Abstract
Background/aim: Fractionated stereotactic radiotherapy (FSRT) is a new treatment for brain tumours that are close to critical structures, such as the visual apparatus. This study aims to assess the visual outcomes for patients with parasellar meningioma following FSRT.
Methods: A retrospective, non-comparative case series of 13 patients with parasellar meningiomas who were treated in one institution with FSRT between January 1995 and January 2001.
Results: 13 patients (26 eyes) were followed for a mean of 2 years. Visual acuity improved in four eyes (12.5%), remained stable in 18 eyes (75%), and worsened in three eyes (12.5%). Visual field improved in 15 eyes (57%), remained stable in six eyes (23%), and worsened in four eyes (15%). No adverse visual outcome occurred as a result of radiation.
Conclusion: These preliminary findings suggest that FSRT is a safe and effective treatment for parasellar meningiomas.
Keywords: fractionated stereotactic radiotherapy, parasellar meningioma, visual outcome, stereotactic radiosurgery
Intracranial meningiomas are slow growing tumours that traditionally have been treated by surgical excision. Parasellar meningiomas often compress the optic nerve, the optic chiasm, or optic tracts, and invade the cavernous sinus. Morbidity rates of 12–30% have been reported with surgical excision despite recent advances in microsurgical techniques.1–3 Other treatments such as radiotherapy and gamma knife surgery are being explored with the aim of effective treatment with maximal preservation of neural and visual function.4–8 Fractionated stereotactic radiotherapy (FSRT) is one of the newer treatments that are changing the traditional neurosurgical approach to meningiomas located at the base of the skull.9,10 Radiotherapy delivered to targets near the optic apparatus has been a concern and reports of radiation injury with visual loss have been reported.11,12
FSRT has been demonstrated to be a safe and an effective refinement of stereotactic radiosurgery for parasellar lesions and lesions involving the optic apparatus, including optic nerve sheath meningioma (ONSM).13,14 We have reviewed the medical records of our practice for patients who were treated with FSRT for parasellar meningioma. Based on encouraging results with ONSM, we report the preliminary findings of the visual outcomes in these patients. This series does not overlap with any of the secondary ONSM patients previously reported.
PATIENTS AND METHODS
The study protocol was approved by the institutional review boards of Wills Eye Hospital and Thomas Jefferson University. We reviewed the medical records of the Neuro-Ophthalmology Service of the Wills Eye Hospital for patients with parasellar meningiomas who underwent FSRT.
Twenty four patients were evaluated between January 1995 and January 2001. One patient died of lung cancer. Another patient was excluded because of a history of radiation for bilateral retinoblastoma during childhood, resulting in light perception vision only in both eyes before FSRT. Five patients did not have a complete pre-FSRT examination. Three patients were lost to follow up. Tumour control was defined as a stable or diminished tumour size when assessed in all orthogonal planes on serial post-treatment magnetic resonance images (MRI). Thirteen patients had a complete baseline neuro-ophthalmic examination including visual acuity, and visual field testing, with the same assessments documented following FSRT. All patients had visual field defects indicative of optic neuropathy and/or chiasmal compression. All patients underwent automated static perimetry (Humphrey 24-2 program), except one who could only be tested with kinetic perimetry and another who had one eye tested by kinetic perimetry initially and on follow up.
Best corrected visual acuity and visual field data were obtained from the evaluation immediately before FSRT and at the last follow up visit
For each patient we defined an improvement in visual acuity as a gain of two or more Snellen type lines, while a loss of two or more lines constituted worsening. In addition, Snellen visual acuity measurements were converted to the logarithm of the minimal angle of resolution scores (logMAR) to calculate the mean pre-FSRT and post-FSRT Snellen visual acuity using the appropriate equation.15
A paired t test was used to compare the mean pre-FSRT with the post-FSRT logMAR score. Two Snellen acuity measurements of one patient (pre-FSRT and post-FSRT of the left eye of patient 1) could not be included in the logMAR calculations because the post-treatment vision was light perception.16
Improvement or worsening in the visual field was defined as an increase or decrease of the mean deviation (MD) by more than 2 dB with automated perimetry. This value has been shown to represent a range for fluctuation in the MD that occurs with repeated automated perimetry testing,17 and this value was established to increase the validity of any change in the MD seen at follow up. For patients who had kinetic perimetry at follow up, a 10 degree expansion or constriction of the isoptres was scored as improvement or worsening, respectively.
