their association with GST-PLC-γ1-SH2C (Top), tyrosine phosphorylation (Middle), and protein levels (Bottom) as above. (D) mFAK variants coexpressed with active Src in COS-7 cells were isolated by immunoprecipitation using 9E10 antibody and were assessed for direct binding of GST-PLC-γ1-SH2C by Far-Western (FW) analysis (Top). The membrane was sequentially stripped and reprobed with 4G10 (Middle) and 9E10 (Bottom) antibodies to determine relative mFAK pTyr and protein levels, respectively. The FAK expression vector alone was used as control for assays in B, C, and D.
To determine the FAK phosphorylation site(s) required for association with PLC-γ1-SH2C, GST-SH2 pull-down assays were performed by using lysates prepared from transfected COS-7 cells expressing either wild-type (WT) FAK or various FAK mutants, including phenylalanine substitutions for known phosphoacceptor tyrosines. The FAK variants were tagged at their N terminus with the c-Myc epitope to permit their specific detection by using monoclonal antibody 9E10. As shown in Fig. 1B Top), WT-FAK was efficiently recognized by PLC-γ1-SH2C. Substitution of FAK Tyr-397 with phenylalanine (F397) was sufficient to completely eliminate the interaction whereas substituting FAK Tyr-407 (F407) had no effect. Phenylalanine substitutions for the activation loop tyrosines (F576/F577) partially inhibited the recovery. Because the F576/F577 substitutions negatively affect FAK kinase activity (9, 10), the reduced interaction of this FAK variant with PLC-γ1-SH2C is likely attributable to reduced autophosphorylation of Tyr-397. Supporting this, the “kinase-dead” Arg substitution for the ATP-binding Lys-454 (R454) was also poorly recovered by PLC-γ1-SH2C, and both F576/F577 and R454 variants exhibited greatly reduced pTyr levels (Fig. 1B). It was important to rule out the possibility that PLC-γ1-SH2C binds to a site other than Tyr-397, but one that becomes phosphorylated as a result of Src-kinases interacting with Tyr-397. Therefore, the PLC-γ1-SH2C pull-down experiments were repeated by using COS-7 cell lysates in which the FAK variants were coexpressed with a catalytically active Src kinase to achieve high levels of FAK tyrosine phosphorylation, even in the absence of FAK kinase activity and Tyr-397 phosphorylation. As shown in Fig. 1C, F397-FAK coexpressed with Src was again unable to interact with PLC-γ1-SH2C. That the other sites of FAK tyrosine phosphorylation became elevated as a result of the Src coexpression is indicated by the high pTyr content of F397-, F576/F577-, and R454-FAK, as well as the now efficient recognition of the latter two variants by PLC-γ1-SH2C.
To determine whether the interaction between tyrosine-phosphorylated FAK and PLC-γ1-SH2C is direct, a Far-Western binding analysis was carried out on the epitope-tagged FAK variants, isolated after coexpression with Src in COS-7 cells, by using GSTPLC-γ1-SH2C as a probe. The probe bound to immobilized WT-FAK as well as to the F407, F576/F577, and R454 variants but was unable to recognize F397-FAK (Fig. 1D). These results further demonstrate the requirement for FAK Tyr-397 in the interaction with PLC-γ1-SH2C and indicate that this interaction is direct.
FAK and PLC-γ1 Associate when Coexpressed in COS-7 Cells.
To determine whether full length PLC-γ1 interacts with FAK in intact cells, HA epitope-tagged PLC-γ1 (HA-PLC-γ1) was coexpressed in COS-7 cells together with WT vs. mutant forms of Myc epitope-tagged FAK, and their association was analyzed by co-immunoprecipitation using antibodies against the tags. (Activated Src was not coexpressed in these experiments.) As shown in Fig. 2A Top), HA-PLC-γ1 is detected in immunoprecipitates of WT- and F407-FAK but not those of F397- and R454-FAK, indicating an interaction mediated by FAK autophosphorylation and PLC-γ1-SH2C binding to pTyr-397. Consistent with previous data indicating lower levels of Tyr-397 phosphorylation in F576/F577-FAK, much less HA-PLC-γ1 was detected in the immunoprecipitates of this variant.
