Abstract
Aim: To generate a mouse model for slow progressive retinal neovascularisation through vascular endothelial growth factor (VEGF) upregulation.
Methods: Transgenic mice were generated via microinjection of a DNA construct containing the human VEGF165 (hVEGF) gene driven by a truncated mouse rhodopsin promoter. Mouse eyes were characterised clinically and histologically and ocular hVEGF levels assayed by ELISA.
Results: One transgenic line expressing low hVEGF levels showed mild clinical changes such as focal fluorescein leakage, microaneurysms, venous tortuosity, capillary non-perfusion and minor neovascularisation, which remained stable up to 3 months postnatal. Histologically, there were some disturbance and thinning of inner and outer nuclear layers, with occasional focal areas of neovascularisation. By contrast, three other lines expressing high hVEGF levels presented with concomitantly severe phenotypes. In addition to the above, clinical features included extensive neovascularisation, haemorrhage, and retinal detachment; histologically, focal to extensive areas of neovascularisation associated with retinal folds, cell loss in the inner and outer nuclear layers, and partial retinal detachment were common.
Conclusions: The authors generated four hVEGF overexpressing transgenic mouse lines with phenotypes ranging from mild to severe neovascularisation. These models are a valuable research tool to study excess VEGF related molecular and cellular changes and provide additional opportunities to test anti-angiogenic therapies.
Keywords: transgenic mouse model, retinal neovascularisation, vascular endothelial growth factor
Retinal neovascularisation, a major cause of blindness in the developed world, is characteristic of many eye diseases including retinopathy of prematurity (ROP), retinal vein occlusion, and diabetic retinopathy (DR).1–3 Retinal neovascularisation is thus an issue of major clinical importance, and for this reason, a variety of animal models have been developed.
The precise mechanisms underlying retinal neovascularisation have not been fully elucidated but have been linked to hypoxia and are thought to be mediated by various growth factors including vascular endothelial growth factor (VEGF).4 VEGF has been a favoured molecule for study since it is hypoxia induced,5 is one of the most potent angiogenic factors known,6 and has a pivotal role during normal retinal vasculature development.7 Furthermore, intravitreal VEGF injections or implants have induced neovascularisation in adult animals.8,9 However, a major shortcoming of these models has been the lack of consistent phenotype and reproducibility.9–12
The development of transgenic mouse models with early retinal neovascularisation13,14 provides an approach to study the damaging phase of retinal neovascularisation and an opportunity for therapeutic evaluation.15–19 However, most of the current ocular neovascularisation models are hampered by both extensive and rapid neovascularisation which result in severe retinal damage.14
The rhodopsin promoter used in existing transgenic mouse models contains regulatory regions known to enhance expression of the associated transgene. In addition, the rhodopsin promoter length has been correlated to the transgene’s expression level.20–22 As our aim was to produce a transgenic mouse model expressing low VEGF levels which would lead to a slow progression of retinal neovascularisation akin to vascular changes observed in DR, we constructed a transgene cassette using the 1.4 kb mouse rhodopsin promoter fragment known to lack transcriptional enhancers23 to drive human VEGF (hVEGF) expression in the transgenic mice.
METHODS
Animal husbandry
All animal procedures were performed in accordance with the ARVO statement for the use of animals in ophthalmic and vision research and with approval from the animal ethics committee at the University of Western Australia, Australia. Mice were housed in cages at a constant temperature of 22°C, with a 12:12 hour light/dark cycle, and food and water were available ad libitum.
