Abstract
Aim: To evaluate the efficacy and safety of a stainless steel miniature glaucoma drainage device (Ex-PRESS R50) for the surgical treatment of primary open angle glaucoma (POAG) and cataract when combined with phacoemulsification.
Methods: Clinical, prospective, multicentre, single treatment arm, non-randomised, non-masked study. The Ex-PRESS device was implanted at the limbus under a conjunctival flap. Phacoemulsification cataract extraction and in the bag IOL implantation were performed through clear cornea temporally. Primary outcome: IOP change; secondary outcomes: side effects and VA changes.
Results: 26 eyes of 25 patients were implanted with the device. The mean (SD) follow up was 23.9 (10.4) months and the mean age was 75.1 (7.1) years. 17/26 eyes have more than 3 years of follow up. One case was discontinued because of device removal, one because of death, and three were lost to follow up. Efficacy: preoperative IOP was 21 (4) mm Hg; at 1, 2, and 3 years IOP was 15.3 (3.1) mm Hg (35% reduction), 16.6 (2.7) mm Hg (29% reduction), and 16 (2.6) mm Hg (22% reduction) respectively. Kaplan-Meyer determined overall success rate (IOP ⩽21 mm Hg at the last visit with or without medications) as 76.9%. The number of antiglaucoma medications was reduced by 95% at year 1. Only six patients (23%) were taking IOP lowering treatment at their last visit, five with one medication and one with two medications. Side effects: early postoperative complications were clinically mild and included six cases of hypotony (IOP <5 mm Hg), three cases of hyphaema (<2 mm) with no clinically significant further effects. Long term complications were two cases (7.7%) of device rotation (one treated by reposition) and three cases (11.5%) of conjunctival erosion at 2 and 3 years.
Conclusions: The Ex-PRESS implant, combined with phacoemulsification cataract extraction, is clinically safe and effective, maintaining in the long term a large reduction in IOP and in the number of antiglaucoma medications.
Keywords: Ex-PRESS implant, glaucoma, glaucoma filtering surgery, intraocular pressure, phacoemulsification
The occurrence of both glaucoma and clinically relevant cataract in the same individual is a frequent condition, especially in the elderly population. Besides age, this might be related to the possible role of antiglaucoma medications in the progression of lens opacity.1–3 When both procedures are required, these can be performed separately or in combination.
Glaucoma filtering surgery is indicated when glaucomatous damage progresses despite the lower level of intraocular pressure (IOP) obtained with pharmacological and/or laser treatment. In the past few years, filtering surgery has improved, with an increased likelihood of successfully lowering the IOP while minimising complications. While full thickness filtration procedures considerably reduced the IOP, they were associated with early postoperative hypotony and related side effects and became progressively less popular.
Trabeculectomy has been the surgical treatment of choice for primary open angle glaucoma (POAG) since the 1970s4; its success rates and complication however are less than ideal.5,6 Early hypotony and bleb infections are still of concern.7,8,9,10,11,12,13
Glaucoma drainage implants, designed to shunt the aqueous posteriorly, represent an alternative method for lowering IOP in glaucomatous patients,14–17 commonly used in refractory glaucoma or after the failure of other surgical approaches.
The Ex-PRESS is a miniature stainless steel glaucoma device, developed as an alternative to trabeculectomy and to the other types of glaucoma filtering surgery for patients with POAG. This procedure would be theoretically more reproducible and simple to perform as well as less traumatic to the ocular tissue than traditional filtering surgery. The implant is inserted at the limbus under a conjunctival flap and diverts the aqueous humour from the anterior chamber to the subconjunctival space, obtaining the formation of a conjunctival filtration bleb, in a similar way to trabeculectomy. This procedure can be performed on its own or in combination with phacoemulsification.
This study is about the long term efficacy and safety results of the Ex-PRESS device (Optonol Ltd) implanted for the surgical treatment of POAG and cataract when combined with phacoemulsification.
PATIENTS AND METHODS
This non-randomised, multicentre, prospective trial was conduced at three different centres. All operations were performed by one experienced ophthalmic surgeon in each centre. Surgeons were instructed in the implantation procedure for the device and practised the procedure on animal eyes before operating on humans. Approval from the institutional review boards was obtained. After receiving a comprehensive explanation of the experimental nature of this procedure, informed consent was obtained from each patient.