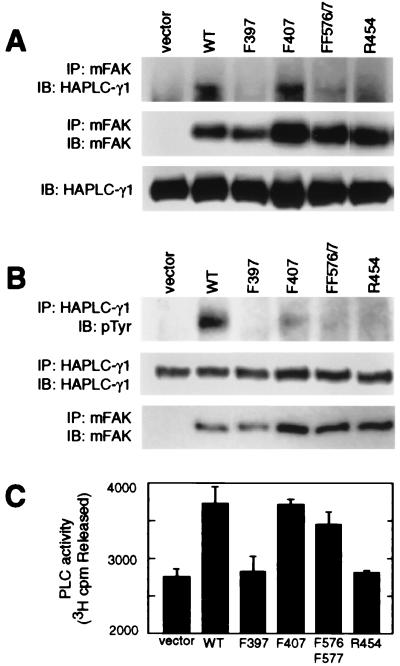
Figure 2.
FAK associates with PLC-γ1 and stimulates PLC-γ1 tyrosine phosphorylation and enzymatic activity in COS-7 cells. (A) Myc-tagged FAK (mFAK) variants (or FAK vector only control) were transiently coexpressed in COS-7 cells together with HA-tagged PLC-γ1, and mFAK variants were immunoprecipitated (IP) with antibody 9E10. Coprecipitation of HA-PLC-γ1 was assessed by probing immunoblots (IB) with anti-HA antibody 12CA5 (Top), and relative amounts of immunoprecipitating mFAK were visualized by immunoblotting with 9E10 (Middle). Expression of HA-PLC-γ1 was assessed by immunoblot analysis of whole cell lysates using 12CA5 (Bottom). (B) HA-PLC-γ1 coexpressed in COS-7 cells with mFAK variants (or vector only) was immunoprecipitated with 12CA5 antibody and was analyzed for pTyr content by immunoblot analysis using anti-pTyr antibody 4G10 (Top). Recovery of HA-PLC-γ1 was determined by probing immunoblots with 12CA5 antibody (Middle), and expression of mFAK was determined by immunoprecipitation/immunoblot analysis using 9E10 antibody (Bottom). (C) HA-PLC-γ1 coexpressed in COS-7 cells with mFAK variants (or vector only) was immunoprecipitated with 12CA5 antibody and was analyzed for hydrolytic activity toward [3H]phosphatidylinositol 4,5-bisphosphate. PLC-γ1 activity is presented as cpm [3H]inositol 1,4,5-trisphosphate released (mean values + SEM from four independent experiments). Separate immunoblots (not shown) confirmed that near-equal amounts of HA-PLC-γ1 were assayed under each condition and that near-equal amounts of mFAK variants were coexpressed.
FAK Stimulates PLC-γ1 Tyrosine Phosphorylation and Enzymatic Activity.
It was of interest to determine whether the ability of FAK to interact with PLC-γ1 was associated with increased PLC-γ1 tyrosine phosphorylation and/or enzymatic activity. The relative pTyr content of HA-PLC-γ1, when coexpressed in COS-7 cells with the epitope-tagged FAK variants, was determined by anti-pTyr immunoblotting. As shown in Fig. 2B, HA-PLC-γ1 pTyr was not evident when the phospholipase was expressed alone (vector lane), indicating that endogenous COS-7 cell tyrosine kinases are unable to bring about significant HA-PLC-γ1 phosphorylation. However, coexpression of either WT- or F407-FAK resulted in detectable tyrosine phosphorylation of HA-PLC-γ1. Expression of either F397-FAK or R454-FAK failed to effectively promote tyrosine phosphorylation of HA-PLC-γ1, indicating a requirement for FAK autophosphorylation. Although some HA-PLC-γ1 could be detected in F576/F577-FAK immunoprecipitates (Fig. 2A), coexpression of this variant was also unable to promote detectable HA-PLC-γ1 tyrosine phosphorylation (Fig. 2B).
To determine whether PLC-γ1 enzymatic activity is elevated in correlation with the FAK interaction, HA-PLC-γ1 was again expressed in COS-7 cells together with the FAK variants (or FAK vector control) and then was recovered by immunoprecipitation, and phospholipase activity was measured. Coexpression of WT- or F407-FAK modestly stimulated PLC-γ1 activity by ≈35% over the activity measured from cells expressing only HA-PLC-γ1 (Fig. 2C). This FAK-enhanced phospholipase activity was not observed on coexpression of either F397- or R454-FAK. Coexpression of F576/F577-FAK also stimulated PLC-γ1 activity, but somewhat less than WT- or F407-FAK. In view of the inability of F576/F577-FAK to effectively promote tyrosine phosphorylation of PLC-γ1, the observed stimulation of PLC-γ1 activity by this variant suggests that the SH2 interaction with FAK may directly activate PLC-γ1, although tyrosine phosphorylation of PLC-γ1 may further elevate activity.
