Abstract
Background/aim: Thyroid associated orbitopathy (TAO) and Graves’ disease (GD) have an autoimmune pathogenesis, possibly related to the thyrotropin receptor (TSHR). The aim of this study was to determine whether TSHR immunoreactivity is correlated with disease severity or serum TSHR antibody (TRAB) levels.
Methods: Orbital tissues from 30 patients with TAO were compared with those of 20 patients with strabismus and four with non-thyroid orbital inflammation. TSHR was detected by immunohistochemistry and TRAB were measured by radioreceptor assay.
Results: No TSHR immunoreactivity was detected in the 24 control orbital tissues, whereas in all TAO biopsies elongated fibroblast-like cells, expressing TSHR, were present. These cells were located between the muscle cells, which were separated by oedema in the acute phase but fibrous tissue in the chronic phase of disease. Semi-thin sections showed numerous mast cells present in the chronic phase and in close contact with adipocytes. The number of TSHR immunostained cells was high in early disease, decreased with disease duration, and was positively correlated with TRAB levels at the onset of TAO.
Conclusion: TSHR immunoreactivity was demonstrated specifically in TAO orbits which highlights the importance of TRAB early in the pathogenesis.
Keywords: thyrotropin receptor, extraocular muscles, thyroid associated orbitopathy
The pathogenic mechanism of thyroid associated orbitopathy (TAO) is still unknown, although several considerations suggest that it is autoimmune. Firstly, TAO occurs almost exclusively in patients with autoimmune thyroid disease. Secondly, there is an association between TAO and serum anti-thyrotropin receptor autoantibodies (TRAB). Thirdly, there is lymphocytic infiltration in orbits of TAO patients, with cytokine production.
The hypothesis is that antigens shared by the thyroid and the orbital tissues are the targets of a cellular autoimmune reaction.1,2 The orbital autoantigen has not been conclusively identified, but a logical candidate is the thyrotropin receptor (TSHR), since TAO is frequently associated with Graves’ disease (GD) and indeed with patients having highest titres of TRAB.3 TSHR transcripts were demonstrated by northern blot in orbital adipose tissue from a patient with TAO, transcripts in normal adipose tissue being at the limit of the detection.4 RT-PCR (real time-polymerase chain reaction) technique also showed TSHR mRNA transcripts in thyroid and extraocular muscles (EOMs), but not in abdominal fat, cardiac muscle, kidney, or brain.5,6
Immunohistochemical analysis using two monoclonal antibodies (Ab) against TSHR has shown that the TSHR transcripts are translated into protein. TSHR immunoreactivity has been reported in the EOMs of patients with TAO,7,8 but a possible correlation with circulatory TRAB or with the severity and duration of TAO has not been examined. Moreover, very few histological studies of TAO have been done.
The purpose of our study was to investigate whether TSHR immunoreactivity found in EOMs of patients with TAO is (1) specific to TAO, (2) quantifiable and possibly related to the inflammatory stage and degree of severity of TAO, (3) correlated to the serum level of TRAB.
PATIENTS AND METHODS
Patients (table 1)
Table 1.
Characteristics of patients with TAO
| Number of patients: n = 30 | |
| Mean (range) age: 55.9 years (28–85) | |
| Sex: female: n = 22, male n = 8 | |
| Smokers: n = 19 | |
| Mean (range) TAO duration: 25.7 months (6–96) | |
| TAO severity: moderate n = 16, severe n = 14 | |
| CAS (0–7): | |
| 0 | n = 23 |
| 1 | n = 2 |
| 2 | n = 1 |
| 3 | n = 1 |
| 4 | n = 0 |
| 5 | n = 2 |
| Unknown in 1 patient | |
| Mean (range) TRAB level at the beginning: | |
| Not detectable | n = 6 |
| Positive | n = 22:50.64 U/l (2.3–212) |
| Unknown in 2 patients | |
| Mean (range) TRAB level at the biopsy: | |
| Not detectable | n = 4 |
| Positive | n = 26:25.392 U/l (2.4–213) |
| Unknown in 2 patients | |
| Number of TSHR+ cells in EOM biopsy: | |
| <5/field | n = 16 |
| 5–10/field | n = 11 |
| >10/field | n = 3 |
CAS, clinical activity score; EOM, extraocular muscles; TAO, thyroid associated orbitopathy; TRAB, thyrotropin receptor autoantibodies; TSHR, thyrotropin receptor.