Patients were recruited prospectively and non-consecutively. Their mean age was 75.1 (SD 7.1) years. Eligibility criteria included the presence of POAG, mobile unscarred conjunctiva for at least two clock hours superiorly, an indication for glaucoma filtering surgery based on uncontrolled IOP despite medications, and the presence of a clinically significant decrease of visual acuity as a result of cataract. Eyes with angle closure glaucoma, neovascular glaucoma, congenital or juvenile glaucoma, or monocular patients were excluded. All patients had visual field examinations within 6 months before the recruitment, and were affected by POAG.
Upon enrolment each patient underwent a complete ophthalmic examination; postoperative evaluations were performed on the first and seventh days and on the first, second, third, sixth, ninth, 12th, 18th, 24th, 30th, 36th, 42nd, and 48th postoperative months, or more often when required. The examination consisted of measurement of best corrected visual acuity, slit lamp biomicroscopy of the anterior and posterior segments, IOP measurement by applanation tonometry, and gonioscopy. History and all medication details were recorded. Standard case report forms (CRF) were used.
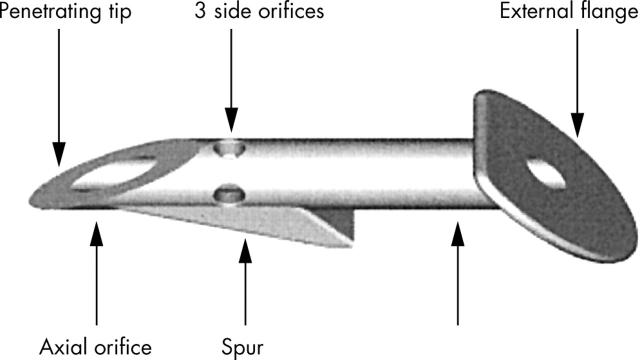
The Ex-PRESS R50 drainage device is an non-valved flow restricting implant, made of implantable stainless steel (316L), which is approved for medical and ophthalmic applications. The R50 device is built with a 3 mm long tube with a 400 μm (27 gauge) external diameter, a 50 μm inner diameter, and a bevelled, sharpened, rounded tip (fig 1). The disc-like flange at the device’s proximal end prevents it being implanted too deeply, while the spur-like projection prevents its extrusion. The external flange and inner spur are angled to conform to the anatomy of the sclera, and the distance between them corresponds to the scleral thickness at the limbus, where the device is implanted. Three holes around the proximal end of the device’s shaft ensure filtration if the main orifice becomes blocked by the iris or other tissues.
Figure 1.
Design of the Ex-PRESS miniature glaucoma implant.
Surgical technique
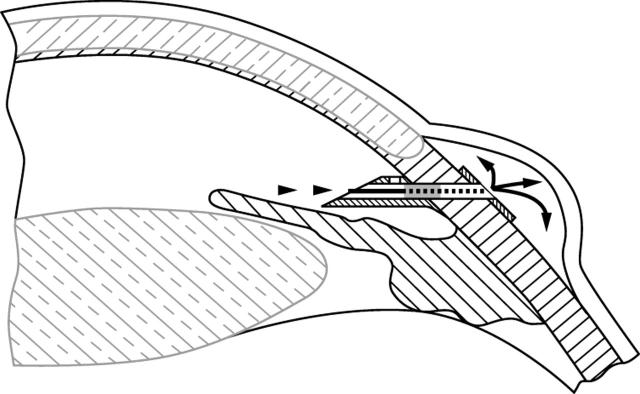
The Ex-PRESS R50 device was implanted under local or topical anaesthesia, using standard ophthalmic microsurgical instruments. The procedure consisted of a 2–4 mm circumferential conjunctiva opening, 10–15 mm posterior to the limbus. The implant, mounted on its introducer, was slid under the conjunctiva and Tenon’s capsule and implanted radial to the limbus and parallel to the iris through a pre-incision performed with a 27 gauge needle. The introducer was then withdrawn. The conjunctiva was closed with a suture at the surgeon’s discretion. Manipulation of the conjunctiva during the introduction was limited to one or two clock hours. At the end of this procedure, a temporal clear cornea phacoemulsification cataract extraction with “in the bag” intraocular lens (IOL) insertion was performed. (A diagrammatic representation of the implant in place is shown in fig 2.)