FAK Does Not Directly Phosphorylate PLC-γ1.
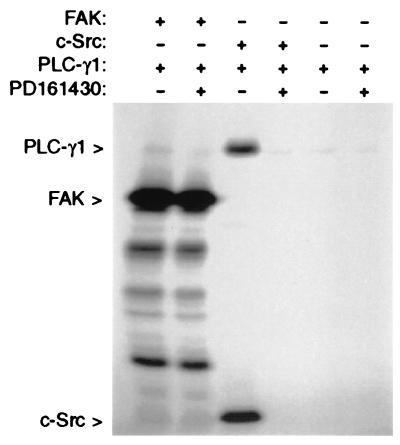
The above observations give no indication as to whether PLC-γ1 is phosphorylated by FAK directly or by another FAK-activated or FAK-associated tyrosine kinase, such as c-Src. To clarify whether FAK can directly phosphorylate PLC-γ1, in vitro kinase assays were conducted by using baculovirus-expressed PLC-γ1 and FAK. To eliminate possible Src-kinase activity associated with the recombinant FAK protein, the Src-selective inhibitor PD161430 was included in the kinase reaction. As shown in Fig. 3, PLC-γ1 was not phosphorylated by FAK, even though strong FAK autophosphorylation activity was observed. Similar experiments carried out with baculovirus-expressed c-Src, however, confirmed previous reports (20, 21) that PLC-γ1 can serve as a Src substrate. These data suggest that the observed ability of FAK to promote PLC-γ1 tyrosine phosphorylation when the two proteins are coexpressed in COS-7 cells is not attributable to direct phosphorylation by FAK but is possibly a result of the ability of FAK to interact with and activate Src-family kinases.
Figure 3.
FAK does not phosphorylate PLC-γ1 in vitro. Recombinant PLC-γ1 was incubated either alone or in the presence of recombinant FAK or recombinant c-Src in a kinase assay reaction mixture containing [γ-32P]ATP. The Src-selective inhibitor PD16430 was included in some reactions to guard against possible contamination of the FAK or PLC-γ1 preparations with Src-family kinases. After the reactions, proteins were separated by SDS/PAGE, and [32P]-labeled proteins were detected by autoradiography.
Cell Adhesion to Fibronectin Stimulates PLC-γ1 Activity and Association with FAK, Dependent on Tyr-397.
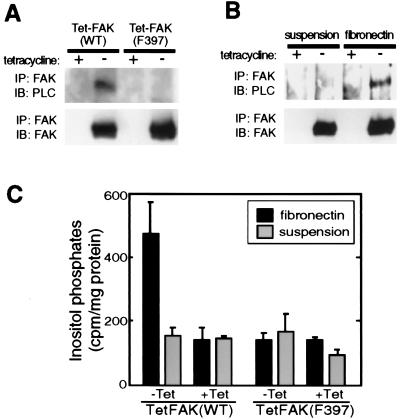
We next investigated the possibility that FAK plays a regulatory role in adhesion-induced PLC-γ1 activation. For these experiments, we used mouse fibroblast cell lines, TetFAK(WT) and TetFAK(F397), that lack endogenous FAK but inducibly express either WT-FAK or F397-FAK, respectively, under control of the tetracycline-repression system (10). As shown in Fig. 4A, endogenous PLC-γ1 is readily detected in the FAK immunoprecipitates of adherent normally growing cells induced to express WT-FAK but could not be similarly detected in cells expressing F397-FAK. Moreover, the coimmunoprecipitation of PLC-γ1 with WT-FAK was abolished when the cells were trypsinized and held in suspension but was reestablished within 30 min of cell replating onto fibronectin (Fig. 4B).
Figure 4.