Radiation technique
The linear accelerator (Linac) radiosurgical technique involves a conventional fraction paradigm to maximise the chance of vision preservation and minimise the chance of radiation induced damage to the optic nerves and the chiasm. This paradigm involves the use of 1.8 Gy/fraction, 28 fraction protocols administered daily, for a cumulative dose of 50.4 Gy, during a 5 week period. We used a Linac designed for radiosurgery (Varian 600 SR; Variann Corp, Palo Alto, CA, USA) that became operational in 1994.18 Imaging data included both computed tomographic and fat suppression MRI data sets that were fused (Radionics, Burlington, MA, USA) for treatment planning and involved the use of a Gill-Thomas-Cosman relocatable frame (Radionics, Burlington, MA, USA), and X-Knife 3 D treatment planning software (Radionics). An average of three isocentres were used, and high conformality was established by non-coplanar arc beam shaping and differential beam weighting as previously described.
RESULTS
Thirteen patients, nine women and four men, comprised our series. The mean age was 57.8 years. The mean follow up period after FSRT was 24.7 (range 11–34) months. Seven patients had previous subtotal resection of the tumour. FSRT was administered to these patients as an adjuvant modality because of tumour re-growth, inability to completely excise the tumour, or historical difference in the preferred treatment method. In six patients (patients 4, 6, 8, 9, 11, 13), FSRT was the primary treatment. Patient 9 had surgery only to biopsy the tumour. In patients who had previous surgery, the mean time from surgery to FSRT was 35.7 (range 2–99) months (table 1).
Table 1.
Clinical characteristics
| Patient No | Age | Sex | Presenting symptom | Tumour location | Previous surgery |
| 1 | 63 | F | Decreased vision | Right cavernous sinus | Transorbital craniotomy |
| 2 | 54 | F | Decreased vision | Planum sphenoidale | Craniotomy |
| 3 | 37 | F | Decreased vision | Suprasellar | Trans-orbital craniotomy |
| 4 | 77 | F | Diplopia | Right sphenoid wing | – |
| 5 | 76 | M | Decreased vision | Right sphenoid wing | Craniotomy |
| 6 | 47 | F | Decreased vision | Left cavernous sinus | – |
| 7 | 69 | F | Pressure in the eye | Left sphenoid wing | Two craniotomies |
| 8 | 55 | M | Decreased vision | Clivus | – |
| 9 | 60 | F | Diplopia | Right sphenoid wing | Craniotomy for biopsy only |
| 10 | 73 | F | Decreased vision | Left sphenoid wing | Craniotomy |
| 11 | 45 | F | Decreased vision | Right sphenoid wing | – |
| 12 | 29 | M | Decreased vision | Left sphenoid wing | Two craniotomies |
| 13 | 67 | M | Decreased | Clivus | – |
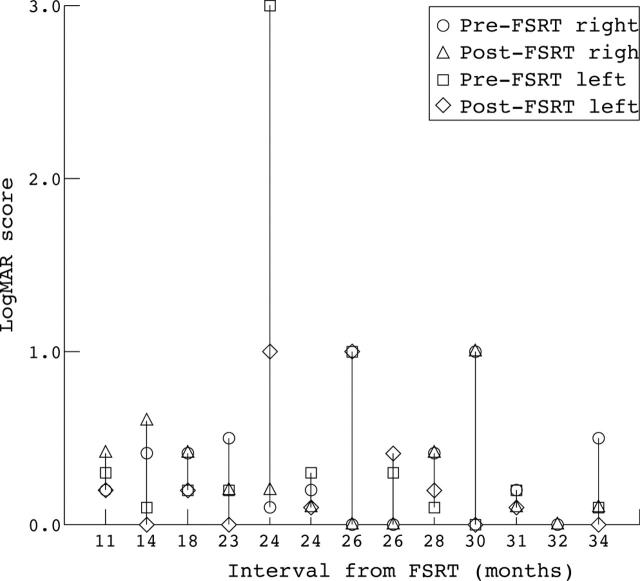