Figure 2.
Schematic drawing of the Ex-PRESS implant in situ.
CRF were used to collect data on the procedure, its duration, and any complications during surgery. Using a semiquantitative scale of 1–5 (where 1 = very easy or very well positioned and 5 = very difficult or very poorly positioned), the surgeon graded the ease of the insertion and positioning of the implant at the limbus. At each follow up, the surgeon carried out a complete ophthalmic examination, recording all data on the CRF, including anterior chamber depth, device position, presence or absence of the bleb, and visual acuity. The IOP and the use of antiglaucoma medications or other IOP reduction procedures during the follow up were primary outcome measures. Secondary outcome measures included the visual acuity and the occurrence of perioperative or postoperative complications.
The procedure was labelled an overall success when IOP was <21 mm Hg with or without antiglaucoma medications; a complete success was defined as an IOP <21 mm Hg without medications. Postoperative bleb management and scarring modulation with needling or 5-fluorouracil (5-FU) injections were not a criterion for failure.
Results were analysed on an “intent to treat” basis. Data were presented as mean (SD), unless otherwise specified. The Wilcoxon signed rank test was used to compare changes from baseline IOP and to compare the number of antiglaucoma medications used by the patient. Time to failure, defined as an IOP >21 mm Hg at two consecutive visits, IOP >21 mm Hg at the last visit, or a need for additional surgery, was evaluated using Kaplan-Meyer survival analysis. A p value of <0.05 was considered statistically significant for all analyses. Statistical analyses were carried out using the SPSS statistical package or Microsoft Excel.
RESULTS
The Ex-PRESS R50 device was implanted into 26 eyes of 25 white patients with POAG and cataract. The mean age of the patients was 75.1 (7.1) years; table 1 details other demographic data.
Table 1.
Patients and methods
| Patients | |
| Number of eyes | 26 |
| Female 15 (57.7%) | Male 11 (42.3%) |
| Age (SD) (years) | 75.1 (7.1) |
| Types of glaucoma | |
| Primary open angle glaucoma | 20 (76.9%) |
| Pseudoexfoliative glaucoma | 5 (19.2%) |
| Ocular hypertension | 1 (3.9%) |
The mean follow up period was 30.2 (14.5) months. Twenty five patients completed 1 year, 18 patients 2 years, nine patients 3 years, and four patients 4 years. Two subjects discontinued the study because of erosion followed by removal and four patients were lost to follow up.
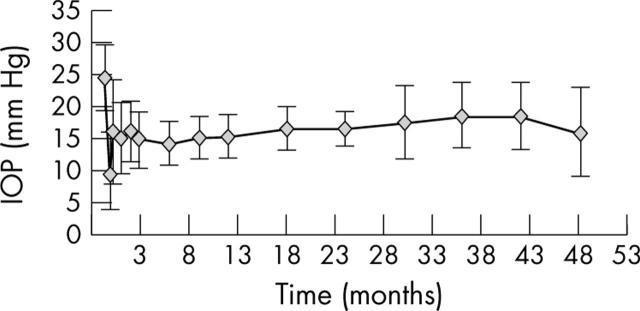
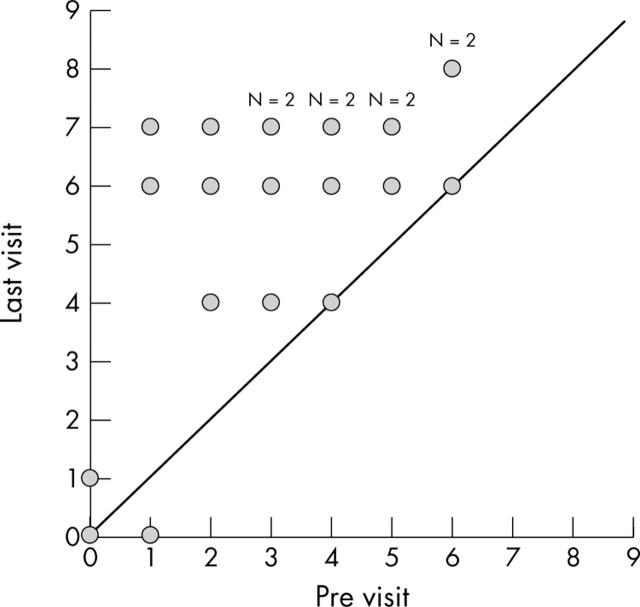
Mean preoperative (baseline) IOP was 21 (SD 4) mm Hg; at 1, 2, and 3 years after surgery IOP was 15.3 (3.1) mm Hg (35% reduction), 16.6 (2.7) mm Hg (29% reduction), and 16 (2.6) mm Hg (22% reduction), respectively (table 2, fig 3). The overall success rate (IOP ⩽21 mm Hg with or without medications) was 94.4% at 2 years (17/18 eyes) and 76.9% at last visit (20/26 eyes). The complete success rate (IOP ⩽21 mm Hg without medications) was 66.7% at 2 years (12/18 eyes) and 65.4% (17/26 eyes) at last visit (table 3).