Transgenic mice generation
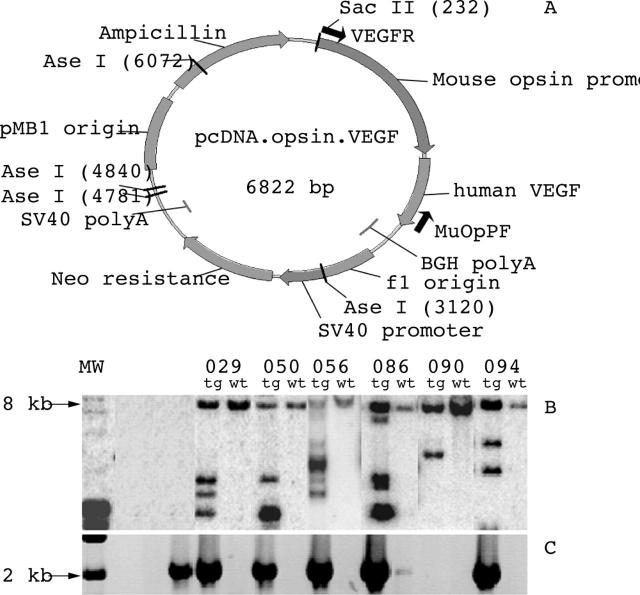
The previously described hVEGF165 isoform24 and truncated mouse rhodopsin promoter23 were used to generate the pcDNA.opsin.VEGF construct (fig 1A). The 2.89 kb AseI/SacII fragment containing the truncated mouse rhodopsin promoter, hVEGF165 and bovine growth hormone polyadenylation signal sequence was used for transgenic mice generation.
Figure 1.
In vitro characterisation of transgenic mice. (A) Diagrammatic representation of the pcDNA.opsin.VEGF construct used for transgenic mice generation. (B) Southern blot analysis showing the banding profiles from the different lines. Pups were screened by Southern blot analysis of EcoRI digested tail DNA and probed with a 1.1 kb [α–32P]dCTP labelled EcoRI fragment of the mouse rhodopsin promoter from pcDNA.opsin.VEGF (A). Lane 1, lambda DNA control; lanes 2 and 3, no DNA control. (C) Agarose gel electrophoresis of PCR products from the different lines. Lane 1, no primer control; lane 2, no DNA control; lane 3, positive control. The PCR conditions consisted of denaturation at 95°C for 15 minutes, 40 cycles of 95°C for 30 seconds, 58°C for 1 minute, and 72°C for 4 minutes, followed by a final extension at 72°C for 7 minutes. MW, molecular weight marker; tg, transgenic mice; wt, non-transgenic littermate.
Pups were first screened by Southern blot analysis. Presence of the 2.1 kb fragment containing the truncated mouse rhodopsin promoter and hVEGF165 fragments in the transgenic mice was then confirmed by polymerase chain reaction (PCR) amplification of tail DNA using the primer pair 5′CGA GGC TCA GAG AGG AAT ACT T3′ and 5′CAC CGC CTC GGC TTG TCA C3′. First generation heterozygote transgenic offspring from backcrossing founders with C57Bl/6J mice were characterised clinically, by colour fundus photography (CFP) and fluorescein fundus angiography (FFA), and histologically with age matched, non-transgenic littermates and C57Bl/6J mice as controls.
Quantification of ocular hVEGF protein
To circumvent complications presented by differences in onset and severity of retinopathy at later time points, 10 day old mouse eyes, an age where the retinopathy was still mild and retinas intact in all the lines, were enucleated for hVEGF quantification using an enzyme linked immunosorbent assay (ELISA; Quantikine, R&D Systems, Minneapolis, MN, USA). Intact mouse eyes were disrupted with a pestle in 50 μl of phosphate buffered saline (PBS) containing protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Total protein in the eye homogenate was quantified by the Bradford method (Protein Assay dye; Bio-Rad, Richmond, CA, USA) with bovine serum albumin as the standard. Ocular hVEGF levels were normalised to 1 mg total protein.