Tumour growth was controlled in all cases when assessed on serial MRI scans, except for one patient (patient 8). Visual acuity improved in four of 26 eyes (15%), remained stable in 19 eyes (73%), and worsened in three eyes (11.5%) (fig 1). Ten of 13 patients had final visual acuity better than or equal to 20/40 in at least one eye. The average preoperative logMAR score improved from 0.39 (Snellen equivalent 20/50, SD 0.6) to a mean postoperative value of 0.27 (Snellen equivalent 20/37, SD 0.32). This improvement did not reach statistical significance by paired t test (p value = 0.16). In the subgroup of patients who had FSRT as a primary treatment (no previous surgery) the mean logMAR score improved from 0.32 (Snellen equivalent 20/42, SD 0.28) to 0.26 (Snellen equivalent 20/37, SD 0.32). This also was not statistically significant (p value = 0.19). Overall, 17 of 26 eyes (65%) had a final visual acuity equal to or better than 20/40. In all patients, visual acuity remained stable in at least one eye. One patient (patient 2) had bilateral improvement in visual acuity (from 20/60 both eyes to 20/25 right eye and 20/30 left eye). That same patient had a bilateral improvement in her visual fields (table 2).
Figure 1.
LogMAR score for each patient before and after FSRT arranged according to the time interval from FSRT. (LogMAR = 0 is equivalent to 20/20 Snellen acuity, logMAR = 1 is equivalent to 20/200 Snellen acuity, logMAR = 3 is equivalent to hand motion vision).
Table 2.
Pretreatment and post-treatment data
| Patient No | Pre-FSRT visual acuity | Post-FSRT visual acuity | Pre-FSRT MD of the visual field | Post-FSRT visual field | ||||
| Right eye | Left eye | Right eye | Left eye | Right eye | Left eye | Right eye | Left eye | |
| 1 | 20/20 | LP | 20/20 | NLP | 0.0 | −26.8 | 2.73 | – |
| 2 | 20/60 | 20/60 | 20/25 | 20/30 | −19.73 | −17.78 | −0.24 | −3.19 |
| 3 | 20/25 | 20/30 | 20/20 | 20/20 | 1. 32 | −3.84 | 2.79 | 0.10 |
| 4 | 20/25 | 20/50 | 20/30 | 20/50 | − 4.27 | −5.45 | −1.78 | −9.69 |
| 5 | HM | 20/25 | 20/200 | 20/30 | constricted kinetic field | −0.44 | Improved kinetic field | −1.87 |
| 6 | 20/20 | 20/20 | 20/20 | 20/20 | −2.01 | −6.03 | 1.60 | 0.79 |
| 7 | 20/200 | 20/40 | 20/200 | 20/50 | −14.91 | −6.12 | −7.93 | −27.25 |
| 8 | 20/30 | 20/50 | 20/50 | 20/50 | −14.86 | −13.48 | −17.32 | −23.97 |
| 9 | 20/40 | 20/30 | 20/30 | 20/30 | −16.56 | 0.0 | −5.86 | 0.34 |
| 10 | 20/50 | 20/200 | 20/80 | 20/200 | Constricted kinetic field | Improved kinetic field | ||
| 11 | 20/25 | 20/20 | 20/20 | 20/20 | −10.73 | −0.65 | −2.08 | −1.25 |
| 12 | 20/30 | 20/30 | 20/25 | 20/25 | −9.63 | −4.83 | −9.63 | −6.38 |
| 13 | 20/40 | 20/30 | 20/25 | 20/25 | −2.36 | −2.37 | 0.64 | 0.52 |
MD, mean deviation of the visual field; LP, light perception only; NLP, no light perception; HM, hand motion.
