Abstract
Aims: To evaluate the relation between refractive error and electrophysiological retinal abnormalities in children referred for investigation of reduced vision.
Methods: The study group comprised 123 consecutive patients referred over a 14 month period from the paediatric service of Moorfields Eye Hospital for electrophysiological investigation of reduced vision. Subjects were divided into five refractive categories according to their spectacle correction: high myopia (⩽−6D), low myopia (>−6D and ⩽−0.75D), emmetropia (>−0.75 and <1.5D), low hyperopia (⩾1.5 and <6D), and high hyperopia (⩾6D). Patients with a specific diagnosis at the time of electrophysiological testing were excluded. Only the first member of any one family was included if more than one sibling had been tested. All tests were performed to incorporate ISCEV standards, using gold foil corneal electrodes where possible. In younger patients skin electrodes and an abbreviated protocol were employed.
Results: The mean age of patients was 7.1 years with an overall incidence of abnormal electrophysiological findings of 29.3%. The incidence of abnormality was higher in high ametropes (13/25, 52%) compared to the other groups (23/98, 23.5%). This difference was statistically significant (χ2 test, p = 0.005). There was also a significant association between high astigmatism (>1.5D) and ERG abnormalities (18/35 with high astigmatism v 20/88 without, χ2 test, p = 0.002). There was no significant variation in frequency of abnormalities between low myopes, emmetropes, and low hyperopes. The rate of abnormalities was very similar in both high myopes (8/15) and high hyperopes (5/10).
Conclusions: High ametropia and astigmatism in children being investigated for poor vision are associated with a higher rate of retinal electrophysiological abnormalities. An increased rate of refractive errors in the presence of retinal pathology is consistent with the hypothesis that the retina is involved in the process of emmetropisation. Electrophysiological testing should be considered in cases of high ametropia in childhood to rule out associated retinal pathology.
Keywords: electrophysiology, myopia, hyperopia, retinal dystrophy
Ametropia has been noted to be a common finding in many retinal disorders.1 Hypermetropia is a well recognised feature of Leber’s congenital amaurosis,2 though not present in all pedigrees.3 More commonly, retinal dystrophies are associated with moderate to high myopic errors. Myopia is a consistent feature of several specific genetically defined dystrophies such as X linked congenital stationary night blindness,4 X linked retinitis pigmentosa,5 cone dystrophies6 and some cone-rod dystrophies.7 Ametropic errors have also been noted to be a common feature of congenital achromatopsia.8
A common feature of the retinal disorders associated with ametropia is that most are congenital or become symptomatic in childhood. High myopic errors presenting in childhood have recently been proposed as a potential marker for associated pathology, both ocular and non-ocular,9 which led to the recommendation that high myopia in childhood merits further clinical investigation. High hyperopia has recently been reported to have a similar incidence of associated ocular abnormalities as high myopia.10 In light of the noted association between retinal disorders and ametropia, this paper addresses the question of whether ametropia, both hyperopic and myopic, in the presence of reduced vision, represents a risk factor for abnormal retinal function.
This study was conducted at a major tertiary referral centre and involved an analysis of the electrophysiological, refractive, and clinical findings of all patients less than 18 years of age that had been referred for investigation of reduced vision by the paediatric ophthalmology service over a 14 month period.
METHODS
Subject selection
A retrospective review was conducted of all patients referred over a 14 month period (June 2000–July 2001) for electrophysiological testing by the paediatric and strabismus service of Moorfields Eye Hospital. Patients had to be ⩽18 years of age and referred for investigation of reduced vision of unknown cause or for the investigation of nystagmus. Specifically excluded were patients referred from other hospitals, or privately, because of potential problems accessing their full medical records. Patients with a known diagnosis at the time of electrophysiological testing or patients involved in specific research projects were also excluded to remove potential biases. Only the first member of any family was included if more than one sibling had been tested as determined by surname and/or address.