Table 2.
Efficacy—IOP summary, long term
| No | IOP (mm Hg) | |||||
| Mean | SD | Median | Min | Max | ||
| Before | 26 | 24.5 | 5.0 | 24 | 17 | 40 |
| 1st year | 25 | 15.3 | 3.1 | 15 | 7 | 22 |
| 2nd year | 18 | 16.7 | 2.7 | 17 | 13 | 25 |
| 3rd year | 9 | 18.6 | 5.1 | 17 | 13 | 28 |
| 4th year | 4 | 16 | 6.9 | 18 | 6 | 22 |
Figure 3.
Efficacy, IOP dynamics (p = 0.004 for IOP reduction >20% paired t test).
Table 3.
Success rate
| Target IOP | Overall success (with or without meds) | Complete success (without meds) | |
| Success rate at 2 years (n = 18) | |||
| ⩽21 mm Hg | 17/18 (94.4%) | 12/18 (66.7%) | |
| ⩽18 mm Hg | 16/18 (88.9%) | 11/18 (61.1%) | |
| Success rate at last visit (n = 26) | |||
| ⩽21 mm Hg | 20/26 (76.9%) | 17/26 (65.4%) | |
| ⩽18 mm Hg | 19/26 (73.1%) | 16/26 (61.5%) | |
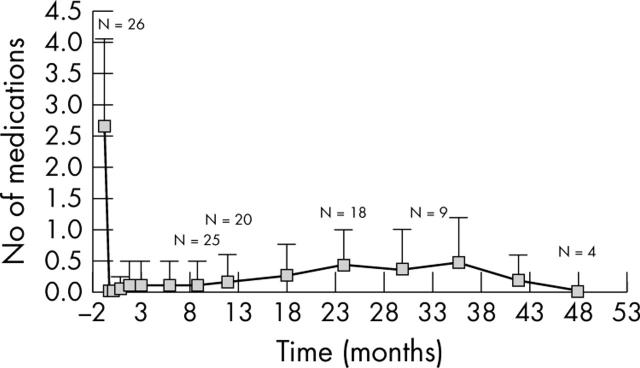
The reduction of antiglaucoma medication use recorded following implantation of the Ex-PRESS device was significant. Six patients were treated pharmacologically at their last visit: five were treated with one medication and one with two medications (fig 4).
Figure 4.
Number of medications over time (p = 0.0001 for IOP reduction in medications, paired t test). Six patients (23%) were treated with medications at their last visit; five were treated with one medication; and one with two medications.
Visual acuity improved in 21 eyes; no change in VA was recorded in four eyes (fig 5). In one patient visual acuity decreased at the last visit (one Snellen line decrease).
Figure 5.
Visual acuity (VA) for patients with a minimum follow up of 2 months (n = 26). VA improvement in 21 eyes; no change in VA, four eyes; VA decrease, one eye (one Snellen line decrease).
Bleb needling in the postoperative period was performed in seven eyes: six of them associated with 5-FU injection. One further patient received an injection of 5-FU without bleb needling (table 4).
Table 4.
