Abstract
Aims: To determine the presence and origin of myofibroblasts in pterygia.
Methods: 86 specimens including head, body, and fibrovascular tissue from 52 primary and 34 recurrent pterygia and five exenterated eyes without pterygia were searched for the origin of myofibroblasts. All tissues were subjected to haematoxylin and eosin staining, immunohistochemistry using antibodies against alpha smooth muscle actin (α-SMA), desmin, vimentin, and caldesmon, and transmission electron microscopy (TEM). The phenotype of fibroblasts subcultured in a serum free medium from pterygium fibrovascular tissues was characterised by the above antibodies. Bundles of dense fibrous tissues were noted in 86% of the fibrovascular tissue specimens evaluated. Cells within these bundles were characterised as myofibroblasts based on positive staining to α-SMA, but negative to desmin and caldesmon, markers for smooth muscle cells. Interestingly, positive α-SMA staining was also found in the periorbital fibroadipose tissue posterior to Tenon’s capsule near the nasal conjunctiva in all exenterated specimens. All first passage fibroblasts expressed vimentin, some were positive to α-SMA, but all were negative to desmin or caldesmon. Cells in pterygium fibrovascular tissues showed ultrastructural features of intracytoplasmic bundles of microfilaments, consistent with myofibroblastic differentiation.
Conclusion: These studies collectively demonstrate the presence of contractile myofibroblasts bundle in pterygia and in the periorbital fibroadipose tissue posterior to Tenon’s capsule of exenterated eyes without pterygium.
Keywords: pterygium, myofibroblasts, recurrence, proliferation
Pterygium has long been considered as a chronic degenerative disease with elastotic degeneration caused by ultraviolet irradiation.1 Nevertheless, more evidence has shown that pterygium is actually a proliferative disease. Histopathological findings reveal epithelial hyperplasia and exuberant fibrovascular tissue in the stroma of pterygia. Importantly, the extent of fibrovascular proliferation in the stroma has been used as a reliable morphological index for predicting the propensity of pterygium recurrence following primary excision.2
The extension of pterygium has been topographically correlated with the degree of with the rule corneal astigmatism.3–6 These findings have prompted Oldenburg et al7 to speculate that the astigmatism associated with pterygia is caused by the contractile forces generated by myofibroblasts in the stroma. Nevertheless, their histopathological and ultrastructural findings did not support this hypothesis.
Previously, we have reported that following the excision of pterygium head and body, a thorough removal of subconjunctival fibrovascular tissue in the adjacent area is important to reduce the recurrence rate.8,9 In this study, we carried out extensive immunohistochemical and ultrastructural characterisation of such removed fibrovascular tissues in a number of primary and recurrent pterygia, and report evidence that contractile myofibroblasts are prevalent in subconjunctival fibrovascular tissue of pterygia, but not in the control without pterygia. Furthermore, we subcultured cells in a serum free media from these fibrovascular tissues and confirmed that they were myofibroblasts. These cells were also traced to the periorbital fibroadipose tissue posterior to Tenon’s capsule. The significance of this finding in pterygium and other cicatricial conjunctival diseases is further discussed.
MATERIALS AND METHODS
Materials
The monoclonal antibodies against α-SMA (1:200), desmin (1:100), caldesmon (1:100), vimentin (1:100), neurofilament (1:100), the polyclonal antibody against S-100 (1:200), the secondary LSAB biotinylated anti-mouse, anti-rabbit and anti-goat secondary antibodies, the biotin blocking system, the streptavidin peroxidase conjugate enzyme were all purchased from Dako Corporation (Carpinteria, CA, USA). 3-Amino-9-ethylcarbazole chromogen (AEC) was purchased from Sigma Chemical (St Louis, MO, USA). Collagenase A was purchased from Roche (Indianapolis, IN, USA).