Patient demographics
A total of 138 met the above criteria for inclusion. Of these, 123 (89%) had satisfactory records of refraction at the time of referral and were included in the full refractive analysis presented below. Results are presented for the right eye of each patient with comparisons given for the left eye where appropriate.
Information sources
The medical charts were examined to determine the visual acuity and refraction data at the time of referral. Additional diagnostic information and clinical observations were obtained from the charts including any subsequent diagnostic information or follow on tests. The reason for referral was determined from the medical charts and the referral form or letter sent to the electrophysiology department. Notes taken at the time of electrophysiological testing were also examined to determine any difficulties performing the test or additional findings such as nystagmus that had not previously been noted. The electrophysiological findings were taken from the authorised report provided by two highly experienced clinical electrophysiologists (AGR, GEH).
Refractive status
Subjects were divided into five refractive categories according to the spherical equivalent of their full spectacle correction: high myopia (⩽−6D), low myopia (>−6D and ⩽−0.75D), emmetropia (>−0.75 and <1.5D), low hyperopia (⩾1.5 and < 6D), and high hyperopia (⩾6D). Refraction was determined from the objective retinoscopic findings under cycloplegia subtracting a 1.5D working distance correction or by the subjective refraction.
Electrophysiological testing
All tests were performed to incorporate ISCEV standards when possible, using gold foil corneal electrodes and mydriasis. In very young patients periorbital surface electrodes, with the active electrode positioned along the lower eyelid, and an abbreviated protocol based on the ISCEV Standard were employed. This first involved recording ERGs to single flash and 30 Hz flicker stimulation under photopic conditions in order to assess generalised cone system function. Children were then dark adapted for a minimum of 5 minutes, and single bright stimuli used to elicit ERGs. Such responses allow assessment of scotopic retinal function relating to photoreceptor and inner retinal layers and are dominated by the rod system. Several repetitions of each response were routinely obtained in order to demonstrate reproducibility. If patient compliance was still satisfactory, further dark adaptation was performed.
Pattern electroretinograms (PERGs) were recorded to high contrast checkerboard reversal in order to assess central retinal function. Multichannel cortical visual evoked potentials (VEPs) were recorded monocularly with occipital scalp electrodes using high contrast checkerboard reversal and/or diffuse flash stimulation, allowing assessment of the integrity and function of the retinocortical visual pathways. Fixation was continuously monitored in all patients undergoing pattern testing.
RESULTS
Age and refraction
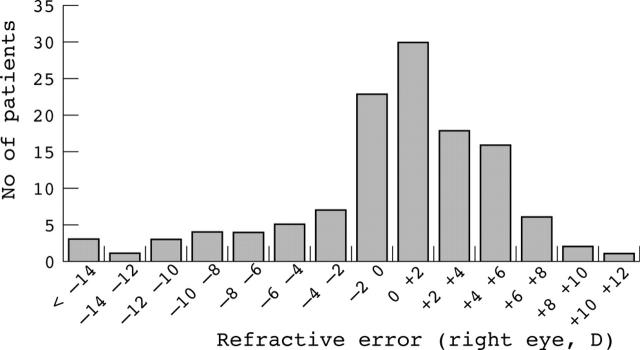
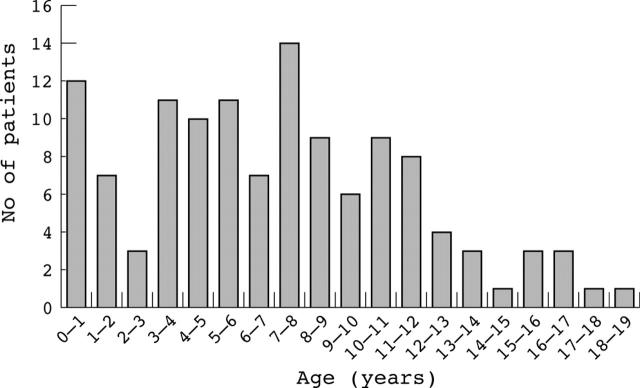
The mean age was 7.1 years (median 7.1 years, SD 4.4 years). The mean refractive error of the right eye was +0.23D with a standard deviation of 5.1D, indicative of the wide spread of refractions. There was no significant difference between the right and left eyes in terms of spherical equivalent refraction with the left eyes showing a mean refraction of +0.45D (SD 4.6D, paired t test, p = 0.15). The distributions of spherical equivalent refractive error and ages are shown in figures 1 and 2. There were 74 males and 49 females in this sample. There was no significant difference in spherical equivalent refraction between males (mean +0.48D, SD 5.1) and females (mean −0.15D, SD 5.1) (unpaired t test, p = 0.50).