Postoperative interventions
| Intervention | Eyes | |
| No | % | |
| 5-FU injection | 1 | 3.8 |
| Needling with 5-FU | 6 | 23.1 |
| Needling without 5-FU | 1 | 3.8 |
| Total | 8 | 30.7 |
Short term complications included three cases of hyphaema (<2 mm), one in the first postoperative day, all spontaneously resolved within 1 week; two cases of device rotation, one noticed at the first at first postoperative month and immediately repositioned and one 2 years after surgery, which caused conjunctival erosion; three cases of erosion: one at the second year of follow up, still now under observation, two at the second and third year respectively, one followed by device removal and one by device replacement; one case of choroidal detachment, probably related to vigorous rubbing of the eye as reported by the patient, spontaneously resolved; one case of iris contact with the implant, at second postoperative month, without sequelae (table 5).
Table 5.
Complications
| Complication | No (%) | Comments |
| Hyphaema <2 mm | 3 (11.5) | 1 day postop; all resolved spontaneously within 1 week |
| Device rotation | 2 (7.7) | 1 month postop treated by repositioning; 2 years postop followed by erosion and is under observation (see below) |
| Choroidal detachment | 1 (3.8) | 21 months postop, due to vigorous rubbing of the eye; spontaneously resolved |
| Device iris touch | 1 (3.8) | 2 months postop; calm eye |
| Device malposition | 2 (7.7) | 1.5 years postop spontaneously resolved; 2.5 years postop with erosion. |
| Erosion | 3 (11.5) | 2 years postop associated with rotation and is under observation (see above); 2.5 and 3 years postop one followed by device removal and one by device replacement |
| Suture granuloma | 1 (3.8) | 2 months postop; spontaneously resolved |
| Visual acuity decrease | 1 (3.8) |
DISCUSSION
The implantation of the Ex-PRESS R50 miniature stainless steel glaucoma drainage device in 26 eyes of 25 patients combined with phacoemulsification cataract extraction produced a cumulative overall success rate of 76.9% using an IOP ⩽21 mm Hg as an end point. Our success rate compares favourably with data reported in the literature for other filtering procedures: 50–100% for trabeculectomy,18–20 up to 87.5% for non-penetrating surgery,21,22 and 25–90% for other glaucoma drainage devices.23–25
Our results confirm those obtained in a small case series using a trabecular meshwork bypass shunt where IOP and glaucoma medications were reduced in patients who were followed for up to 9 months.26
In studies comparing viscocanalostomy with trabeculectomy, the latter procedure has been shown to be more effective in controlling IOP while viscocanalostomy had fewer postoperative complications.21,22,27–30 A study comparing trabeculectomy (retrospectively) in one eye and deep sclerectomy with collagen implant (prospectively) in the other eye of 20 patients found comparable success rates with IOP <21 mm Hg obtained in 40% of the deep sclerectomy eye group and 45% of the trabeculectomy eye group31; comparable IOP reductions were also reported in a prospective study of 78 eyes.32 However, another study noted lower levels of IOP with trabeculectomy although there were fewer complications with deep sclerectomy.33 Preliminary results from a study comparing deep sclerectomy with Ex-PRESS implant surgery indicated that the two procedures were comparable at 6 months of follow up.34
In our study, implantation of the Ex-PRESS device produced a low rate of short term complications, such as a shallow or flat chamber and hyphaema, similar to other filtering surgeries; there were no long term complications. Only six patients in our study required antiglaucoma medications at their last visit; comparatively better than commonly reported after trabeculectomy.6,20,35
Bleb failure due to fibroblast proliferation is one of the main causes of failed filtration. In order to minimise the risk of scarring, the use of antimetabolites preoperatively and postoperatively is common.
CONCLUSIONS
In summary, results from our study demonstrate that implantation of the Ex-PRESS device is safe and is not associated with significant complications, both in short and long term. The cases of conjunctival erosion can be prevented by optimal intraoperative positioning of the device, mainly avoiding too anterior implantation. The correct positioning of the implant is the most crucial consideration for preventing complications.
This stainless steel device is safe and effective for reducing IOP and antiglaucoma medications in combination with cataract extraction.
Our results indicate that this procedure can be considered as an alternative to conventional filtering surgery for the control of IOP in patients with open angle glaucoma. However, further studies that directly compare the Ex-PRESS implant with trabeculectomy and other procedures are still required to assess precisely the role and relative advantages and disadvantages of each technique.
Abbreviations
5-FU, 5-fluorouracil
CRF, case report forms
IOL, intraocular lens
IOP, intraocular pressure
POAG, primary open angle glaucoma
Competing interests: Professor Carlo E Traverso is also a clinical investigator for other surgical drainage glaucoma devices.