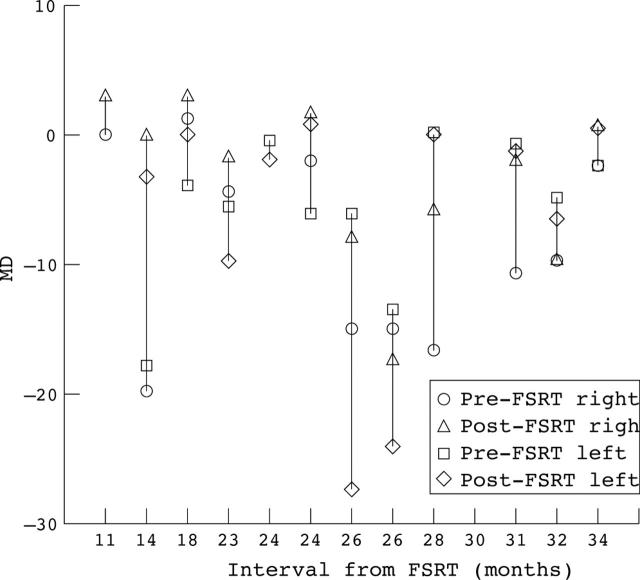
Visual field improved in 15 of 26 eyes (57%) (12 eyes followed by static and three eyes by kinetic perimetry), remained stable in six eyes (23%), and worsened in four eyes (15 %) (fig 2). Visual field data were not available for one eye because of light perception vision. The visual field improved in at least one eye in 11 out 13 patients, and in four patients the improvement was bilateral. One patient (patient 8) had bilateral worsening of his visual field that required surgical decompression 2 years after his FSRT (table 2).
Figure 2.
Pre-FSRT and post-FSRT mean deviation (MD) of all patients arranged according to interval from FSRT. Only eyes that had automated static perimetry are shown.
DISCUSSION
We were not able to find any report in the literature which details the visual outcomes following treatment for parasellar meningiomas with FSRT. Our study indicates that FSRT can be a safe and effective method for the primary treatment of parasellar meningioma and as an adjuvant modality following incomplete surgical excision. FSRT should not be confused with stereotactic radiosurgery, which unlike FSRT applies a higher single radiation dose in one session. As a result, stereotactic radiosurgery can be associated with a higher risk of damage to the retina, optic nerve, or chiasm.19
Our findings showed that there is a trend towards improvement or stabilisation of the visual function following FSRT, results which are similar to our results with ONSM.13 The improvement of visual acuity did not reach statistical significance most probably because of the small sample size and the fact that eight out of the 12 patients had an initial visual acuity equal to or better than 20/40 in at least one eye initially. It is possible that we did not have enough patients with visual acuity between 20/60–20/100 who could show either improvement or worsening more readily.
The visual field data were a more sensitive measure of a change in the visual function signifying either improvement or worsening. Since these tumours are slow growing and regress slowly following radiation treatment, longer follow up may show more improvement in visual acuity. No patient in our series had an adverse visual outcome (damage to the optic nerve, chiasm, or retina) as a result of radiation when examined with serial neuro-ophthalmological examinations and MRI scans. Selch et al have reviewed 45 patients who had FSRT for cavernous sinus meningioma who were followed over a median period of 36 months. Sixteen patients received FSRT as a primary treatment and 29 received it after incomplete surgical excision or recurrence following surgery. The median radiation dose used was 5040 cGy. Although the visual outcome was not one of their outcome measures, they reported local tumour control in 80%, stable neurological status in 80% and no treatment induced cranial neuropathy, endocrine dysfunction, cognitive decline, or secondary malignancy. They concluded that FSRT is safe and effective in treating cavernous sinus meningioma. We have experienced similar results in our series.20
Patients with parasellar meningiomas often present with visual acuity and visual field deficits. Therefore, one of the primary aims of treatment is the preservation of visual function. Klink et al21 reported on the long term visual outcome following non-radical microsurgery for parasellar meningioma in a series of 29 patients followed over 10 years. Seven patients in this series had adjuvant conventional postoperative radiotherapy; 93% of the subjects retained 20/40 vision in at least one eye and 14% had improvement of the visual field in one eye. However, only gross visual field changes were considered significant and automated static perimetry was not performed.