Fluorescein labelled dextran perfusion and retina flat mount preparation
Six week old deeply anaesthetised mice were perfused with PBS (pH 7.4) followed by 2 ml fluorescein isothiocynate labelled dextran (FITC-dextran, 50 mg/ml, MW 2.0×106; Sigma, St Louis, MO, USA). Eyes were enucleated, fixed in 2% paraformaldehyde for 30 minutes, and flat mounted for fluorescence microscopy.25
Histology
Eyes from each transgenic line were enucleated at between 1 week and 12 weeks postnatal following euthanasia with pentobarbital (Lethabarb; Virbac, NSW, Australia) and fixed for 4 hours in 4% neutral buffered formalin before paraffin embedding. Five μm thick paraffin sections were stained with haematoxylin and eosin (H&E) for light microscopy.
RESULTS
Characterisation of transgenic mice
Six founders, designated 029, 050, 056, 086, 090, and 094 (fig 1B), were identified by Southern blot analysis. Subsequent PCR amplification showed that the 2.1 kb DNA fragment was present in all but line 090 (fig 1C). Of these, lines 029, 050, 056, and 094 showed varying degrees of neovascularisation and pathology (see below). Lines 086 and 090 appeared normal even at 10 months postnatal and hence are not discussed further.
Features of diabetic retinopathy
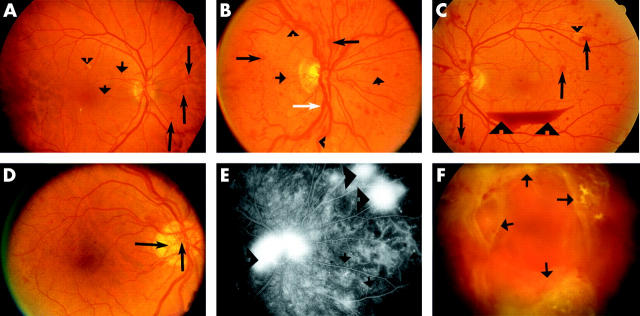
The most prevalent disease associated with retinal neovascularisation is DR.3 In this study, we used classic clinical features of DR26 to determine whether retinal vascular changes observed in DR also occurred in our transgenic lines (table 1). Representative images showing pathological features associated with non-proliferative diabetic retinopathy (NPDR; microaneurysm, exudates, haemorrhage, venous abnormalities) and proliferative diabetic retinopathy (PDR; neovascularisation, preretinal haemorrhage, fibrovascular proliferation) are summarised and documented in figure 2.
Table 1.
Characterisation of retinal vasculature in the transgenic mouse lines
| Retinal vasculature characteristics | Line 029 | Line 050/056 | Line 094 |
| Capillary non-perfusion | + | + | + |
| Microaneurysms | + | + | + |
| Venous dilatation | – | + | + |
| Tortuosity of blood vessels | + | – | + |
| Haemorrhages | ± | ++ | ++ |
| Neovascularisation | + | ++ | ++ |
| Preretinal haemorrhage | – | + | ++ |
| Vitreous haemorrhage | – | + | ++ |
| Fibrovascular proliferation | – | + | + |
| Cataracts* | – | +++ | + |
| Retinal detachment | – | +++ | ++ |
*Other pathological features observed through clinical and histological examinations.
Figure 2.
Colour fundus photography (CFP) and fluorescein fundus angiography (FFA) showing different features of diabetic retinopathy (DR). (A) An eye with mild non-proliferative diabetic retinopathy (NPDR) presented with microaneurysms (short arrows), haemorrhages (long arrows), as well as hard and soft exudates (arrowhead). (B) An eye with severe NPDR showing a greater number of microaneurysms (short arrows), haemorrhages (long arrows), and also venous abnormalities such as venous dilatation (white arrow) and tortuosity (arrowheads). (C) and (D) Eyes with high risk proliferative diabetic retinopathy (PDR). Haemorrhages (long arrows), hard exudates (small arrowhead), venous beading on the inferior arcade, new vessels (hallmark for PDR) and preretinal haemorrhage (large arrowheads) are present in (C) and new vessels at the disc (long arrows) are seen in (D). (E) A late phase FFA of an eye with PDR showing leakage from the new vessels (arrowheads) and small spots of hyperfluorescence representing microaneurysms (arrows). Dark patches near the right edge are zones of capillary non-perfusion. (F) Eye with advanced PDR showing extensive fibrovascular proliferation (arrows).