Informed consent: Approved by local ethics committees.
Declared interests: Carlo E Traverso, Audrey Messas-Kaplan, Philippe Denis, Shmuel Levartovsky, Eric Sellem, Zbigniew Zagorski, and Alain Bron have received funding from Optonol Ltd as clinical investigators for carrying out this study. Michael Belkin has proprietary interest in Optonol Ltd.
This work was presented as a poster at the Association for Research in Vision and Ophthalmology Annual Meeting 25–29 April 2004, Ft Lauderdale, FL, USA
REFERENCES
- 1.The Ocular Hypertension Treatment Study. A randomised trial determines that topical ocular hypotensive medication delays or prevents the onset of POAG. Arch Ophthalmol 2002;120:701–3. [DOI] [PubMed] [Google Scholar]
- 2.The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 8. Risk of cataract formation after trabeculectomy. Arch Ophthalmol 2001;119:1771–80. [DOI] [PubMed] [Google Scholar]
- 3.Lichter PR, Musch DC, Gillespie BW, et al. and the CIGTS Study Group. Interim clinical outcomes in the collaborative initial glaucoma treatment study comparing initial treatment randomized to medication or surgery. Ophthalmology 2001;108:1943–53. [DOI] [PubMed] [Google Scholar]
- 4.Cairns JE. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol 1968;66:673–9. [PubMed] [Google Scholar]
- 5.Rothman RF, Liebmann JM, Ritch R. Low-dose 5-fluorouracil trabeculectomy as initial surgery in uncomplicated glaucoma: long-term follow up. Ophthalmology 2000;107:1184–90. [DOI] [PubMed] [Google Scholar]
- 6.The Fluorouracil Filtering Surgery Study Group. Fluorouracil filtering surgery study one-year follow-up. Am J Ophthalmol 1989;108:625–35. [DOI] [PubMed] [Google Scholar]
- 7.Mermoud A, Schnyder CC, Sickenberg M, et al. Comparison of deep sclerectomy with collagen implant and trabeculectomy in open-angle glaucoma. J Cataract Refract Surg 1999;25:323–31. [DOI] [PubMed] [Google Scholar]
- 8.El-Sayyad F, Helal M, El-Kholify H, et al. Non penetrating deep sclerectomy versus trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology 2000;107:1671–4. [DOI] [PubMed] [Google Scholar]
- 9.Watson PG, Jakeman C, Ozturk M, et al. The complications of trabeculectomy (a 20 year follow-up). Eye 1990;4:425–38. [DOI] [PubMed] [Google Scholar]
- 10.Quaranta L, Hitchings RA, Quaranta CA. Ab-interno goniotrabeculectomy versus mitomycin C trabeculectomy for adult open-angle glaucoma. Ophthalmology 1999;106:1357–62. [DOI] [PubMed] [Google Scholar]
- 11.Higginbotham EJ, Stevens RK, Musch DC, et al. Bleb-related endophthalmitis after trabeculectomy with mitomycin C. Ophthalmology 1996;103:650–6. [DOI] [PubMed] [Google Scholar]
- 12.Wolner B, Liebmann JM, Sassani JW, et al. Late bleb-related endophthalmitis after trabeculectomy with adjunctive 5-fluorouracil. Ophthalmology 1991;98:1053–60. [DOI] [PubMed] [Google Scholar]
- 13.Khaw PT, Wells AP, Lim KS. Surgery for glaucoma in the 21st century. Br J Ophthalmol 2002;86:710–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molteno ACB. New implant for drainage in glaucoma. Animal trial. Br J Ophthalmol 1969;53:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krupin T, Podos SM, Becker B, et al. Valve implants in filtering surgery. Am J Ophthalmol 1976;81:232–5. [DOI] [PubMed] [Google Scholar]
- 16.Krupin T, Kaufman P, Mandell A, et al. Filtering valve implant surgery for eyes with neovascular glaucoma. Am J Ophthalmol 1980;89:338–43. [DOI] [PubMed] [Google Scholar]
- 17.Van de Veen G, Jongebloed WL, Worst JG. The gonioseton, a surgical treatment for chronic glaucoma. Doc Ophthalmol 1990;75:365–75. [DOI] [PubMed] [Google Scholar]
- 18.The European Glaucoma Society. Incisional surgery. In: Terminology and guidelines for glaucoma. 2nd ed. Savona, Italy: Dogma, The European Glaucoma Society 2003, Ch 3,:33–6.