FAK Tyr397 is required for cell adhesion-promoted FAK:PLC-γ1 association and inositol phosphate production. (A) TetFAK(WT) and TetFAK(F397) cells maintained in the presence or absence of tetracycline were lysed at subconfluent density, and FAK immunoprecipitates (IP) were examined for the presence of PLC-γ1 (Upper) or FAK (Lower) by immunoblot (IB) analysis. (B) The same cells, maintained in the presence or absence of tetracycline, were serum-starved (20 hr in DMEM containing 0.5% FBS) and then were detached by trypsin and either were maintained in suspension or were replated onto fibronectin-coated dishes and allowed to spread for 30 min. Both suspended and fibronectin-replated cells then were lysed, and FAK immunoprecipitates were subjected to immunoblot analysis using antibodies against either PLC-γ1 (Upper) or FAK (Lower). (C) Serum- and inositol-starved adherent TetFAK(WT) and TetFAK(F397) cells were labeled with myo-(2-3H) inositol, were harvested by trypsinization, and were resuspended in serum- and inositol-free DMEM. One half of the cell suspension was replated onto a fibronectin-coated cell culture dish and was allowed to attach and spread whereas the other half was held in suspension. After 30 min, free inositol phosphates of both suspended and fibronectin-adherent cells were extracted, and [3H]inositol phosphates were quantitated by scintillation counting. The relative production of total inositol phosphates is shown as cpm per mg of total residual cellular protein. Data presented are means (+SEM) from four independent measurements.
To determine whether the adhesion-dependent interaction of FAK with PLC-γ1 is associated with increased PLC-γ1 activity, inositol phosphate production was measured in induced vs. uninduced TetFAK(WT) and TetFAK(F397) cells. Cells were labeled with [3H]myo-inositol and either were trypsinized and held in suspension or were replated onto fibronectin for 30 min before total inositol phosphates were extracted, were separated on anion-exchange columns, and were measured by liquid scintillation counting. Cells expressing WT-FAK and plated onto fibronectin produced ≈2.5× the amount of inositol phosphates as compared with the same cells held in suspension during the same time period [Fig. 4C, TetFAK(WT), −Tet]. This adhesion-stimulated increase in inositol phosphate production was not observed for the uninduced (+Tet) TetFAK(WT) cells, indicating that FAK expression was required for the response. Moreover, cells expressing F397-FAK also failed to show the adhesion-stimulated increase in inositol phosphate production [Fig. 4C, TetFAK(F397), −Tet]. Thus, FAK Tyr-397 mediates a stable, adhesion-induced association with PLC-γ1 in cultured fibroblasts, and this is associated with activation of PLC activity.
DISCUSSION
The major finding of this study is that FAK plays a signaling role leading to activation of PLC-γ1 in response to integrin-mediated cell adhesion. This response requires FAK Tyr-397 and apparently results from a direct interaction between pTyr-397 and PLC-γ1-SH2C. Experimental evidence for PLC-γ1 activation resulting from integrin interactions has accumulated over recent years. Antibody-crosslinking of β2 integrin on T cells (22) and β1 integrin on pancreatic acinar cells (23) stimulates tyrosine phosphorylation of PLC-γ1 associated with Ca2+ mobilization. Adhesion of rat glomerular epithelial cells to collagen, mediated in part by β1 integrins, increases membrane association and tyrosine phosphorylation of PLC-γ1, and production of inositol phosphates and diacylglycerol (24, 25). Tyrosine phosphorylation of PLC-γ1 also has been observed in response to β1 integrin-mediated adhesion of human skin fibroblasts to collagen (26) and in intestinal epithelial cells migrating in a monolayer wound model (27). And rapid elevation of diacylglycerol has been observed after fibronectin plating of vascular smooth muscle cells (28). Our results provide possible mechanistic insight into these observations and are compatible with other studies showing in vitro binding of PLC-γ1 SH2 domains to FAK (11) and in vivo association of FAK and PLC-γ1 after integrin-mediated lipocyte adhesion (29).