Thirty patients with TAO were studied; 26 had GD, two Hashimoto’s thyroiditis, and two were euthyroid; the mean age was 55.9 years (range 28–85), 22 patients were women (mean age 57.63 year old), and eight were men (mean age 51.12 year old).
Patients had varying degrees of TAO severity: 16 had moderate TAO (soft tissues involvement, proptosis less than or equal to 23 mm, and/or moderate restriction of ocular motility) and 14 had severe TAO (soft tissue involvement, proptosis more than 23 mm, severe ocular restriction motility, and varying degree of compressive optic neuropathy). The activity of eye disease was assessed using the clinical activity score (CAS), based on the classic signs of inflammation: orbital pain (two items: spontaneous pain or pain during eye movements), redness of the conjunctiva (one) or eyelids (one), swelling of caruncle (one) or eyelids (one), or chemosis (one). The score ranges from 0 to 7.9
Except for two patients who had the EOM biopsy early in the disease with a CAS of 5, all had a low CAS (⩽3).
Twenty patients received corticosteroid treatment. In severe TAO cases, it consisted of intravenous methylprednisolone (1 g/day for 3 days) followed by oral prednisolone (1 mg/kg/day tapered in 3–4 months). Six patients with moderate TAO received oral prednisolone (at the same dosage), associated with radiation therapy in one patient (20 Gy administered in 10 days on both orbits). Six patients with moderate TAO received only radiation therapy at the same dosage of 20 Gy. Corticotherapy was discontinued 11.5 months (range 1–72) and radiation therapy 12.4 months (range 5–48) before EOM biopsy. Four patients with moderate TAO received neither corticotherapy nor radiation therapy.
The mean duration of TAO was 25.7 months (range 6–96 months). Nineteen patients were smokers.
All patients had a biopsy in the third anterior portion of the EOM, as far as possible from the tendon, at the chronic, end stage of TAO, during surgery for diplopia; except for two patients who also has a biopsy during orbital decompression for compressive optic neuropathy.
All patients were euthyroid at the time of EOM biopsy: 12 received antithyroid drugs, eight thyroidectomy, four radioiodine and thyroidectomy, two radioiodine only. The two patients with thyroiditis had only thyroid hormones, and two euthyroid patients had no treatment. TRAB were measured in the serum by radioreceptor assay (Selco TRAB-Medipan) (normal value <12 U/l) at initial diagnosis in 28/30 patients and in all patients at the time of biopsy.
Processing of EOM for morphology and immunohistochemistry
All EOM specimens were processed either for light microscopy or for immunohistochemistry as previously described.10 For light microscopy, some samples were fixed in 10% formaldehyde and embedded in paraffin. Sections 5 µm thick were stained with haematoxylin-eosin-safran. Other samples were fixed in 2.5 % glutaraldehyde and then in 1% osmium tetroxide. After embedding in resin, sections (0.5 µm thick) were stained with toluidine blue.
The sample designed for immunohistochemistry was inserted into a liver fragment, embedded in Tissue-Tek and rapidly frozen in isopentane cooled in liquid nitrogen to generate 5 µm thick cryostat sections.
The frozen sections were subjected to indirect immunoperoxidase staining, the peroxidase activity being revealed by the DAB (3-3′-diaminobenzidine tetrahydrochloride)—H2O2 reaction giving a brown staining.10 Another immunodetection method used was the En Vision technique. The secondary antibody was a goat anti-mouse immunoglobulin conjugated to peroxidase labelled polymer (En Vision +, Dako, Copenhagen, Denmark). The chromogen used for revelation was AEC (3-amino-9-ethylcarbazole) which produces a red stain.
We have used three different monoclonal antibodies specific for TSHR. BA8 and 3G411 were used at a dilution of 1:250, and NCL-TSH-R2 (Novocastra Laboratories, Newcastle upon Tyne, UK) at a dilution of 1:100.