Chicani et al22 reported the long term visual outcome of a series of 18 patients with suprasellar meningioma treated surgically; 56% of the patients retained stable vision in both eyes over 10 years of follow up. Only one of the patients had FSRT after three craniotomies.
More recently, radiation therapy following surgery has been shown to reduce the risk of recurrence or progression of meningiomas.23,24 However, conventional radiotherapy carries the risk of blindness in one or both eyes by causing radiation optic neuropathy and retinopathy in addition to hypopituitarism, dementia, delayed radiation injury to the brain, and the induction of secondary tumours.25 In this series, we did not see an adverse event associated with radiation such as radiation retinopathy, radiation optic neuropathy, or chiasmal radionecrosis developing during the follow up period. Recently, a case of radiation retinopathy occurring 22 months after FSRT for optic nerve sheath meningioma has been reported.26 Even though we had no adverse events over a comparable follow up period, all potential complications should be discussed with the patient before treatment.
Like any retrospective case series, our study has limitations. In addition to its relatively small size, 11 (45%) of our 24 patients who were treated with FSRT for parasellar meningioma could not be included for reasons mentioned above. We have used the MD of the visual field since it a single measurement that serves as a “global” index of the automated field. It is possible that at least part of the improvement seen in visual fields in this series could be the result of a “learning effect” or “long term fluctuation.” Finally, the mean follow up period was approximately 2 years. Longer follow up will be needed to determine the precise role of FSRT as either a primary or an adjuvant modality in treatment of parasellar meningiomas. Complete surgical excision of parasellar meningioma sparing the visual apparatus is ideal, but not always technically feasible. We believe the FSRT may have a role in the treatment of primary or recurrent meningiomas that are technically difficult to excise because of their proximity of the visual apparatus.
Abbreviations
FSRT, fractionated stereotactic radiotherapy
MD, mean deviation
MRI, magnetic resonance image
ONSM, optic nerve sheath meningioma
The authors have no proprietary interest in any of the instruments used or any other aspect of this study.
REFERENCES
- 1.Chang SD, Adler JR. Treatment of cranial base meningiomas with linear accelerator radiosurgery. Neurosurgery 1997;41:1019–27. [DOI] [PubMed] [Google Scholar]
- 2.Liscack R, Simonova G, Vymazal J, et al. Gamma knife radiosurgery of meningioma in the cavernous sinus region. Acta Neurochir 1999;141:473–80. [DOI] [PubMed] [Google Scholar]
- 3.Sekhar LN, Patel S, Cusimano M, et al. Surgical treatment of meningiomas involving the cavernous sinus: evolving ideas based on ten year experience. Acta Neurochir 1996; 65 :58–62. [DOI] [PubMed]
- 4.Al-Mefty O . Clinoidal meningioma. J Neurosurg 1990;73:840–8. [DOI] [PubMed] [Google Scholar]
- 5.Al-Mefty O, Kersh JE, Routh A, et al. The long-term side effects of radiation therapy for beingn brain tumors in adults. J Neurosurg 1990;73:502–12. [DOI] [PubMed] [Google Scholar]
- 6.Chan RC, Thompson GB. Morbidity, mortality, and quality of life following surgery for intracranial meningioma. J Neurosurg 1984;60:52–60. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki M, Mizio K, Yoshimoto T. Should meningiomas involving the cavernous sinus be totally resected? Surg Neurol 1995;44:3–13. [DOI] [PubMed] [Google Scholar]