Our data suggesting a direct interaction between FAK pTyr-397 and the C-terminal SH2 domain of PLC-γ1, when considered with previous findings that FAK pTyr-397 interacts with SH2 domains of Src-family kinases (6, 7) and the p85 subunit of PI3K (8), emphasize a multifunctional signaling role for the FAK autophosphorylation site. The observed apparent affinities of FAK pTyr-397 with these SH2 domains (Fig. 1A) is generally consistent with past observations on SH2 binding specificity and structure. The strong interaction between FAK and c-SrcSH2 is predicted by the resemblance of the optimal SrcSH2 recognition motif (pTyr-Glu-Glu-Ile) (30) to the FAK pTyr-397 site (pTyr-Ala-Glu-Ile) and importance of +3 Ile interactions in high-affinity binding (31, 32). The weaker interactions of the p85 SH2 domains likely reflects the bulky FAK pTyr-397 +3 Ile being poorly accommodated by the narrow +3 binding pockets of these SH2 domains (33). Because both PLC-γ1 SH2 domains select +1 and +3 hydrophobic residues, and PLC-γ1-SH2N shows additional selectivity for acidic residues at the +2 position (30), our data indicating poor recognition of FAK by PLC-γ1-SH2N were unexpected. However, structural studies on PLC-γ1-SH2C (34) suggest that the +2 position of a high-affinity peptide is relatively unimportant in stabilizing the interaction. Of the PLC-γ1-SH2C residues making contact with the high-affinity peptide, those interacting with the +1 residue (Ile) are most poorly conserved in PLC-γ1-SH2N, suggesting this position is important in differential peptide recognition by PLC-γ1 SH2 domains. It is thus notable that the FAK pTyr-397 +1 Ala is structurally more similar to the Val optimally selected (30) at the +1 position by PLC-γ1-SH2C than the Leu optimally selected by PLC-γ1-SH2N.
It is well established that the interaction of PLC-γ1 with receptor tyrosine kinases promotes PLC-γ1 activity, and this likely involves structural changes in the PLC-γ1 catalytic domain brought about by both SH2 domain interactions with receptor autophosphorylation sites and receptor-mediated tyrosine phosphorylation of PLC-γ1 (reviewed in refs. 35 and 36). Our observations of FAK-enhanced PLC-γ1 tyrosine phosphorylation and activation dependent on Tyr-397 suggest that similar mechanisms are involved in adhesion-induced activation of PLC-γ1 signaling. However, FAK does not directly phosphorylate PLC-γ1. Nor does the ability of FAK to promote PLC-γ1 activity appear to be solely attributable to PLC-γ1 tyrosine phosphorylation because expression of F576/F577-FAK stimulated PLC-γ1 activity in COS-7 cells while not effectively elevating PLC-γ1 pTyr levels. Also, we have been unable to detect significant changes in total PLC-γ1 pTyr levels associated with adhesion-stimulation of PLC-γ1 activity in induced vs. noninduced Tet-FAK(WT) cells, although we noted increased recovery of tyrosine-phosphorylated PLC-γ1 from an insoluble cytoskeletal fraction after fibronectin-replating of the induced, relative to noninduced, Tet-FAK(WT) cells (data not shown). Nevertheless, PLC-γ1 bound to FAK may be phosphorylated by Src-family kinases present at sites of cell adhesion, and this may contribute to PLC-γ1 activation. The Src-family kinases could be brought to the adhesion complexes, and activated, through their SH2/SH3-mediated interactions with other FAK molecules in the complex (e.g., those not bound to PLC-γ1 or PI3K) or with FAK-associated proteins such as p130Cas and paxillin (3). The demonstrated ability of Src to phosphorylate (refs. 20 and 21; Fig. 3) and activate (37) PLC-γ isoforms supports this mechanism, as do observations of PLC-γ1 recruitment to focal adhesion complexes, along with c-Src and FAK, on integrin-mediated cell adhesion (38). Evidence indicating that growth factor stimulation of PLC-γ1 activity may result in part from plasma membrane translocation via its pleckstrin homology domain interacting with phosphatidylinositol 3,4,5-trisphosphate (39) raises the possibility that the ability of FAK to activate PI3K (8) also may play a role leading to integrin stimulation of cellular PLC-γ1 activity. Additional studies will be needed to clarify the mechanism by which FAK pTyr397 promotes PLC-γ1 activity.
FAK signaling, requiring Tyr-397, enhances integrin-dependent cellular functions, including cell spreading (10, 40), migration (10, 41), and survival (42). Our observations raise the possibility that PLC-γ1 acts downstream of FAK and contributes to these responses. PLC-γ1 activity has been previously implicated in pathways leading to cell spreading and/or migration. PLC-γ1-null (knockout) fibroblasts exhibit a more rounded morphology than their normal counterparts (43). And receptor tyrosine kinase-mediated PLC-γ1 activation plays a role in platelet-derived growth factor- and epidermal growth factor-induced motility (27, 44, 45). The mechanism by which PLC-γ1 could stimulate cell spreading or migration is presently unclear, but pathways regulated by protein kinase C have been implicated (28, 46–48), and both phosphatidylinositol 4,5-bisphosphate hydrolysis and Ca2+ elevation are known to regulate actin-binding proteins (e.g., profilin, gelsolin, vinculin) involved in the cytoskeletal remodeling associated with these processes (49, 50). Our findings also raise the possibility that PLC-γ1 signaling could contribute to the ability of FAK ability to stimulate ERK kinase activity and progression through G1 phase of the cell cycle (51, 52). Future studies aimed at determining the mechanisms by which FAK signaling affects cell behavior should consider the possible role of PLC-γ1.