Different immunohistochemical controls were performed: by omission of the first monoclonal antibody, or of the first and second antibodies, by substituting an IgG2a of the same isotype than BA8 and NCL-TSH-R2. The number of cells positively expressing the TSHR were counted on the whole section in at least 10 microscopic fields at a magnification ×250. Results were expressed as follows: >10 labelled cells/field, 5–10 labelled cells/field, <5 labelled cells/field.
To assess the specificity of TSHR expression in TAO EOMs, we analysed EOM biopsies, taken in the anterior portion of the muscle during surgery, from:
three patients with non-thyroid orbital inflammatory disease (idiopathic inflammatory orbital pseudotumour in one patient, lymphoma or lymphoid hyperplasia in one patient, and idiopathic myositis in one patient),
a fourth patient with an ischaemic oculomotor palsy and amiodarone induced hyperthyroidism without TRAB in the serum.
All of these patients have been treated with a high dose of oral corticosteroids for several months, before surgery.
20 healthy patients with an essential strabismus (mean age 23.55 year, range 2–72), had a biospy in the anterior portion of their EOM itself (not in the tendon), during strabismus surgery.
Written consent for EOM biopsy was obtained from all patients. Ethical rules were respected according to guidelines of the ethics committee of the Université Catholique de Louvain.
Statistical analysis
Correlations between continuous or ordinal variables were made with the Spearman correlation coefficient. Comparisons between groups were done using the Wilcoxon test for continuous variables and with the Fisher’s exact test for proportions. SAS (Statistical Analysis Software, version 6.0) was used for calculation.
RESULTS
Morphological analysis
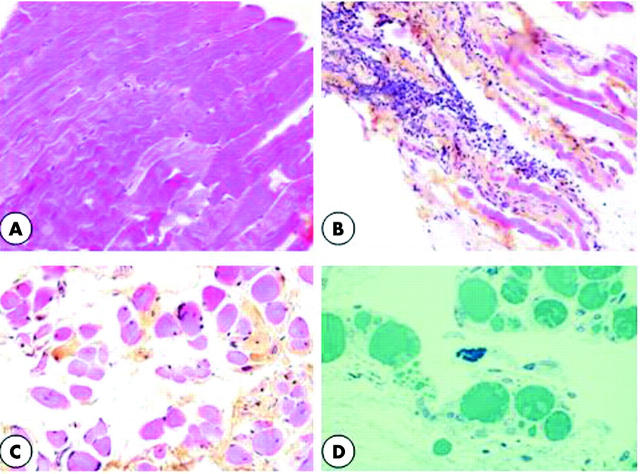
In the control strabismus group (fig 1A), the muscular cells were tightly associated, only separated by a few fibroblasts.
Figure 1.
(A) A 5 µm thick section (haematoxylin-eosin-safran staining). Control strabismic patients. Muscular cells are tightly associated (×150). (B) 5 µm thick section (haematoxylin-eosin-safran staining). Patient in active stage of TAO. Muscular cells are dissociated by oedema and an infiltrate of inflammatory cells (×150). (C) 5 µm thick section (haematoxylin-eosin-safran staining). Patient in chronic stage of TAO. Muscular cells are separated by fibrous tissues with collagen bundles stained in yellow (×150). (D) 0.5 µm thick section (toluidine blue staining). Patient in chronic stage of TAO. Mast cells identified by their dense granules filling the cytoplasm are often in close vicinity with adipocytes (×350).
In EOMs from patients in the active stage of TAO (fig 1B), the muscular cells were dissociated by oedema and lymphatic infiltration.
The chronic stage of TAO was characterised by a fibrotic reaction with numerous elongated fibroblasts among collagen bundles and by the presence of adipocytes (fig 1C). The lymphocytes infiltrate was reduced, but there were numerous mast cells, identified by their dense granules filling the cytoplasm. They were often in close proximity to adipocytes and signs of degranulation were frequently observed (fig 1D).
Immunodetection of TSHR expression
The specificity of TSHR immunostaining was assessed on human thyroid. The labelling was located at the baso-lateral pole of the thyroid cells (fig 2A). There was no staining when omitting the first antibody (fig 2B).
Figure 2.