Figure 1.
Histogram showing the distribution of refractive errors in the tested subjects.
Figure 2.
Histogram showing the distribution of ages of subjects tested (n = 123).
Frequency and nature of flash ERG abnormalities
Abnormal flash ERG findings were present in 29.3% (36/123) of subjects. The nature of the abnormality was classified as undetectable/minimal response in at least one test condition in nine patients. Thirteen patients showed abnormal b-wave to a-wave amplitude ratios or abnormal on-off pathway responses to long duration stimulation indicative of post-receptoral deficits. Nine patients showed primarily amplitude reductions in one or more tests and five patients had an abnormality primarily in response timing. There was no clear pattern of rod or cone defects as a function of refraction with the majority of subjects showing defects in both pathways. Inner retinal dysfunction appeared more common in myopes than in hyperopes. The variation in electrophysiological abnormality are summarised in table 1.
Table 1.
Electrophysiological abnormality in each refractive group
| Isolated rod | Isolated cone | Combined rodand cone | Inner retinal dysfunction | |
| High myopia | 1 | 1 | 6 | 4 |
| Low myopia | 0 | 1 | 5 | 3 |
| Emmetropia | 0 | 3 | 2 | 2 |
| Low hyperopia | 2 | 2 | 6 | 3 |
| High hyperopia | 0 | 1 | 3 | 1 |
In addition to the electrophysiological diagnoses, a specific final clinical diagnosis of retinal dysfunction was recorded in the clinical notes at the most recent follow up in 11 cases. These comprised two cone dystrophies, four cone-rod dystrophies, one case of Batten’s disease, one case of congential stationary night blindness, two rod monochromats, and one case of suspected oligocone syndrome.
Flash ERG and spherical equivalent refraction
An increased proportion of abnormal ERG results were obtained in patients with high ametropia (>6D of spherical equivalent error). In high ametropes 52% (13/25) showed an abnormal ERG compared to 25.5% (25/98) among emmetropes and low ametropes. This difference was statistically significant (χ2, p = 0.005). The odds ratio for retinal abnormalities in high ametropes compared to low ametropes and emmetropes was 3.53 (95% CI: 1.42 to 8.80). The left eye results did not significantly differ from those of the right eye, with a significant excess of abnormal ERGs in high ametropes (χ2, p = 0.0003).
There was no significant variation in frequency of abnormalities between low myopes, emmetropes, and low hyperopes, but low myopes had a higher proportion of abnormal ERG findings (31.6%, 6/19) than the emmetropes (20%, 7/35) and low hyperopes (22.7%, 10/44). The rate of abnormalities was similar in both high myopes (8/15) and high hyperopes (5/10). These findings are summarised in table 2.
Table 2.