- 8.Wilson CB. Meningiomas: genetics, malignancy and the role of radiation in induction and treatment. J Neurosurg 1994;81:666–75. [DOI] [PubMed] [Google Scholar]
- 9.Roche PH, Regis J, Dufour H, et al. Gamma knife radiosurgery in the management of cavernous sinus meningioma. J Neurosurg 2000;93:68–73. [DOI] [PubMed] [Google Scholar]
- 10.Villavicencio AT, Black PM, Shrieve DC, et al. Linac radiosurgery for skull base meningiomas. Acta Neurochir 2001;143:1141–52. [DOI] [PubMed] [Google Scholar]
- 11.Leber KA, Bergloff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cafernous sinus to stereotactic radiosurgery. J Neurosurg 1998;88:43–50. [DOI] [PubMed] [Google Scholar]
- 12.Mitsumori M, Shrieve DC, Alexander E, et al. Initial clinical results of LINAC-based stereotactic radiosurgery and steroetactic radiotherapy for pituitary adenomas. Int J Radiat Oncol Biol Phys 1998;42:573–80. [DOI] [PubMed] [Google Scholar]
- 13.Andrews DW, Faroozan R, Yang BP, et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery 2002;51:890–904. [DOI] [PubMed] [Google Scholar]
- 14.Pitz S, Becker G, Schiefer U, et al. Stereotactic fractionated irradiation of optic nerve sheath meningioma: a new treatment alternative. Br J Ophthalmol 2002;86:1265–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey IL, Bullimore MA, Raasch TW. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci 1991;32:422–32. [PubMed] [Google Scholar]
- 16.Holladay JT, Prager TC. Snellen equivalent for Bailey-Lovie acuity chart. Arch Ophthalmol 1989;107:955. [DOI] [PubMed] [Google Scholar]
- 17.Brenton RS, Argus WA. Fluctuations on the Humphrey and Octopus perimeters. Invest Ophthalmol Vis Sci 1987;28:767–71. [PubMed] [Google Scholar]
- 18.Das IJ, Downes MB, Corn BW, et al. Characteristics of a linear accelerator-based Stereotactic radiosurgery-radiotherapy unit. Radiother Oncol 1996;38:61–8. [DOI] [PubMed] [Google Scholar]
- 19.Kondziolka D, Niranjan A, Lunsford LD, et al. Stereotactic radiosurgery for meningiomas. Neurosurg Clin N Am 1999;18:117–20. [PubMed] [Google Scholar]
- 20.Selch MT, Ahn E, Laskari A, et al. Stereotactic radiotherapy for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 2004;59:101–11. [DOI] [PubMed] [Google Scholar]
- 21.Klink DF, Sampath P, Miller NR, et al. Long term visual outcome after non-radical microsurgery in patients with parasellar meningiomas. Neurosurgery 2000;47:24–32. [PubMed] [Google Scholar]
- 22.Chicani CF, Miller NR. Visual outcome in surgically treated suprasellar meningiomas. J Neuroophthalmol 2003;23:3–10. [DOI] [PubMed] [Google Scholar]
- 23.Lunsford LD. Contemporary management of meningiomas: radiation therapy as an adjuvant and radiosurgery as an alternative to surgical removal? J Neurosurg 1994;80:187–90. [DOI] [PubMed] [Google Scholar]
- 24.Mirabell R, Lingood RM, de La Monta S, et al. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol 1992;13:157–64. [DOI] [PubMed] [Google Scholar]
- 25.Corn BW, Curran WJ Jr, Shrieve DC, et al. Stereotactic radiosurgery and radiotherapy: new developments and new directions. Sem Oncol 1997;24:707–14. [PubMed] [Google Scholar]
- 26.Subramanian PS, Bressler NM, Miller NR. Radiation retinopathy after fractionated stereotactic radiotherapy for optic nerve sheath meningioma. Ophthalmology 2004;111:565–7. [DOI] [PubMed] [Google Scholar]