Acknowledgments
We thank Tom Polte for constructing FAK expression plasmids, David Fry for providing the Src-selective inhibitor, Samyukta Reddy for technical assistance, and Brent Polk for helpful discussions. This work was supported by National Institutes of Health Grants GM49882 (to S.K.H.) and CA75195 (to G.C.).
ABBREVIATIONS
- FAK
focal adhesion kinase
- PI3K
phosphatidylinositol 3-kinase
- PLC
phospholipase C
- pTyr
phosphotyrosine
- SH
Src homology
- GST
glutathione S-transferase
- WT
wild-type
References
- 1.Rosales C, O’Brien V, Kornberg L, Juliano R. Biochim Biophys Acta. 1995;1242:77–98. doi: 10.1016/0304-419x(95)00005-z. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 3.Hanks S K, Polte T R. BioEssays. 1997;19:137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- 4.Schaller M D, Borgman C A, Cobb B S, Vines R R, Reynolds A B, Parsons J T. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanks S K, Calalb M B, Harper M C, Patel S K. Proc Natl Acad Sci USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaller M D, Hildebrand J D, Shannon J D, Fox J W, Vines R R, Parsons J T. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polte T R, Hanks S K. Proc Natl Acad Sci USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H-C, Appeddu P A, Isoda H, Guan J-L. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 9.Calalb M B, Polte T R, Hanks S K. Mol Cell Biol. 1995;15:954–963. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen J D, Ruest P J, Fry D W, Hanks S K. Mol Cell Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlaepfer D D, Hanks S K, Hunter T, van der Geer P. Nature (London) 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 12.Guan K L, Dixon J E. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 13.Polte T R, Hanks S K. J Biol Chem. 1997;272:5501–5509. doi: 10.1074/jbc.272.9.5501. [DOI] [PubMed] [Google Scholar]
- 14.Hildebrand J D, Schaller M D, Parsons J T. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horstman D A, Ball R, Carpenter G. Protein Expression Purif. 1995;6:278–283. doi: 10.1006/prep.1995.1036. [DOI] [PubMed] [Google Scholar]
- 16.Withers B E, Keller P R, Fry D W. Protein Expression Purif. 1996;7:12–18. doi: 10.1006/prep.1996.0002. [DOI] [PubMed] [Google Scholar]
- 17.Hamby J M, Connolly C J, Schroeder M C, Winters R T, Showalter H D, Panek R L, Major T C, Olsewski B, Ryan M J, Dahring T, et al. J Med Chem. 1997;40:2296–2303. doi: 10.1021/jm970367n. [DOI] [PubMed] [Google Scholar]
- 18.Nishibe S, Wahl M I, Hernandez-Sotomayor S M, Tonks N K, Rhee S G, Carpenter G. Science. 1990;250:1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- 19.Downes C P, Hawkins P T, Irvine R F. Biochem J. 1986;238:501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao F, Shin H S, Rhee S G. Biochem Biophys Res Commun. 1993;191:1028–1033. doi: 10.1006/bbrc.1993.1320. [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi O, Shibasaki F, Hidaka M, Homma Y, Takenawa T. J Biol Chem. 1993;268:10754–10759. [PubMed] [Google Scholar]
- 22.Kanner S B, Grosmaire L S, Ledbetter J A, Damle N K. Proc Natl Acad Sci USA. 1993;90:7099–7103. doi: 10.1073/pnas.90.15.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wrenn R W, Creazzo T L, Herman L E. Biochem Biophys Res Commun. 1996;226:876–882. doi: 10.1006/bbrc.1996.1443. [DOI] [PubMed] [Google Scholar]
- 24.Cybulsky A V, Carbonetto S, Cyr M- D, McTavish A J, Huang Q. Am J Physiol. 1993;264:C323–C332. doi: 10.1152/ajpcell.1993.264.2.C323. [DOI] [PubMed] [Google Scholar]
- 25.Cybulsky A V, McTavish A J, Papillon J. Am J Physiol. 1996;271:F579–F587. doi: 10.1152/ajprenal.1996.271.3.F579. [DOI] [PubMed] [Google Scholar]