Surgical specimens
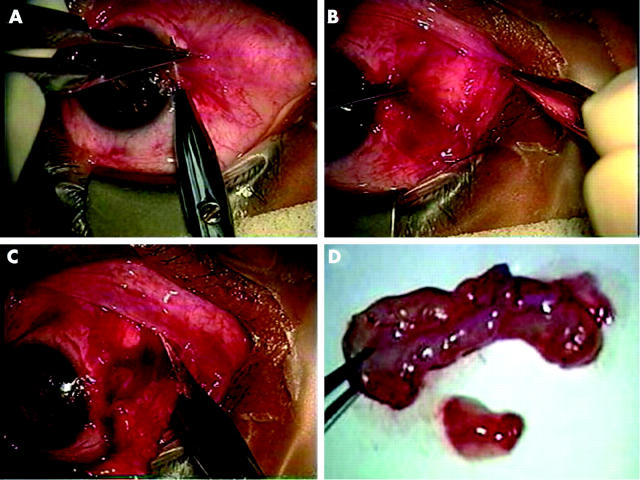
In compliance with the provisions of the Declaration of Helsinki, relevant patient demography, diagnosis, clinical data, and surgical procedure were retrospectively retrieved from 86 eyes of 76 patients, who underwent surgeries to remove the head, body, and fibrovascular tissue from 52 primary pterygia, and 34 recurrent pterygia. All 86 specimens were studied for morphological evidence of myofibroblasts. All surgeries were performed at the Bascom Palmer Eye Institute from November 1997 to March 1999 by one surgeon (SCGT) to ensure consistency in the extent of tissue removal. Figure 1 shows how head and body were excised in one piece by dissection and how subconjunctival tissue was removed from the fornix and the nasal caruncle. The removed subconjunctival fibrovascular tissue frequently contained some fat tissue in the edge bordering with the nasal caruncle.
Figure 1.
Surgical procedures of removing subconjunctival fibrovascular tissue. In this representative case, the head is avulsed from the corneal surface from the limbus by a blunt dissection (A). The subconjunctival fibrovascular tissue grabbed by a fine forceps is dissected with a sharp scissors from the overlying epithelial tissue, which is also grabbed with another fine forceps (B). The subconjunctival tissue is then transected from the fornix, which was infiltrated with fat tissue in the nasal caruncle (C). The transected subconjunctival fibrovascular tissue is of several folds in size compared to that of the head and body (D).
In addition, we retrospectively retrieved a total of five exenteration pathological specimens from three patients with basal cell carcinoma, one patient with melanoma of the eyelid, and one patient with adenoid cystic carcinoma of the lacrimal gland.
Cell cultures of pterygium fibrovascular tissue
With patient’s consent and authorisation, the fibrovascular tissue removed from one eye with primary and two eyes with recurrent pterygium was cut into explants of approximately 1×1 mm, treated with collagenase A in DMEM (1 mg/ml), and incubated for 2 hours at 37°C under 95% humidity with 5% CO2. After centrifugation at 800×g, the residual tissue was seeded in serum free defined KSFM (keratinocyte serum free medium) on a 100 mm plastic dish. The medium was changed every 2 days. At 80% to 90% confluence, fibroblasts were passaged with 1:3 split using 0.25% trypsin and 1 mM EDTA in HBSS. Fibroblasts derived from the first passage were used for immunostaining.
Histology and immunostaining
Histology was performed in a traditional manner and stained with haematoxylin and eosin. Immunoreactivity of α-SMA, desmin, caldesmon, vimentin, and neurofilament S-100 was detected in 3 µm paraffin sections using a conventional immunohistochemistry. Briefly, after endogenous peroxidase and non-specific avidin-biotin binding were blocked, deparaffinised and rehydrated sections were incubated with primary antibodies against the corresponding antigens for 30 minutes, washed in phosphate buffered saline, and incubated with the biotinylated linking secondary antibody for 22 minutes. Immunoreactivity was detected using a streptavidin peroxidase enzyme conjugate reagent (1:100) for 22 minutes and AEC, counterstained with haematoxylin and photographed. Immunofluorescence staining was performed on cultured fibroblasts by incubating them with the above primary antibodies for 1 hour, followed by fluorescein isothiocyanate (FITC) conjugated antibody for 45 minutes. For all staining, incubation without primary antibody was used as a negative control, and paraffin embedded sections of the lid and conjunctival fibroblast from patients with conjunctivochalasis were used as a control.
Transmission electron microscopy
Tissue samples were fixed by immersion in 2% paraformaldehyde and 2.5% glutaraldehyde in Millonig’s phosphate buffer for 1 hour. Fixed samples were rinsed in saline solution, post fixed with 1% osmium tetroxide for 1 hour, dehydrated in a serial of ethanol dilutions, infiltrated in a 1:1 ratio of resin to dehydrate, embedded in 100% resin, for 1–2 hours. Thick (1 μm) and thin (100 nm) sections were cut and stained with 2% methanolic uranyl acetate. Ultrathin sections were studied under the Jeol CX100 TEM (Jeol Ltd, Akishima, Japan).
RESULTS
Table 1 summarises demographic data of these 76 patients. They were 52 men and 24 women, with a mean age of 47.5 (SD 15.8) years (range 22–79 years). There were a total of 52 eyes with primary pterygium, among which 10 eyes had a double head pterygium, and 34 eyes had recurrent pterygia, among which five eyes had a double head pterygium.
Table 1.
Demographic data
| Total | Primary | Recurrent | |
| No of eyes | 86 | 52 | 34 |
| Right eye | 35 | 25 | 10 |
| Left eye | 34 | 18 | 16 |
| Male | 52 | 32 | 20 |
| Female | 24 | 15 | 9 |
| Age (mean (SD)) | 47.5 (15.8) | 51.1 (15.8) | 41.8 (14.4) |
| (range) | (22–79) | (22–79) | (27–79) |
| Motility restriction | 2 | 0 | 2 |
Myofibroblasts in pterygium fibrovascular tissues
In the stroma of the fibrovascular tissue, the most striking finding under haematoxylin and eosin staining was eosinophilic bundles of dense fibrous tissues in 86% of the specimens evaluated (table 2, also see an example in fig 2A). Under higher magnification, these bundles were distinguishable from the surrounding connective tissue (fig 2B). Such bundles were only found in one body specimen of a primary pterygium, but in none of the head specimens (table 2). The stroma of the fibrovascular tissue did not reveal any actinic damage.
Table 2.
Grading of eosinophilic bundles in specimens
| Grading of eosinophilic bundles | Total | Primary | Recurrent | ||||||
| H | B | FVT | H | B | FVT | H | B | FVT | |
| 0 absent | 0 | 0 | 12 | 0 | 0 | 10 | 0 | 0 | 2 |
| 1+ mild presence | 0 | 0 | 25 | 0 | 0 | 17 | 0 | 0 | 8 |
| 2+ moderate presence | 0 | 1 | 23 | 0 | 1 | 10 | 0 | 0 | 13 |
| 3+ abundant presence | 0 | 0 | 26 | 0 | 0 | 15 | 0 | 0 | 11 |
H, head; B, body; FVT, fibrovascular tissue.
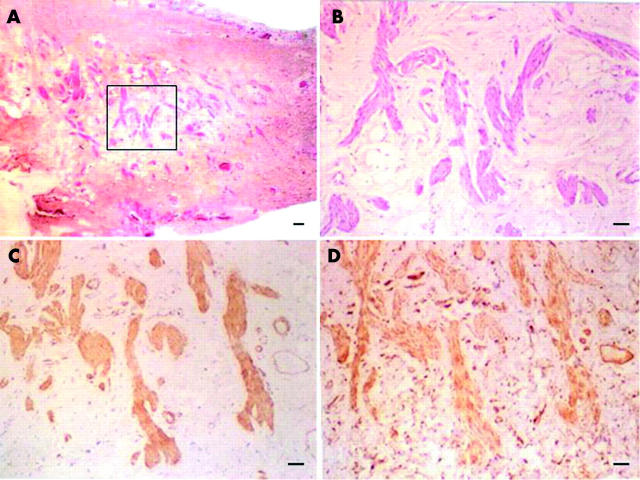
Figure 2.
Immunohistochemical characterisation of myofibroblasts in pterygium fibrovascular tissues. Eosinophilic bundles of dense connective tissue are found in the stroma of fibrovascular tissue (A). Higher magnification showed that cells present in these bundles (B) are positive to staining to α-SMA (C) and vimentin (D), while cells in the rest of the connective tissue are only positive to vimentin (D). The bar represents 50 μM.
To characterise the phenotype of the cells within these bundles, we conducted immunostaining with a battery of antibodies. The result showed that these cells were positively stained for α-SMA (fig 2C) and vimentin (fig 2D), but negative to desmin, and caldesmon (not shown)—that is, markers for smooth muscle cells, and negative to the nerve sheath marker S-100 protein (not shown). In contrast, cells in the rest of the connective tissue of the stroma were positive to vimentin staining (fig 2D). Therefore, these results collectively indicated that these bundles, although morphologically similar to nerve tissues, were not nerves but consisted primarily of myofibroblasts.
Myofibroblasts in cultured fibrovascular tissues
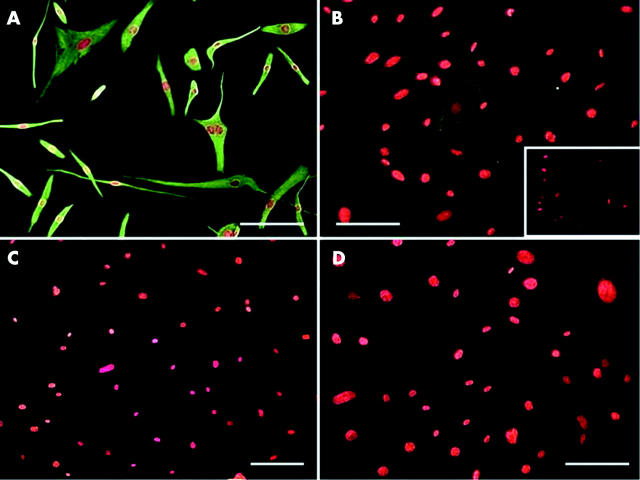
To further confirm the myofibroblast phenotype, we established serum free primary cultures from pterygium fibrovascular tissues. As shown in figure 3, all cultured cells showed uniform positive staining to vimentin (fig 3A), while some cells showed positive staining to α-SMA (fig 3B). All cultured cells were negative to desmin (fig 3C) and caldesmon (fig 3D). The same results were obtained in three different surgical specimens—that is, one primary and two recurrent pterygia. These results supported that myofibroblasts were present in pterygial fibrovascular tissues.
Figure 3.
Immunostaining of cultured cells derived from pterygium fibrovascular tissue. All cells are uniformly positive to vimentin (A), while some are positive to α-SMA (B), negative control to α-SMA in conjunctivochalasis fibroblast (inset) but are negative to desmin (C) and caldesmon (D). The bar represents 50 μM.
Ultrastructural characterisation of myofibroblasts
Transmission electron microscopy revealed that cells in the bundle of fibrovascular tissues manifested ultrastructural features of myofibroblasts, which included intracytoplasmic bundles of microfilaments that ran parallel to the long axis of the cell at the periphery (stars fig 4C), numerous nuclear indentations and deep folds that collectively gave indirect evidence of contraction (arrows, fig 4C).10–12 In addition, pseudopodal projections were noticed (fig 4A and D).
Figure 4.
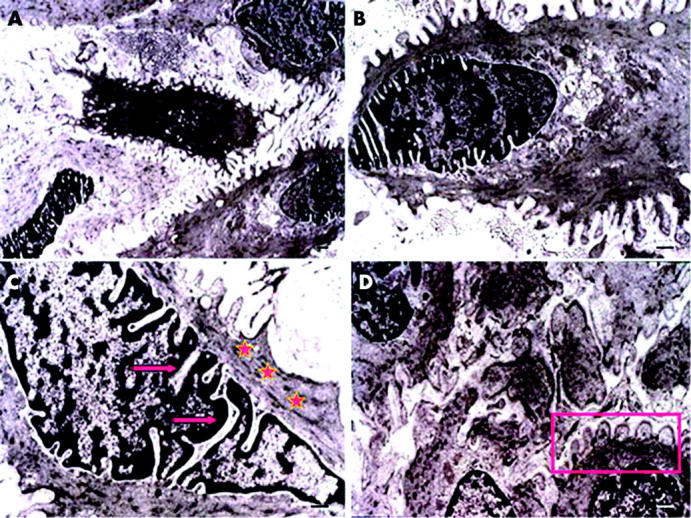
Ultrastructural features of cells in pterygium fibrovascular tissue. Cells show intracytoplasmic bundles of microfilaments (stars) arranged in parallel to the axis of the cell at the periphery (A), numerous nuclear indentations (arrows) and deep folds that give indirect evidence of contraction (C), and active pseudopodal activity (B and D).
Localisation of myofibroblasts in the periorbital fibroadipose tissue
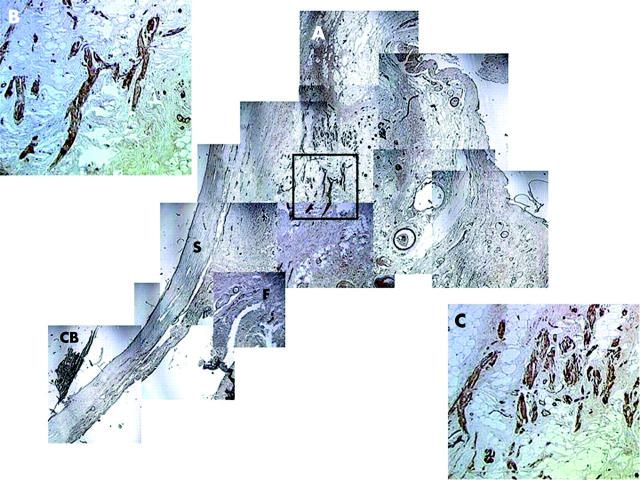
We then wondered whether myofibroblasts were normally present in the eye, and if so, where these cells were. We thus surveyed the expression of the above cell markers in five exenterated eyes that did not have any clinical evidence of pterygium. In all five exenterated specimens, we found myofibroblasts in the periorbital fibroadipose tissue posterior to Tenon’s capsule (fig 5A). These cells were positive to α-SMA (fig 5B) and vimentin (fig 5C) staining, but were negative to desmin and caldesmon staining (not shown).
Figure 5.
Immunohistochemical staining of extenterated eyes. (A) Myofibroblasts are found in the periorbital fibroadipose tissue posterior to Tenon’s capsule (F: fornix, CB: ciliary body, S: sclera). These cells were positive to α-SMA (B) and vimentin (C) staining.
DISCUSSION
Using immunostaining with cell specific markers and ultrastructural analysis, we provide strong evidence supporting the important finding that myofibroblasts are present in the fibrovascular tissue of primary and recurrent pterygia. The presence of myofibroblasts helps explain why pterygium results in corneal astigmatism and regional fornix shortening. In this study, myofibroblasts were found predominantly (86%) in fibrovascular tissues around the head and body, but only in one body specimen, and absent in the head specimens. Because Oldenburg et al7only studied the head and body they did not detect myofibroblasts.
Myofibroblasts are heterogeneous when defined by the expression of different types of cytoskeletons.13 In normal human and animal tissues, myofibroblasts may express vimentin only (V phenotype), vimentin and desmin (VD phenotype), and vimentin and α-SMA (VA phenotype). However, in pathological situations, myofibroblasts express vimentin, α-SMA, and desmin (VAD phenotype) or vimentin, α-SMA, desmin, and smooth muscle myosin heavy chains (VADM phenotype).13–16 When grown in culture, subpopulations of fibroblasts derived from normal tissues acquire several phenotypic features of myofibroblasts.17–19 In this study, we noted that myofibroblasts derived from fibrovascular tissues of primary and recurrent pterygia expressed vimentin and α-SMA—that is, VA phenotype, in vivo and in culture. We used a serum free culture medium to avoid artificial activation of fibroblasts into myofibroblasts in culture.
The presence of myofibroblasts in pterygia raises one important question about their origin. One possibility is that myofibroblasts are activated from the resident fibroblasts in the region by fibrogenic stimuli such as transforming growth factor β, connective tissue growth factor, and platelet derived growth factor (for reviews see Leask et al,20 Tomasek et al,21 Ina et al,22 and Kissin and Korn23)—that is, factors previously detected in the pterygium tissue.24 Because these factors are rich in the head and body,24 one would predict that myofibroblasts should be readily found in the head and the body of the pterygium. This theory is less likely because we found myofibroblasts in the body of only one specimen. In this study we did not demonstrate pterygium myofibroblast migration from deep periorbital tissue, as an alternative theory we speculate that myofibroblasts already present in the normal tissue might be activated to migrate into the pterygium tissue. We favour this theory because we found myofibroblasts in normal periorbital fibroadipose tissue posterior to Tenon’s capsule near the nasal conjunctiva with all five exenterated whole eyes specimens examined. Because this region is contiguous to pterygial fibrovascular tissue, future studies are needed to investigate whether these resident myofibroblasts actually contribute to the formation of contractile fibrovascular tissue of the pterygium.
Abbreviations
AEC, 3-amino-9-ethylcarbazole chromogen
FITC, fluorescein isothiocyanate
α-SMA, alpha smooth muscle actin
TEM, transmission electron microscopy
Financial support in part by an unrestricted grant from Ocular Surface Research and Education Foundation, Miami, FL, USA.
Conflict of interest: None.
Presented in part at the Annual Meeting of International Ocular Surface Society, May 2002 in Fort Lauderdale, FL, and at the ARVO Meeting, May 2003 in Fort Lauderdale, FL, USA.
REFERENCES
- 1.Austin P, Jakobiec FA, Iwamoto T. Elastodysplasia and elastodystrophy as the pathogenic bases of ocular pterygia and pinguecula. Ophthalmology 1983;90:96–109. [DOI] [PubMed] [Google Scholar]
- 2.Tan D, Chee S-P, Dear K, et al. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol 1997;115:1235–40. [DOI] [PubMed] [Google Scholar]
- 3.Tomidokoro A. Effects of pterygium on corneal spherical power and astigmatism. In: Miyata K, Sakaguchi Y, Samejima T, et al, eds. Ophthalmology 2000;107:1568–71. [DOI] [PubMed] [Google Scholar]
- 4.Stern GA. Effect of pterygium excision on induced corneal topographic abnormalities. In: Lin A, ed. Cornea 1998;17:23–7. [DOI] [PubMed] [Google Scholar]
- 5.Lin A. Correlation between pterygium size and induced corneal astigmatism. In: Stern G, ed. Cornea 1998;17:28–30. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay RG. Pterygium-induced corneal astigmatism. In: Sullivan L, ed. Clin Exp Optom 2001;84:200–3. [DOI] [PubMed] [Google Scholar]
- 7.Oldenburg JB, Garbus J, McDonnell JM, et al. Conjunctival pterygia. Mechanism of corneal topographic changes. Cornea 1990;9:200–4. [PubMed] [Google Scholar]
- 8.Prabhasawat P, Barton K, Burkett G, et al. Comparison of conjunctival autografts, amniotic membrane grafts and primary closure for pterygium excision. Ophthalmology 1997;104:974–85. [DOI] [PubMed] [Google Scholar]
- 9.Solomon A, Pires R, Tseng SCG. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology 2001;108:449–60. [DOI] [PubMed] [Google Scholar]
- 10.Ryan G. Myofibroblasts in human granulation tissue. Hum Pathol 1974;5:55–67. [DOI] [PubMed] [Google Scholar]
- 11.Gabbiani G. Dupuytren’s contracture: fibroblast contraction? An ultrastructural study. Am J Pathol 1972;66:131–46. [PMC free article] [PubMed] [Google Scholar]
- 12.Gabbiani G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971;27:549–50. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt-Graff A, Desmouliére A, Gabbiani G. Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch 1994;425:3–24. [DOI] [PubMed] [Google Scholar]
- 14.Menzel T. The emerging role of myofibroblasts in soft tissue neoplasia. Am J Clin Pathol 1997;107:2–5. [DOI] [PubMed] [Google Scholar]
- 15.Schurch W. The intermediate filament cytoskeleton of myofibroblasts: an immunofluorescence and ultrastructural study. Virchows Arch A Pathol Anat Histopathol 1984;403:323–36. [DOI] [PubMed] [Google Scholar]
- 16.Skalli O. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest 1989;60:275–85. [PubMed] [Google Scholar]
- 17.Desmouliére A, Rubbia-Brandt L, Abdiu A, et al. α-Smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated by gamma-interferon. Exp Cell Res 1992;201:64–73. [DOI] [PubMed] [Google Scholar]
- 18.Desmouliere A, Gabbiani G. Modulation of fibroblastic cytoskeletal features during pathological situations: the role of extracellular matrix and cytokines. Cell Motil Cytoskeleton 1994;29:195–203. [DOI] [PubMed] [Google Scholar]
- 19.Sappino A. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest 1990;63:144–61. [PubMed] [Google Scholar]
- 20.Leask A, Holmes A, Abraham D. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Rheumatol Rep 2002;4:136–42. [DOI] [PubMed] [Google Scholar]
- 21.Tomasek J, Vaughan M, Haaksma C. Cellular structure and biology of Dupuytren’s disease. Hand Clin 1999;15:21–34. [PubMed] [Google Scholar]
- 22.Ina K, Kitamura H, Tatsukawa S, et al. Transformation of interstitial fibroblasts and tubulointerstitial fibrosis in diabetic nephropathy. Med Electron Microsc 2002;35:87–95. [DOI] [PubMed] [Google Scholar]
- 23.Kissin EY, Korn JH. Fibrosis in scleroderma. Rheum Dis Clin North Am 2003;29:351–69. [DOI] [PubMed] [Google Scholar]
- 24.Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-β and tumor necrosis factor-α in the pterygium. Acta Histochem (Jena) 1996;98:195–201. [DOI] [PubMed] [Google Scholar]