ERG findings by refractive group
| Refraction | High myopia | Low myopia | Emmetropia | Low hyperopia | High hyperopia |
| ⩽−6D | >−6 to ⩽−0.75D | >−0.75 to −<1.5D | >1.5D to <6D | ⩾+ 6D | |
| ERG findings | |||||
| Normal | 7 | 13 | 28 | 34 | 5 |
| Abnormal | 8 | 6 | 7 | 10 | 5 |
| Total | 15 | 19 | 35 | 44 | 10 |
| % abnormal ERG findings | 53.33 | 31.58 | 20.00 | 22.73 | 50.00 |
Flash ERG and sex
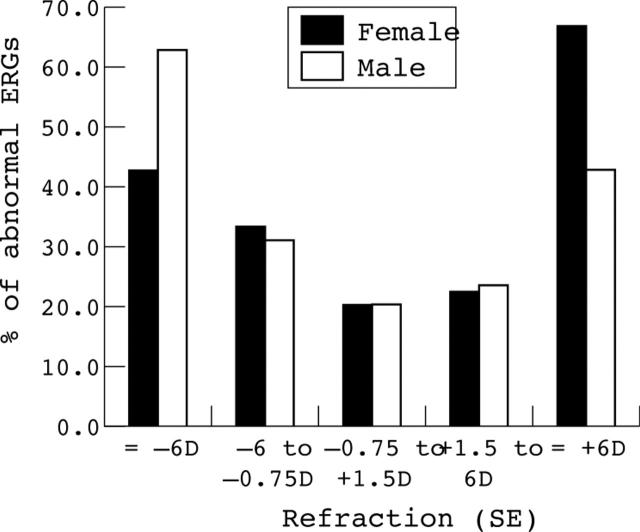
The proportion of abnormal ERG results was almost identical for males (28.6% n = 74) and females (29.7% n = 49). There was a slightly greater proportion of abnormal ERGs in males in the highly myopic group and a higher proportion of abnormal ERGs in females in the highly hyperopic group (see table 3). The proportion of abnormal ERGs in low myopes, emmetropes, and low hyperopes was almost identical in males and females. The distribution of abnormal ERG findings by refraction and sex is shown in figure 3.
Table 3.
Abnormal ERG findings by refractive group and sex
| Refraction | High myopia | Low myopia | Emmetropia | Low hyperopia | High hyperopia |
| ⩽−6D | >−6 to ⩽−0.75D | >−0.75 to <1.5D | >1.5D to < 6D | ⩾+ 6D | |
| Abnormal ERG findings | |||||
| Male | 5 | 4 | 4 | 6 | 3 |
| Female | 3 | 2 | 3 | 4 | 2 |
| Total | 8 | 6 | 7 | 10 | 5 |
| % Abnormal | |||||
| Male | 62.5 | 30.8 | 20.0 | 23.1 | 42.9 |
| Female | 42.9 | 33.3 | 20.0 | 22.2 | 66.7 |
Figure 3.
Variation of percentage of patients with an abnormal ERG by sex and refraction.
Flash ERG and astigmatism
Abnormal flash ERGs were also more common in eyes with high astigmatism. For right eyes with ⩾1.5D of astigmatism 51% (18/35) of eyes had abnormal flash ERGs compared with 23% (20/88) of eyes with <1.5D of astigmatism (χ2, p = 0.002; odds ratio 3.6, 95% CI: 1.57 to 8.25). A significant association was also present for the left eye (χ2, p = 0.016).
Pattern ERG
Abnormalities of the pattern ERG in the presence of a normal flash ERG, indicative of retinal dysfunction limited to the macula, were obtained in five patients (three high myopes, one emmetrope, and one low hyperope). Combining flash and pattern ERG abnormalities accentuated the observed increase in abnormal electrophysiological findings among ametropic eyes leading to a highly significant difference between high ametropia and low/normal refractive errors (χ2, p = 0.0006).
Flash and pattern VEP
Compared to the very high interocular concordance noted for flash ERG with only two patients having contrasting findings in the two eyes, a much greater interocular asymmetry was noted for VEP findings with 12 patients having unilateral VEP abnormalities. A total of 30 patients had abnormal cortical evoked potentials with normal ERG and pattern ERG, indicative of post-retinal dysfunction in at least one eye. There was no excess of abnormal responses in high ametropes in either eye. In the right eye only three subjects in this group showed isolated VEP abnormalities compared to 22 within the emmetropic and low ametropic groups. In the left eye only one high ametrope showed an isolated VEP abnormality compared to 20 within the emmetropic and low ametropic groups.