- 26.Langholz O, Roeckel D, Petersohn D, Broermann E, Eckes B, Krieg T. Exp Cell Res. 1997;235:22–27. doi: 10.1006/excr.1997.3640. [DOI] [PubMed] [Google Scholar]
- 27.Polk D B. Gastroenterology. 1998;114:493–502. doi: 10.1016/s0016-5085(98)70532-3. [DOI] [PubMed] [Google Scholar]
- 28.Haller H, Lindschau C, Maasch C, Olthoff H, Kurscheid D, Luft F C. Circulation Res. 1998;82:157–165. doi: 10.1161/01.res.82.2.157. [DOI] [PubMed] [Google Scholar]
- 29.Carloni V, Romanelli R G, Pinzani M, Laffi G, Gentilini P. Gastroenterology. 1997;112:522–531. doi: 10.1053/gast.1997.v112.pm9024306. [DOI] [PubMed] [Google Scholar]
- 30.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, et al. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 31.Waksman G, Shoelson S E, Pant N, Cowburn D, Kuriyan J. Cell. 1993;72:779–790. doi: 10.1016/0092-8674(93)90405-f. [DOI] [PubMed] [Google Scholar]
- 32.Eck M J, Shoelson S E, Harrison S C. Nature (London) 1993;362:87–91. doi: 10.1038/362087a0. [DOI] [PubMed] [Google Scholar]
- 33.Breeze A L, Kara B V, Barratt D G, Anderson M, Smith J C, Luke R W, Best J R, Cartlidge S A. EMBO J. 1996;15:3579–3589. [PMC free article] [PubMed] [Google Scholar]
- 34.Pascal S M, Singer A U, Gish G, Yamazaki T, Shoelson S E, Pawson T, Kay L E, Forman-Kay J D. Cell. 1994;77:461–472. doi: 10.1016/0092-8674(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 35.Kamat A, Carpenter G. Cytokine Growth Factor Rev. 1997;8:109–117. doi: 10.1016/s1359-6101(97)00003-8. [DOI] [PubMed] [Google Scholar]
- 36.Singer W D, Brown H A, Sternweis P C. Annu Rev Biochem. 1997;66:475–509. doi: 10.1146/annurev.biochem.66.1.475. [DOI] [PubMed] [Google Scholar]
- 37.Arkinstall S, Payton M, Maundrell K. Mol Cell Biol. 1995;15:1431–1438. doi: 10.1128/mcb.15.3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plopper G E, McNamee H P, Dike L E, Bojanowski K, Ingber D E. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falasca M, Logan S K, Lehto V P, Baccante G, Lemmon M A, Schlessinger J. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson A, Malik R K, Hildebrand J D, Parsons J T. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cary L A, Chang J F, Guan J-L. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 42.Frisch S M, Vuori K, Ruoslahti E, Chan-Hui P-Y. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji Q-S, Ermini S, Baulida J, Sun F-L, Carpenter G. Mol Biol Cell. 1998;9:749–757. doi: 10.1091/mbc.9.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kundra V, Escobedo J A, Kazlauskas A, Kim H K, Rhee S G, Williams L T, Zetter B R. Nature (London) 1994;367:474–476. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- 45.Chen P, Xie H, Sekar M C, Gupta K, Wells A. J Cell Biol. 1994;127:847–857. doi: 10.1083/jcb.127.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vuori K, Ruoslahti E. J Biol Chem. 1993;268:21459–21462. [PubMed] [Google Scholar]
- 47.Myat M M, Anderson S, Allen L H, Aderem A. Curr Biol. 1997;7:611–614. doi: 10.1016/s0960-9822(06)00262-4. [DOI] [PubMed] [Google Scholar]
- 48.Rigot V, Lehmann M, Andre F, Daemi N, Marvaldi J, Luis J. J Cell Sci. 1998;111:3119–3127. doi: 10.1242/jcs.111.20.3119. [DOI] [PubMed] [Google Scholar]
- 49.Gilmore A P, Burridge K. Nature (London) 1996;381:531–535. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- 50.Toker A. Curr Opin Cell Biol. 1998;10:254–261. doi: 10.1016/s0955-0674(98)80148-8. [DOI] [PubMed] [Google Scholar]
- 51.Schlaepfer D D, Broome M A, Hunter T. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J-H, Reiske H, Guan J-L. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]