Evaluation of mouse eyes and comparison with human DR eyes
CFP and FFA demonstrated the fundus and retinal vasculature of C57Bl/6J mice (fig 3A and B) and non-transgenic littermates (data not shown) to be normal at all ages examined. The C57Bl/6J mouse eye has even calibre vessels radiating from a well defined optic nerve head. FITC-dextran perfusion and histological examination of control eyes showed intact retinal vessels (fig 3C) and normal retinal morphology (fig 3D).
Figure 3.
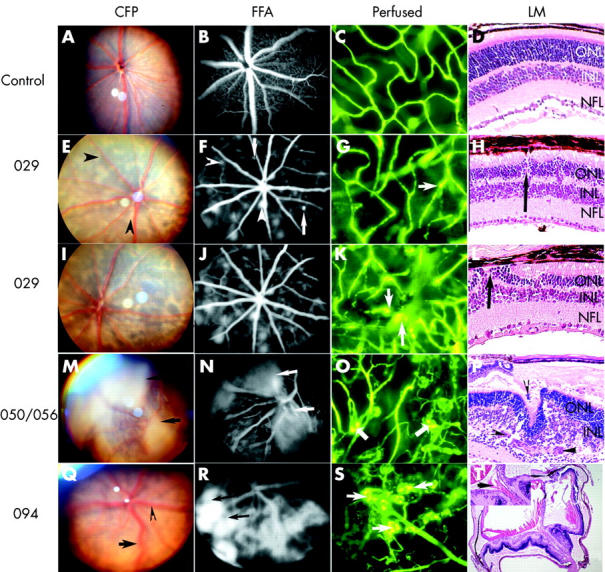
Characterisation of the different transgenic mouse lines by colour fundus photography (CFP) (A, E, I, M, and Q), fluorescein fundus angiography (FFA) (B, F, J, N, and R), fluorescence micrographs of flat mounted, fluorescein labelled dextran perfused eyes (C, G, K, O, and S), and light micrographs (LM) of haematoxylin and eosin stained paraffin sections (D, H, L, P, and T). C57Bl/6 control mouse eye at 4 weeks (A–C) and at 3 weeks postnatal (D). Line 029 transgenic mouse eye at 4 weeks (E–G) and at 3 weeks (H) postnatal. Line 029 transgenic mouse eyes at 12 weeks (I–J) and at 10 weeks (K–L) postnatal. Line 050/056 transgenic mouse eye at 4 weeks (M–O) and at 3 weeks (P) postnatal. Line 094 transgenic mouse eyes at 4 weeks (Q–S) and at 3 weeks (T) postnatal. Arrowhead in (F) demonstrates focal hyperfluorescence which corresponds to pale lesions in (E). Arrows in (N) and (R) point to intense and extensive fluorescein leakage. The arrows in (G), (K), (O), and (S) point to focally dilated vessels (G), microaneurysm (K), and glomerular tufts of hyperfluorescence (O and S). The arrows in (H), (L), (P), and (T) point to new vessels. Arrowhead and arrow in (Q) show venous dilatation and tortuosity, respectively.