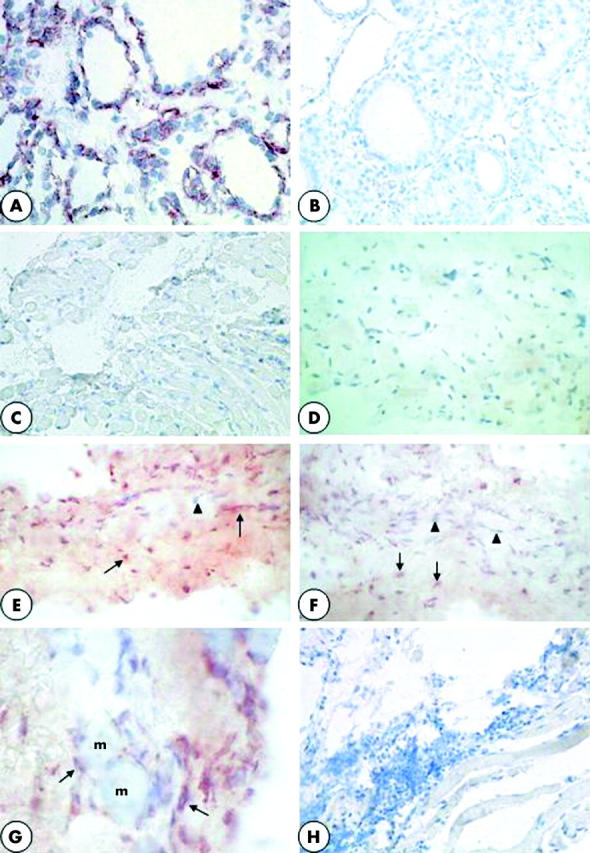
(A) Normal human thyroid as positive immunostaining control. TSHR is located at the baso-lateral pole of thyroid cells (×250). (B) Normal human thyroid. There is no labelling after omission of the first anti-TSHR mAb (×250). (C) Control strabismic patient. The muscular cells, in longitudinal section, are tightly associated. There are no TSHR+ cells (×120). (D) Patient operated on for a non-thyroid pathology. The muscular cells, in transversal section, are tightly associated. There are no TSHR+ cells (×120). (E) Patient in an active stage of TAO. There are numerous TSHR+ cells, stained in red (arrows), between the muscular cells, dissociated by oedema. Arrowhead indicates nuclei of muscular cells stained in blue (×250). (F) Patient in a chronic stage of TAO. TSHR+ cells, stained in red (arrows), are located in the fibrous tissue between the muscular cells, but they are less numerous than in (D). Arrowheads indicate nuclei of cells which do not express TSHR (×250). (G) Patient in a chronic stage of TAO. TSHR+ cells, stained in red (arrows), are elongated and look like fibroblasts. They are located in the fibrous tissue separating the muscular cells (m) (×400). (H) Patient in an active stage of TAO. No cells were positively stained when omitting the first anti-TSHR antibody (×120).
In EOM from strabismic patients (fig 2C), and from patients with a non-thyroid orbital inflammation (fig 2D), we did not observe any cells expressing TSHR. On the contrary, in all the EOM samples from TAO patients, there were cells positively immunostained for TSHR (fig 2E–G). The same results were obtained with the three different monoclonal antibodies used. No staining was observed when using IgG2a instead of the first anti-TSHR antibody (fig 2H).
The number of TSHR+ cells was very high in patients in the active stage of TAO (fig 2E) and systematically lower in patients in the chronic stage (fig 2F).
The cells expressing TSHR were elongated and looked like fibroblasts. They were located in the fibrous tissue dissociating the muscular cells (fig 2G).
Correlation between the number of TSHR positive cells (table 2) or serum TRAB levels (table 3) and the evolution of TAO
Table 2.
Correlation with the number of TSHR+ cells at EOM biopsy
| r | p Value | |
| Age | 0.14 | NS |
| TAO duration | −0.36 | 0.05 |
| TAO stage | 0.15 | NS |
| CAS (clinical activity score) | 0.27 | NS |
| TRAB level at the beginning | 0.20 | NS |
| TRAB level at biopsy | 0.14 | NS |
Spearman’s correlation coefficient, r, and p value (significance level < or = 0.05).
Table 3.
Correlation with serum TRAB level at the beginning of TAO
| r | p Value | |
| Age | −0.07 | NS |
| TAO stage | 0.35 | 0.05 |
| CAS | 0.04 | NS |
| Smoking | 0.17 | NS |
Spearman’s correlation coefficient, r, and p value (significance level < or = 0.05).
A negative significant correlation was observed between the number of TSHR+ cells in the EOM samples and the duration of TAO disease (p = 0.05). We had the opportunity to biopsy one patient twice, and another three times with a delay between the biopsies from 6 months to 12 months; both showed a dramatic reduction in the number of TSHR+ cells and an increase in fibrosis (fig 2G).
The number of TSHR+ cells in TAO EOM did not correlate with the TAO stage (p = 0.42), and the disease inflammation activity, assessed by the CAS (p = 0.15).
But a very high number of TSHR+ cells (>10 cells/field) was observed in only three patients (four biopsies) with severe TAO.
The TRAB level in the serum at the time of EOM biopsy was not significantly correlated with the number of TSHR + cells or with the TAO stage.
However, TRAB levels at the beginning of TAO presented a significant correlation with the TAO stage (r = 0.35, p = 0.05) and were significantly higher in women than in men (p = 0.02). The values were 54.69 U/l (0–212) for the women (n = 22) and 7.11 U/l (0–26.9) for the men (n = 8).
The duration of interruption of the corticosteroids and radiation therapy did not seem to influence the number of TSHR+ cells in the EOM.
Three patients with moderate TAO received neither corticotherapy nor radiation therapy, but all had TSHR+ cells in their biopsy, two with a moderate (5–10 cells/field) and one with a low (<5 cells/field) number. However, we did not find a significant difference in the number of TSHR+ cells between treated and untreated patients.
DISCUSSION
There is increasing evidence that the TSHR is present in orbital tissue. TSHR mRNA has been found in tissue homogenates by polymerase chain reaction (PCR),6 and immunohistochemistry has shown that orbital fibroblasts express TSHR at certain stages of their maturation.12 As recently shown, 20% of mice immunised with TSHR developed GD and orbital pathology,13–15 suggesting that an autoimmune reaction against TSHR is the first event in TAO.
The morphological alterations that we observed in the human EOM biopsies were very similar to those described in the animal models but changed as the disease progressed. The active stage was characterised by infiltration of polymorphonuclear cells and lymphocytes, proliferation of fibroblasts, and oedema. The chronic stage was marked by fibrosis and adipogenesis and the infiltrating cells are mainly lymphocytes and mast cells. Mast cells have been previously reported in human TAO biopsies16 and this was confirmed in our study. Their close proximity to adipocytes and the signs of degranulation could be suggestive of their participation in the TAO process.
Our work demonstrates that TSHR protein expression in the orbit is confined to patients with TAO and is associated with elongated fibroblast-like cells. Since immunostaining was obtained with three different TSHR monoclonals, this suggests that it is the TSHR itself rather than a cross reacting antigen.
During adipogenesis induced in vitro, TSHR expression is upregulated17 but this feature is not unique to pre-adipocytes from TAO or even confined to the orbit.8,18 The difference between the results of these studies and ours is probably because of the in vitro versus in vivo nature of the tissues. Our findings confirm results obtained by quantitative PCR analysis of in vivo tissues, in which TSHR transcripts were detected only in TAO orbital fat and cervical fat from GD patients; all other human tissues tested were at the limit of detection.
Our results showed that the number of TSHR+ cells in EOM is particularly high in early TAO. This observation, as in the study of Wakelkamp et al19 where the TSHR mRNA in orbital fat was observed in the active stage but lost in inactive TAO, lends support for TSHR being a primary element for the initiation of TAO.
We did not observe any significant correlation between the number of TSHR+ cells and the CAS, but the large majority of our patients had biopsies at the chronic stage of the disease.
However, on the basis of the Rundle’s curve20 of disease activity, the negative correlation observed between the reduction in the number of TSHR+ cells and the duration of TAO might suggest a possible relation between the number of TSHR+ cells and the early phase of TAO usually characterised by a high inflammatory activity.
Most of our TAO patients were treated with corticosteroids or by radiation; only three patients did not receive any medication. We cannot exclude the possible influence of anti-inflammatory therapy on TSHR expression in EOMs. However, all four of the patients with a non-thyroidal orbital inflammation have been treated with corticosteroids for several months, and none of them display any TSHR immunoreactivity. Location of the biopsy in the EOM might also influence TSHR expression, but multiple biopsies in the same orbit are impossible to obtain because of ethical constraints.
The low correlation between the number of TSHR+ cells in EOM and the TRAB serum rate at the time of EOM biopsy might be explained by the fact that the EOM biopsies were mainly obtained at the late chronic stage of TAO. During long term treatment serum TRAB values decline in most patients, but the extent of the decline varied substantially.21–23
Gerding et al observed a strong correlation between TSI and TBII in the serum and TAO activity, assessed with CAS, but only in patients with ophthalmopathy of short duration.24
These authors related a significant correlation between TSI/TBII and severity of proptosis and of soft tissue involvement. Our results were consistent with those of Gerding et al,24 since we observed at the initial diagnosis of TAO a high correlation between the TRAB level and its severity stage.
Our data did not show a greater disease severity in male or in older patients as previously reported by several authors,25,26 and we did not observe any sex or age influence on the number of TSHR+ cells in EOM. However, women showed a significantly higher level of TRAB at the beginning of the eye disease, without any relation with smoking, age, or thyroid disease.
With the absence of any TSHR immunostaining in the EOM specimens from the patients with non-thyroid orbital inflammation and from one patient with non-Basedow thyroid dysfunction, we might confirm the specificity of TSHR in TAO.
Ocular manifestations of TAO are well known; however, their clinical picture can be highly variable. The diagnosis may easily be overlooked especially in the early stage, in unilateral, and/or asymmetric TAO cases as well as in the absence of thyroid dysfunction. TSHR immunoreactivity testing might become a useful additional method to differentiate TAO from other inflammatory orbital conditions.
In conclusion, until now our study is the largest study of TAO biopsies and it brings strong evidence that TSHR protein is present in the orbit and is specific for TAO. All the results corroborate the important and specific role of TSHR expression in the orbital tissues and of TRAB for the early process of TAO pathogenesis.
Abbreviations
Ab, antibodies
CAS, clinical activity score
EOM, extraocular muscles
GD, Graves’ disease
RT-PCR, real time-polymerase chain reaction
TAO, thyroid associated orbitopathy
TRAB, thyrotropin receptor autoantibodies
TSHR, thyrotropin receptor
REFERENCES
- 1.Heufelder AE. Involvement of the orbital fibroblast and TSH receptor in the pathogenesis of Graves’ ophthalmopathy. Thyroid 1995;5:331–40. [DOI] [PubMed] [Google Scholar]
- 2.Förster G, Otto E, Hansen C, et al. Analysis of orbital T cells in thyroid-associated ophthalmopathy. Clin Exp Immunol 1998;112:427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoo DHC, Ho SC, Seah LL, et al. The combination of absent thyroid peroxidase antibodies and high thyroid-stimulating immunoglobulin levels in Graves’ disease identifies a group at markedly increased risk of ophthalmopathy. Thyroid 1999;9:1175–80. [DOI] [PubMed] [Google Scholar]
- 4.Crisp MS, Lane C, Halliwell M, et al. Thyrotropin receptor transcripts in human adipose tissue. J Clin Endocrinol Metab 1997;82:2003–5. [PubMed] [Google Scholar]
- 5.Busuttil BE, Frauman AE. Extrathyroidal manifestations of Graves’ disease: the thyrotropin receptor is expressed in extraocular, but not cardiac muscle tissues. J Clin Endocrinol Metab 2001;86:2315–19. [DOI] [PubMed] [Google Scholar]
- 6.Feliciello A, Porcellini A, Ciullo I, et al. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet 1993;342:337–338. [DOI] [PubMed] [Google Scholar]
- 7.Ludgate M, Crisp M, Lane C, et al. The thyrotropin receptor in thyroid eye disease. Thyroid 1998;8:411–13. [DOI] [PubMed] [Google Scholar]
- 8.Crisp M, Starkey KJ, Ham J, et al. Adipogenesis in thyroid eye disease. Invest Ophthalmol Vis Sci 2000;41:3249–55. [PubMed] [Google Scholar]
- 9.American Thyroid Association. European Thyroid Association, Latin-American Thyroid Association, Japanese and Asia-Oceania Thyroid Association Classification of eye changes of Graves’ disease. Thyroid 1992;2:235–6. [DOI] [PubMed] [Google Scholar]
- 10.Many MC, Maniratunga S, Varis I, et al. Two-step development of Hashimoto-like thyroiditis in genetically autoimmune prone non-obese diabetic mice: effects of iodine-induced cell necrosis. J Endocrinol 1995;147:311–20. [DOI] [PubMed] [Google Scholar]
- 11.Costagliola S, Rodien P, Many MC, et al. Genetic immunization against the human thyrotropin receptor causes thyroiditis and allows production of monoclonal antibodies recognizing the native receptor. J Immunol 1998;160:1458–65. [PubMed] [Google Scholar]
- 12.Bahn RS, Dutton CM, Natt N, et al. Thyroptropin receptor expression in Graves’ orbital adipose/connective tissues: potential autoantigen in Graves’ ophthalmopathy. J Clin Endocrinol Metab 1998;83:998–1002. [DOI] [PubMed] [Google Scholar]
- 13.Costagliola S, Many MC, Denef JF, et al. Genetic immunization of outbred mice with thyrotropin receptor cDNA provides a model of Graves’ disease. J Clin Invest 2000;105:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Many MC, Costagliola S, Detrait M, et al. Development of an animal model of autoimmune thyroid eye disease. J Immunol 1999;162:4966–74. [PubMed] [Google Scholar]
- 15.Wall JR. Graves’disease is a multi-system autoimmune disorder in which extra ocular muscle damage and connective tissue inflammation are variable features. Thyroid 2002;12:35–6. [DOI] [PubMed] [Google Scholar]
- 16.Hufnagel TJ, Hickey WF, Cobbs WH, et al. Immunochemical and ultrastructural studies on the exenterated orbital tissues of a patient with Graves’disease. Ophthalmology 1984;91:1411–19. [DOI] [PubMed] [Google Scholar]
- 17.Valyasevi R, Erickson D, Harteneck D, et al. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab 1999;84:2557–62. [DOI] [PubMed] [Google Scholar]
- 18.Bell A, Gagnon A, Grunder L, et al. Functional TSH receptor in human abdominal preadipocytes and orbital fibroblasts. Am J Physiol Cell Physiol 2000;279:335–40. [DOI] [PubMed] [Google Scholar]
- 19.Wakelkamp IMMJ, Wiersinga WM, Prummel MF. TSH-R mRNA expression in orbital tissue of Graves’ ophthalmopathy patients is related to disease activity, to Th1 and proinflammatory cytokines. J Endocrinol Invest 2001;24:122. [Google Scholar]
- 20.Hales IB, Rundle FF. Ocular changes in Graves’ disease: a long-term follow-up study. QJ Med 1960;29:113–26. [PubMed] [Google Scholar]
- 21.Pinchera A, Liberti P, Martino E. Effects of antithyroid therapy on the long-acting thyroid stimulor and the antithyroglobulin antibodies. J Clin Endocrinol Metab 1969;29:231. [DOI] [PubMed] [Google Scholar]
- 22.Fenzi GF, Hashizume K, Roudebousch CP. Changes in thyroid-stimulating immunoglobulins during antithyroid therapy. J Clin Endocrinol Metab 1979;48:572–6. [DOI] [PubMed] [Google Scholar]
- 23.Feldt-Rasmussen U, Schleusser H, Carayon P. Meat analysis evaluation of the impact of thyrotropin receptor antibodies on long term remission after medical therapy. J Clin Endocrinol Metab 1994;78:98. [DOI] [PubMed] [Google Scholar]
- 24.Gerding MN, van der Meer JWC, Bakker O, et al. Association of thyrotropin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol 2000;52:267–71. [DOI] [PubMed] [Google Scholar]
- 25.Perros P, Crombie, AL, Matthews AJS, et al. Age and gender. Clin Endocrinol 1993;38:367–72. [DOI] [PubMed] [Google Scholar]
- 26.Kendler DL, Lippa J, Rootman J. The initial clinical characteristics of Graves’ orbitopathy vary with age and sex. Arch Ophthalmol 1993;111:197–201. [DOI] [PubMed] [Google Scholar]