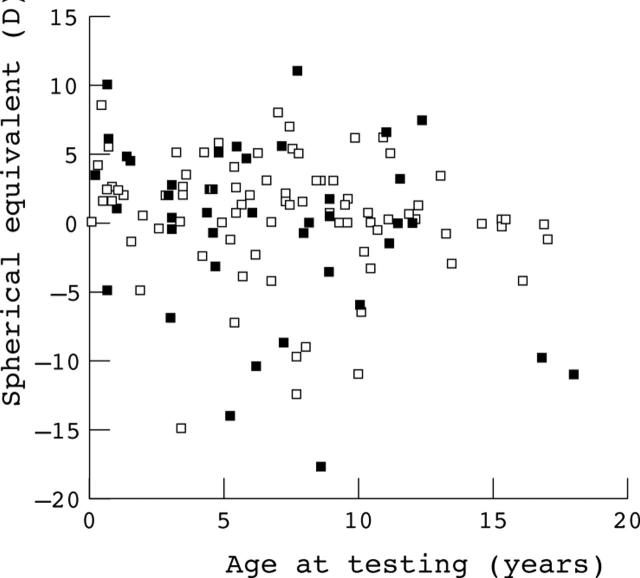
Age
There was no significant association between refractive error and age among subjects with normal ERG findings (r2 = 0.090) or among subjects with abnormal ERG findings (r2 = 0.023). Figure 4 shows a scatter plot of spherical equivalent refraction v age for subjects with normal ERG findings (open symbols) and abnormal ERG findings (closed symbols). Analysis of low v high myopes with ERG abnormalities did reveal that low myopes were younger than high myopes (mean age 6.4 years v 9.9 years) but this was not significant (two tailed t test p = 0.21). This may suggest that over time some of the low myopes with electrophysiological abnormalities may progress to higher levels of myopia but longitudinal studies will be required to address this question.
Figure 4.
Scatter plot showing the spherical equivalent refractive error and age for subjects with normal electroretinogram findings (open symbols) and for subjects with abnormal electroretinogram findings (closed symbols).
Nystagmus
Nystagmus of any type (including latent) was noted in 35% of subjects (43/123). A higher proportion of patients with nystagmus had ERG abnormalities (40%, 17/43) compared to patients without nystagmus (26%, 21/80) but this difference was not statistically significant (χ2, p = 0.13). There were no differences in the frequency of nystagmus in high ametropia (36%, 9/25) compared to low ametropes and emmetropes (34.7%, 34/98). A slightly higher proportion of all hyperopes (42.6%, 23/54) had nystagmus compared to all myopes (32%, 11/34) but this difference was not statistically significant (χ2, p = 0.34).
No relation was seen between the presence or absence of nystagmus and the level of astigmatism. The mean cylinder magnitude in patients with nystagmus was 1.01D compared to 1.07D in patients without nystagmus (t test, p = 0.77). A slightly lower proportion of patients with higher levels of astigmatism (⩾1.5D) were noted to have nystagmus (23%) compared to those with lower levels of astigmatism (<1.5D) where the proportion of nystagmus was 30.1%, but this difference was not statistically significant (χ2 test, p = 0.37).
DISCUSSION
Patients with high spherical equivalent refractive errors, both hyperopic and myopic, and astigmatism, were associated with a significantly higher rate of retinal dysfunction than patients with lower refractive errors. This finding has implications for both clinical practice and also in relation to our understanding of the regulation of eye growth and refractive development.
In relation to clinical practice, high refractive errors in association with reduced vision may be wrongly diagnosed as ametropic amblyopia in the absence of electrophysiological testing. Misdiagnosis or delayed diagnosis of a genetically determined, potentially progressive disorder has implications both in terms of genetic counselling for future pregnancies and also in terms of educational planning. High refractive errors should increase clinical suspicion of retinal disorders.
In light of the fact that several of the well defined retinal disorders linked to refractive errors display X linked inheritance, an excess of males might have been expected in the high ametropia group that displayed retinal abnormalities. Although there was an excess of males overall, no significant sex differences were found between high ametropia and lower refractive errors. There was an excess of males with high myopia and retinal abnormalities compared to females but subdividing groups by sex, refraction, and ERG findings led to small subgroups precluding further analysis.
The question arises as to whether the changes observed in high ametropes represent true retinal dysfunction or simply reflect the altered physiology of highly ametropic eyes. A recent study has defined the normal variations of electrophysiological findings in myopes without maculopathy.11 This study revealed no changes in a-wave or b-wave implicit times or b-wave to a-wave ratio, but did show a small reduction of a-wave and b-wave amplitudes with increased axial length. Therefore, although potentially present, ERG changes in myopia are relatively subtle and do not approach the level of abnormality present in this cohort of patients. A study of predominantly hyperopic ametropes12 showed some hyperopes with b-wave to a-wave ratio abnormalities (both increased and decreased b-a ratios) that did not correlate with axial length or degree of hyperopia. In the current study a greater proportion of inner retinal changes were observed in myopes and only a single high hyperope had a subnormal b-wave to a-wave ratio compared to four low hyperopes. Overall, the data suggest that the observed changes in both high myopes and high hyperopes, compared to low ametropes, represent abnormal retinal function rather than the degree of ametropia.
During the past 15 years there have been significant advances in our understanding of the regulation of eye growth and refractive development in animal models.13 There is now a large body of evidence that retinal image quality affects eye growth and hence refraction. Furthermore, the link between the retinal image and eye growth seems to result from interactions between the retina, choroid and sclera that do not involve central visual pathways. Studies with tetrodotoxin, a neurotoxin which abolishes action potentials, in both birds and mammals reveal that experimental image deprivation myopia and subsequent recovery is mediated by retinal cells that generate graded potentials.14,15 Pharmacological studies of experimental myopia have also implicated amacrine cells on the basis of localisation of VIP (vasoactive intestinal peptide) and dopamine to this complex class of cells.16,17 In keeping with these animal studies, the association of refractive errors and abnormal electrophysiological findings in this study was only noted in terms of retinal dysfunction; optic nerve dysfunction, as suggested by pattern or flash VEP, showed no such association with higher ametropia.
In normal human refractive development both spherical and astigmatic refractive errors decrease during early childhood, a process termed emmetropisation.18 The observed association of ametropia and astigmatism with retinal dysfunction in this study is consistent with hypothesis that the retina is involved in human emmetropisation, in that abnormal retinal function may be expected to disrupt this process.19 With the recent advances in the understanding of the genetic basis of retinal dystrophies, it is possible that future clinical studies may be able to provide valuable information into how the retina modulates eye growth and refraction. Detailed electrophysiological studies of genetically defined and homogeneous retinal disorders comparing those linked with refractive abnormalities to those without refractive abnormalities may shed light on the role of the retina in human refractive development.
CONCLUSIONS
High ametropia (spherical equivalent ⩾+6D or ⩽−6D) and >1.5D of astigmatism in children being investigated for poor vision is associated with a significantly higher rate of retinal electrophysiological abnormalities compared to lower refractive errors and emmetropia. Electrophysiological testing should be considered in cases of high ametropia in childhood to rule out associated retinal pathology. An increased frequency of refractive errors in the presence of retinal pathology is consistent with the hypothesis that the retina is involved in the regulation of eye growth and emmetropisation.
Abbreviations
PERG, pattern electroretinogram
VEP, visual evoked potential
REFERENCES
- 1.Laties AM, Stone RA. Ametropia in retinal disorders. Ch 35. In: Anderson RE, Hollyfield JG, LaVail MM, eds. Retinal degenerations Boca Raton, CRC Press 1991:383–90.
- 2.Fulton AB, Hansen RM, Mayer DL. Vision in Leber congenital amaurosis. Arch Ophthalmol 1996;114:698–703. [DOI] [PubMed] [Google Scholar]
- 3.Hirashima S, Ohba N. A pedigree of Leber’s congenital amaurosis. Ophthalmic Paediatr Genet 1988;9:29–36. [DOI] [PubMed] [Google Scholar]
- 4.Zito I, Allen LE, Patel RJ, et al. Mutations in the CACNA1F and NYX genes in British CSNBX families. Hum Mutat 2003;21:169. [DOI] [PubMed] [Google Scholar]
- 5.Flaxel CJ, Jay M, Thiselton DL, et al. Difference between RP2 and RP3 phenotypes in X linked retinitis pigmentosa. Br J Ophthalmol 1999;83:1144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadowski B, Zrenner E. Cone and rod function in cone degenerations. Vis Res 1997;37:2303–14. [DOI] [PubMed] [Google Scholar]
- 7.Gregory-Evans K, Kelsell RE, Gregory-Evans CY, et al. Autosomal dominant cone-rod retinal dystrophy (CORD6) from heterozygous mutation of GUCY2D, which encodes retinal guanylate cyclase. Ophthalmology 2000;107:55–61. [DOI] [PubMed] [Google Scholar]
- 8.Haegerstrom-Portnoy G, Schneck ME, Verdon WA, et al. Clinical vision characteristics of the congenital achromatopsias. I. Visual acuity, refractive error, and binocular status. Optom Vis Sci 1996;73:446–56. [DOI] [PubMed] [Google Scholar]
- 9.Marr JE, Halliwell-Ewen J, Fisher B, et al. Associations of high myopia in childhood. Eye 2001;15:70–4. [DOI] [PubMed] [Google Scholar]
- 10.Marr JE, Harvey R, Ainsworth JR. Associations of high hypermetropia in childhood. Eye 2003;17:436–7. [DOI] [PubMed] [Google Scholar]
- 11.Westall CA, Dhaliwal HS, Panton CM, et al. Values of electroretinogram responses according to axial length. Doc Ophthalmol 2001;102:115–30. [DOI] [PubMed] [Google Scholar]
- 12.Perlman I, Meyer E, Haim T, et al. Retinal function in high refractive error assessed electroretinographically. Br J Ophthalmol 1984;68:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troilo D. Neonatal eye growth and emmetropisation—a literature review. Eye 1992;6 (Pt 2) :154–60. [DOI] [PubMed] [Google Scholar]
- 14.Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis Neurosci 1994;11:143–53. [DOI] [PubMed] [Google Scholar]
- 15.McBrien NA, Moghaddam HO, Cottriall CL, et al. The effects of blockade of retinal cell action potentials on ocular growth, emmetropization and form deprivation myopia in young chicks. Vis Res 1995;35:1141–52. [DOI] [PubMed] [Google Scholar]
- 16.Stone RA, Laties AM, Raviola E, et al. Increase in retinal vasoactive intestinal polypeptide after eyelid fusion in primates. Proc Natl Acad Sci USA 1988;85:257–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XX, Schaeffel F, Kohler K, et al. Dose-dependent effects of 6-hydroxy dopamine on deprivation myopia, electroretinograms, and dopaminergic amacrine cells in chickens. Vis Neurosci 1992;9:483–92. [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich DL, Braddick OJ, Atkinson J, et al. Infant emmetropization: longitudinal changes in refraction components from nine to twenty months of age. Optom Vis Sci 1997;74:822–43. [DOI] [PubMed] [Google Scholar]
- 19.Goss DA, Wickham MG. Retinal-image mediated ocular growth as a mechanism for juvenile onset myopia and for emmetropization. A literature review. Doc Ophthalmol 1995;90:341–75. [DOI] [PubMed] [Google Scholar]