The four transgenic lines were distinguished by the onset and severity of neovascular changes (table 1). When examined by CFP, FFA, FITC-dextran perfusion, and histology, line 029 eyes showed consistently milder changes at early stages (3–4 weeks). Using CFP, the fundus had pale lesions (fig 3E); some were flat at the level of the outer retina and some were raised when examined stereoscopically. The optic nerve head and retinal vasculature appeared similar to the controls. FFA demonstrated multiple areas of focal hyperfluorescence (fig 3F, arrowhead). Upon stereoscopic examination, these lesions were seen at the level of the capillary bed in the inner retina (fig 3F, arrows), resembling microaneurysms. In the FITC-dextran perfused eyes the retinal vasculature appeared normal, except for some hyperfluorescent spots, focally dilated vessels (fig 3G, arrow) and occasional saccular structures resembling microaneurysms. Although line 029 transgenic mouse eyes had distinct, full thickness and normal appearing inner nuclear (INL), outer nuclear (ONL) and nerve fibre layers (NFL), there were focal areas of disturbance in the retina (fig 3H, arrow).
Clinically (CFP and FFA), changes in line 029 transgenic mice remained relatively stable with some increases in size and numbers of hyperfluorescent spots in follow up examinations at 10–12 weeks postnatal (fig 3I and J). By this age, the presence of dilated, tortuous capillaries with hyperfluorescent saccular structures resembling microaneurysms (fig 3K, arrows) and areas of capillary non-perfusion were evident from FITC-dextran perfusion. Histologically, there was significant disturbance in the ONL and INL which also appeared thinner compared to control eyes (compare fig 3D and 3L). Frequent focal areas of neovascularisation were present in the ONL and INL (fig 3L arrow). These vascular changes, such as microaneurysms, fluorescein leakage, and tortuous vessels, were similar to those observed in NPDR (fig 2A and B) and very early stages of PDR (fig 2C and D).
By 3–4 weeks postnatal, lines 050 and 056 demonstrated severe neovascularisation (fig 3M–P) and line 094 presented with retinal changes (fig 3Q–T) ranging from mild to severe. CFP revealed broad opaque sheets of pale tissue (fig 3M, arrows), venous dilatation (fig 3Q, arrowhead), and tortuosity (fig 3Q, arrow). In addition, FFA revealed intensely hyperfluorescent lesions (fig 3N, arrows and 3R, arrows) associated with haemorrhage. FITC-dextran perfusion showed tortuous vessels and glomerular tufts of hyperfluorescence in the central and peripheral retina that varied in size and morphology (fig 3O and 3S, arrows). In addition, lines 050, 056, and 094 presented with extensive areas of capillary non-perfusion (fig 3O and 3S, dark regions). The presence of extensive subretinal and intraretinal neovascularisation (fig 3P, arrowheads) was associated with the formation of folds and cell loss in the INL and ONL. Partial retinal detachment with subretinal exudate was also present. Preretinal haemorrhage (fig 3T inset, arrow) was observed in line 094 eyes with severe retinopathy. The above described features are similar to changes associated with severe neovascularisation in patients with PDR (fig 2C and 2E, arrowheads).
Although neovascular changes were present in all four lines examined, their choroidal vasculature appeared normal. In addition, their progression to the end stage of the disease, represented by severe fundal depigmentation, retinal vessel narrowing with abnormal capillary bed, scarring or retinal detachment varied between the lines. The disease progression was slow in line 029 (8 months) but was rapid in lines 050 (3 months), 056 (3 months), and 094 (4 months).
Quantification of hVEGF protein
A correlation between VEGF levels and the severity of neovascularisation was seen. hVEGF levels in line 029 (35.5 (SD 13.8) pg VEGF/mg total protein) were significantly lower (p<0.05) than those in lines 050 and 094 (410.6 (52.6) pg and 375.8 (49.0) pg VEGF/mg total protein, respectively). hVEGF protein was not detected in eyes of age matched, non-transgenic littermates or C57Bl/6J eyes (n = 4 for each line).
DISCUSSION
We generated a mouse model that clearly demonstrated that relatively low hVEGF protein levels were sufficient to produce clinical and pathological changes consistent with slowly developing retinal neovascularisation. We used the mouse rhodopsin promoter to ensure inclusion of intrinsic regulatory elements to drive transgene expression. In addition, this promoter was truncated to exclude the enhancer region (−1575 to −1477) with the aim of driving lower hVEGF levels to induce less severe neovascular changes compared to those in previously described transgenic mice.14 Indeed, using the truncated proximal promoter region, we generated one transgenic line (029) with almost 10-fold lower hVEGF expression than in the other three transgenic lines. The fact that only one out of four lines demonstrated moderate hVEGF expression suggested that as long as transgene construct integration remained random, the use of truncated species specific promoters was not sufficient to strictly control transgene expression levels. Fortunately, variability in transgene expression associated with random chromosomal integration can now be circumvented using the more expensive knock-in technologies.27
Line 029 clearly demonstrated that relatively low hVEGF protein levels were sufficient to produce clinical and pathological changes consistent with those seen in NPDR and very early stages of PDR, as well as other ocular neovascular disorders. The ability of exogenous VEGF to induce retinal ischaemia and neovascularisation had previously been reported in monkeys.8,28 It was hypothesised that VEGF may trigger retinal ischaemia since it upregulates retinal ICAM-1 (intercellular adhesion molecule) expression, which in turn may lead to retinal leucosis and death of endothelial cells and pericytes.29
The presence of areas of capillary non-perfusion in the eyes of our transgenic lines may be the result of VEGF triggered retinal ischaemia. It is possible that, once ischaemia is initiated, a cycle begins whereby VEGF induced neovascularisation is outstripped by VEGF induced ischaemia. It has been shown that, in addition to microaneurysm and microvessel obliteration, the earliest clinical manifestation of NPDR arises directly or indirectly as a result of VEGF upregulation.30 Indeed, VEGF has been shown to be upregulated in Müller cells, astrocytes, and some endothelial cells in retinas of diabetics with NPDR31 and in Goto-Kakisaki rats before observable vascular changes.32 Taken together, line 029 demonstrated several changes that have been observed in the retinal vasculature of patients with NPDR, such as microaneurysms, venous abnormalities (dilatation, tortuosity, and focal constrictions) and, at later stages, capillary non-perfusion (fig 2B and C). Further studies on line 029 will be necessary to elucidate the possible role of moderate hVEGF levels on a variety of retinal cell types and how such expression leads to sequelae such as capillary occlusion, hypoxia, microaneurysms, and venous dilatation.
We anticipated that, following moderate photoreceptor specific hVEGF upregulation, the principal pathological change would be neovascularisation restricted to the outer retina. This was observed in line 029 where neovascularisation was mild and may represent the very early stages of PDR. However, in the other lines, severe neovascularisation occurred in the outer retina as well as in the inner retina and at subretinal and preretinal locations. Our observation of preretinal neovascularisation in these lines contrasts with other mouse models with severe retinopathy,13,14 but which lacked preretinal haemorrhage, a hallmark of PDR.26
The photoreceptor specific hVEGF upregulation did not induce choroidal neovascularisation (CNV) in our and other transgenic mouse models.13,14 Similarly, transgenic mouse models with retinal pigment epithelial (RPE) cell specific VEGF upregulation did not develop CNV.33,34 It was suggested that the intact RPE cells and Bruch’s membrane may act as barriers and unless they are perturbed, as through subretinal injection,35,36 the upregulated hVEGF is not sufficient to cause CNV. In our transgenic mice, the RPE and Bruch’s membrane were intact even in lines showing severe retinal neovascularisation. In addition, in spite of the severe retinal neovascularisation present at 3–4 weeks postnatal, the changes in lines 050, 056, and 094 did not in any way resemble the regression and massive overgrowth of abnormal vessels across the surface of the retina, as characterised in ROP.37
The relatively mild degree of neovascularisation and its slow onset in line 029, as well as stable retinopathy for at least 3 months provide a valuable model for the development and evaluation of therapeutic treatments for retinal neovascularisation. The gradual changes observed in line 029 were similar to our previous mouse model in which VEGF upregulation was mediated by a recombinant adeno associated virus vector.38 However, it differed from an existing VEGF transgenic mouse model,13 which demonstrated earlier onset and more severe retinopathy. Likewise, a previously reported inducible VEGF transgenic model39 had variable pathology even when using the same doxycyclin dosage and neovascularisation was rapid and severe with complete retinal detachment.14
Although photoreceptors are not the main VEGF expression site during DR, many resultant pathological changes observed in our transgenic mice, particularly line 029, resembled NPDR or very early stages of PDR. Similar to other VEGF models, our transgenics are not hyperglycaemic but rather target the most damaging end stage of DR—namely, retinal neovascularisation. Transgenic VEGF models therefore may not provide information on the sequence of events leading to VEGF upregulation in DR and other diseases. However, the regulatory sequelae of excess VEGF production are a potential area for further study which may provide valuable information relevant to DR, as well as a wide variety of other ocular diseases in which retinal neovascularisation is a major feature.
Acknowledgments
The authors acknowledge OzGene Pty Ltd, Western Australia, for generating the transgenic colony, and the Animal Resource Centre, Western Australia, and the Sir Charles Gairdner Hospital Animal Resource Centre, Western Australia, for maintenance of the transgenic mice. This project was the research effort of the Diabetic Retinopathy Consortium, Perth, Australia.
Abbreviations
CFP, colour fundus photography
CNV, choroidal neovascularisation
DR, diabetic retinopathy
ELISA, enzyme linked immunosorbent assay
FFA, fluorescein fundus angiography
FITC, fluorescein isothiocyanate
hVEGF, human vascular endothelial growth factor
INL, inner nuclear layer
NFL, nerve fibre layer
NPDR, non-proliferative diabetic retinopathy
ONL, outer nuclear layer
PBS, phosphate buffered saline
PCR, polymerase chain reaction
PDR, proliferative diabetic retinopathy
RPE, retinal pigment epithelium
ROP, retinopathy of prematurity
VEGF, vascular endothelial growth factor
Sponsor details. This study was supported by the Juvenile Diabetes Research Foundation International (USA), National Health and Medical Research Council (Australia) and Westpac Foundation (Australia).
Ethical approval. Ethical approval (AEC 03/300/016 and 03/100/107) for breeding of and experimentation on animals has been granted by the Animal Ethics Committee at the University of Western Australia, Australia.
REFERENCES
- 1.Battaglia Parodi M, Iacono P, Di Crecchio L, et al. Clinical and angiographic features in nasal branch retinal vein occlusion. Ophthalmologica 2004;218:210–13. [DOI] [PubMed] [Google Scholar]
- 2.Recchia FM, Baumal CR, Sivalingam A, et al. Endophthalmitis after pediatric strabismus surgery. Arch Ophthalmol 2000;118:939–44. [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984;102:527–32. [DOI] [PubMed] [Google Scholar]
- 4.Witmer AN, Vrensen GF, Van Noorden CJ, et al. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 2003;22:1–29. [DOI] [PubMed] [Google Scholar]
- 5.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 1996;16:4604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Senger DR, Connolly DT, Van de Water L, et al. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res 1990;50:1774–8. [PubMed] [Google Scholar]
- 7.Feeney SA, Simpson DA, Gardiner TA, et al. Role of vascular endothelial growth factor and placental growth factors during retinal vascular development and hyaloid regression. Invest Ophthalmol Vis Sci 2003;44:839–47. [DOI] [PubMed] [Google Scholar]
- 8.Tolentino MJ, Miller JW, Gragoudas ES, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 1996;103:1820–8. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki H, Hayashi H, Vinores SA, et al. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res 1997;64:505–17. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti S, Sima AA, Tze WJ, et al. Prevention of diabetic retinal capillary pericyte degeneration and loss by pancreatic islet allograft. Curr Eye Res 1987;6:649–58. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T, Kubo E, Takahashi Y, et al. Retinal vessel changes in galactose-fed dogs. