We have followed with interest the discussion ignited by the paper by Reinhard et al1 by way of editorial comments from Horton2 and Plant.3 As co-authors of the paper by Reinhard et al1 and collaborators on that study, we have no objections to the data as presented. However, Horton’s interpretation that these data indicate that “no therapeutic intervention … can correct effectively the underlying visual field deficit” after post-chiasmatic injury is not supported by current scientific evidence. On the contrary, a comprehensive and critical review of the literature reveals that there is a sound scientific basis for recommending vision restoration therapy (VRT) for some patients with hemianopia.
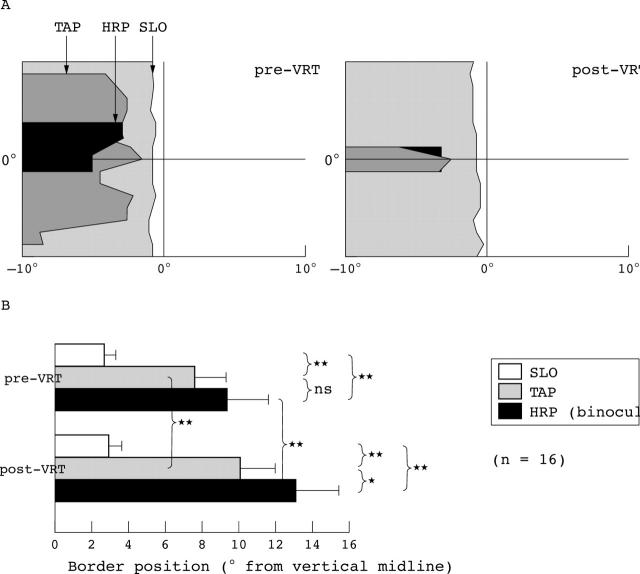
The Reinhard study1 used scanning laser ophthalmoscopy (SLO) to evaluate visual fields before and after a 6 month course of VRT and found no change in the size of the blind field detected by this methodology. An important point well taken by Horton is that rather than relying on the VRT computer based tests alone, it would be “more compelling if visual field improvements could be demonstrated with any standard clinical perimeter.” Although not reported in the Reinhard article, the same patients were also tested by two other perimetric methods: the Tübingen automated perimeter (TAP) and high resolution perimetry (HRP, which is a campimetric visual field test).4 We acknowledge that Horton did not have access to this important information which was in press at the time. We believe that not considering these other perimetric data could lead to incorrect conclusions. Even before VRT began, the SLO border was already located significantly closer to the vertical midline than the absolute TAP and HRP borders (fig 1). After VRT, the SLO border was unchanged, but the absolute TAP and HRP borders had significantly shifted, confirming improvement on these measures.4 Similar enlargement of the visual field after therapy has been demonstrated on “standard clinical perimetry” by various investigators and laboratories.5–9
Figure 1.
This graph (adapted from Sabel et al4) displays the visual field border position in the right eye as assessed by the three perimetric tests. (A) The results of patient CH where grey areas represent the area of the defect. A mismatch in perimetric fields was noted even before therapy. After VRT, the HRP and TAP border shifted away from the vertical meridian whereas the SLO border remained roughly in the same position, exaggerating the border mismatch. (B) Shows the absolute visual field border for SLO, TAP, and HRP in the central 10° region in degrees of visual angle from the 0 vertical meridian before and after VRT (mean (SEM)). Whereas the SLO border was almost identical pre-VRT compared with post-VRT, the HRP and TAP borders were not only significantly different before VRT (mismatch), but also both shifted significantly after VRT, producing a visual field enlargement.4
This apparent discrepancy between the conventional perimetric data and the SLO findings, both at baseline and after therapy, probably reflects the comparatively greater task difficulty of the SLO. It is well known that perimetric performance is task dependent, and the size of the visual field depends critically on stimulus characteristics. In the joint study of the Tübingen-Magdeburg groups1,4 a single near threshold (TAP) or superthreshold bright dot (HRP) was presented on a dark or grey background and the patients had to respond to single stimuli by pressing a button. Contrast these techniques with SLO in which three black dots (a reverse stimulus) were presented on a bright red background which perceptually flickers because it is created by parallel laser lines (the “McKay effect”, see Sabel et al4). Furthermore, patients had to verbally (that is, consciously) report what they were seeing while the experimenter interpreted their verbal reports. Simultaneous stimulus discrimination and detection of negative stimuli on a bright background are probably tasks beyond the abilities of a damaged visual system. It makes a dramatic difference what kind of psychophysical task is being used during perimetric testing and the results have to be interpreted in this context. Plant himself makes a very insightful comment when he says that “it remains possible that improvements may have been in the nature of relative defects which would have not been detected by the method employed in this study to detect absolute defects.”3 This is indeed confirmed by the data analysis of the SLO study patients4: when “relative defects” in TAP and HRP were calculated, the “relative” border was found to be roughly identical to the SLO border. The SLO method appears to be insensitive to relative defects describing areas with residual function as being absolutely “blind.”
Both commentators2,3 erroneously assume that just because the SLO study showed no visual field expansion, eye movement artefacts must have caused the VRT effects on other forms of perimetry. However, the task difficulty and the superior fixation control of the SLO are independent variables. The SLO study leaves unanswered the question if or to what extent eye movements contribute to the VRT effects. Fortunately, there are several other parameters measured in the same patients which help clarify this issue. First of all, most of the patients showed excellent fixation on the SLO, even after VRT, and none of the patients showed stable eccentric fixation on SLO.1 Secondly, both TAP fixation performance and HRP fixation performance were unchanged after VRT, and both used standard, clinically verified fixation control measures.4 Additionally, Trauzettel-Klosinski and Reinhard,10 two of the authors on the study in question, have previously stated that lack of a shift in the blind spot position is a good indicator that fixation is not eccentric. In 12 out of the 16 patients in the SLO study, the blind spot position remained identical after VRT. Among the only four patients who showed a small shift of the blind spot on SLO, none profited from VRT on the other forms of perimetry either. Finally, if eye movements were the cause of visual field expansion, one would expect the entire visual field border to shift. In most patients this is not what is seen. A dramatic example of this is the recently reported selective border shift only within the region of an attention cue.8 Or take the patient shown in figure 1, in which the visual field defect shrank by shifting of the horizontal border without affecting the vertical border, and the deficit next to the fixation spot was unchanged. If eye movement artefacts had occurred, the reverse would be expected: a shift of the vertical border and no change in the horizontal border. Such border dynamics are incompatible with eye movement artefacts.
Horton is concerned that VRT improvements may simply be a result of placebo effects. However, the study by Kasten et al6 described two independent clinical trials in which the placebo effect was controlled for by a randomised, placebo controlled trial and showed that the placebo treatment had no effect in the post-chiasmatic group and only a small effect in the optic nerve group. In this study6 and in others,5,7 patients also reported subjective benefits after VRT, including improved visual function in reading, navigation, and confidence. We agree it is essential to further investigate VRT effects on standardised functional measures of visual performance on everyday life tasks in addition to just perimetry.
There is increasing evidence supported by controlled clinical trials and functional neuroimaging that neuroplasticity is active in many regions of the brain. Training paradigms are now standard in the field of rehabilitation medicine. They are not limited to locomotion therapy, but well established in other functional domains as well (for example, cognitive therapy, memory therapy, speech therapy, auditory therapy, etc). There is no reason why the visual system should be the great exception from all other functional systems of the brain. After all, normal adult subjects are capable of perceptual learning,11 and there is an entire body of evidence on activity dependent use and neuroplasticity, such as studies on adult receptive field expansions following retinal or brain lesions.12–15 We also should remember that the visual system is not purely “sensory.” It utilises many cognitive mechanisms as seen, for example, in the phenomenon of physiological blind spot “filling in” and in the many other mechanisms that contribute to visual perception such as lateral interactions and contour integration.11
The precise mechanisms of visual neuroplasticity in the human are not yet defined. Horton believes that in patients with complete hemianopia there is “no fringe of injured but salvageable tissue.” This assumption may be true in some patients, but most patients actually have incomplete hemianopia where residual neurons survive within or near the damaged zones (“relative defects”). Even patients with “complete” V1 damage have some preserved visual functions. For example, patients can show non-conscious visual responses (blind sight) which are mediated either by surviving primary cortical afferents16 and islands of residual vision17 or by undamaged projections via the colliculus and pulvinar.18,19 This latter pathway has most recently been discovered to relay attention relevant information to the eye movement control system20 and attentional networks are now known to contribute to VRT induced recovery.8 There is yet another pathway bypassing V1 altogether, as elegantly described by Hortońs group21: a direct projection from lateral geniculate neurons to the motion sensitive area MT (V5). Thus, there are apparently multiple pathways whereby visual information can reach higher cortical regions without involving V1. Whether or not such pathways have a role in VRT induced visual field enlargements is currently not known, but the search for neurobiological mechanisms of vision restoration deserves further study.
Sensational support of or enthusiastic opposition to a viable technique can only be justified after a meticulous analysis of the complete data in order to enhance scientific discourse. It is true that VRT does not assist all patients. Predictors of recovery have not been completely defined, except that the size of the relative defect tends to correlate with recovery. VRT has now been applied in over 700 patients with confirmation of its effectiveness from several independent studies and laboratories. The FDA has cleared VRT to be offered in the United States and has done so in recognition of the results from the Tübingen-Magdeburg trial. Several clinical centres throughout the United States are now beginning to observe similar improvements with their first patients, confirming the approach to be helpful to patients. Clearly, the relation of objective and subjective visual function after VRT needs further clarification and the role of eye movement compensation in individual hemianopic patients is of interest. However, many hemianopic patients, especially those with partial deficits, benefit from VRT. The evidence supports the conclusion that some visual improvement is possible.
REFERENCES
- 1.Reinhard J, Schreiber A, Schiefer U, et al. Does visual restitution training change absolute homonymous visual field defect? A fundus controlled study. Br J Ophthalmol 2005;89:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horton JC. Disappointing results from NovaVision’s visual restoration therapy. Br J Ophthalmol 2005;89:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plant GT. A workout for hemianopia. Br J Ophthalmol 2005;89:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabel BA, Kenkel S, Kasten E. Vision restoration therapy (VRT) efficacy as assessed by comparative perimetric analysis and subjective questionnaires. Restor Neurol Neurosci 2004;22:399–420. [PubMed] [Google Scholar]
- 5.Julkunen L, Tenovuo O, Jääskeläinen S, et al. Rehabilitation of chronic post-stroke visual field defect with computer-assisted training. Restor Neurol Neurosci 2003;21:19–28. [PubMed] [Google Scholar]
- 6.Kasten E, Wüst S, Behrens-Baumann W, et al. Computer-based training for the treatment of partial blindness. Nat Med 1998;4:1083–7. [DOI] [PubMed] [Google Scholar]
- 7.Mueller I, Poggel DA, Kenkel S, et al. Vision restoration therapy (VRT) after brain damage: subjective improvements of activities of daily life and their relationship to visual field enlargements. Visual Impairm Res 2003;5:157–78. [Google Scholar]
- 8.Poggel DA, Kasten E, Sabel BA. Attentional cueing improves vision restoration therapy in patients with visual field loss. Neurology 2004;63:2069–76. [DOI] [PubMed] [Google Scholar]
- 9.Zihl J, von Cramon D. Visual field recovery from scotoma in patients with postgeniculate damage. A review of 55 cases. Brain 1985;108:335–65. [DOI] [PubMed] [Google Scholar]
- 10.Trauzettel-Klosinski S, Reinhard J. The vertical field border in hemianopia and its significance for fixation and reading. Invest Ophthalmol Vis Sci 1998;39:2177–86. [PubMed] [Google Scholar]
- 11.Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron 2001;31:681–97. [DOI] [PubMed] [Google Scholar]
- 12.Eysel T, Gonzalez-Aguilar F, Mayer U. A functional sign of reorganization in the visual system of adult cats: lateral geniculate neurons with displaced receptive fields after lesions of the nasal retina. Brain Res 1980;181:285–300. [DOI] [PubMed] [Google Scholar]
- 13.Chino YM, Smith EG 3rd, Kaas JH, et al. Receptive-field properties of deafferentated visual cortical neurons after topographic map reorganization in adult cats. J Neurosci 1995;15:2417–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature 1992;356:150–2. [DOI] [PubMed] [Google Scholar]
- 15.Kaas JH, Krubitzer LA, Chino YM, et al. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science 1990;248:229–31. [DOI] [PubMed] [Google Scholar]
- 16.Wüst S, Kasten E, Sabel BA. Blindsight after optic nerve injury indicates functionality of spared fibers. J Cogn Neurosci 2002;14:243–53. [DOI] [PubMed] [Google Scholar]
- 17.Fendrich R, Wessinger CM Gazzaniga MS. Residual vision in a scotoma: implications for blindsight. Science 1992;258:27. [DOI] [PubMed] [Google Scholar]
- 18.Pöppel E, Held R, Frost D. Residual visual functions after brain wounds involving the central visual pathways in man. Nature 1973;243:295–6. [DOI] [PubMed] [Google Scholar]
- 19.Weiskrantz L, Warrington EK, Sanders MD, et al. Visual capacity in the hemianopic field following a restricted occipital ablation. Brain 1974;97:709–28. [DOI] [PubMed] [Google Scholar]
- 20.Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci 2004;24:11236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sincich LC, Park KF, Wohlgemuth MJ, et al. Bypassing V1: a direct geniculate input to area MT. Nat Neurosci 2004;7:1123–8. [DOI] [PubMed] [Google Scholar]