- 19.Goldenfeld M, Krupin T, Ruderman JM, et al. 5-fluorouracil in initial trabeculectomy. A prospective, randomized, multicentre study. Ophthalmology 1994;101:1024–9. [DOI] [PubMed] [Google Scholar]
- 20.Mastropasqua L, Carpineto P, Ciancaglini M, et al. Delayed post-operative use of 5-fluorouracil as an adjunct in medically uncontrolled open angle glaucoma. Eye 1998;12:701–6. [DOI] [PubMed] [Google Scholar]
- 21.Jonescu-Cuypers C, Jacobi P, Krieglstein G. Primary viscocanalostomy in white patients with open-angle glaucoma: a randomized clinical trial. Ophthalmology 2001;108:254–8. [DOI] [PubMed] [Google Scholar]
- 22.Welsh NH, DeLange J, Wasserman P, et al. The “deroofing” of Schlemm’s canal in patients with open-angle glaucoma through placement of a collagen drainage device. Ophthalmic Surg Lasers 1998;29:216–26. [PubMed] [Google Scholar]
- 23.Wilson MR, Mendis U, Smith SD, et al. Ahmed glaucoma valve implant vs trabeculectomy in the surgical treatment of glaucoma: a randomized clinical trial. Am J Ophthalmol 2000;130:267–73. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd MA, Sedlak T, Heuer DK, et al. Clinical experience with the single-plate Molteno implant in complicated glaucomas. Update of a pilot study. Ophthalmology 1992;99:679–87. [DOI] [PubMed] [Google Scholar]
- 25.Valimaki J, Tuulonen A, Airaksinen PJ. Outcome of Molteno implantation surgery in refractory glaucoma and the effect of total and partial tube ligation on the success rate. Acta Ophthalmol Scand 1998;76:213–19. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel D, Kobuch K. Trabecular meshwork bypass tube shunt: initial case series. Br J Ophthalmol 2002;86:1228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luke C, Dietlein TS, Jacobi PC, et al. A prospective randomised trial of viscocanalostomy versus trabeculectomy in open-angle glaucoma: a 1-year follow-up study. J Glaucoma 2002;11:294–9. [DOI] [PubMed] [Google Scholar]
- 28.O’Brart DP, Rowlands E, Islan N, et al. A randomised, prospective study comparing trabeculectomy augmented with antimetabolites with a viscocanalostomy technique for the management of open angle glaucoma uncontrolled by medical therapy. Br J Ophthalmol 2002;86:748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sunaric-Megevand G, Leuenberger PM. Results of viscocanalostomy for primary open-angle glaucoma. Am J Ophthalmol 2001;132:221–8. [DOI] [PubMed] [Google Scholar]
- 30.Stegmann R, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg 1999;25:316–22. [DOI] [PubMed] [Google Scholar]
- 31.Ambresin A, Shaarawy T, Mermoud A. Deep sclerectomy with collagen implant in one eye compared with trabeculectomy in the other eye of the same patient. J Glaucoma 2002;11:214–20. [DOI] [PubMed] [Google Scholar]
- 32.El Sayyad F, Helal M, El-Kholify H, et al. Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology 2000;107:1671–4. [DOI] [PubMed] [Google Scholar]
- 33.Chiselita D. Non-penetrating deep sclerectomy versus trabeculectomy in primary open-angle glaucoma surgery. Eye 2001;15 (Pt 2) :197–201. [DOI] [PubMed] [Google Scholar]
- 34.Zarnowski T, Kawa P, Rakowska E, et al. Comparison of deep sclerectomy and miniature Ex-PRESS drainage device in open angle glaucoma. Ophthalmic Res 2001;33 (Suppl 1) :183. [Google Scholar]
- 35.Hitchings R. Efficacy of glaucoma treatment: the role of trabeculectomy. In: Jonsson B, Krieglstein G, eds. Primary open-angle glaucoma. Oxford: ISIS Medical Media, 1999:153–61.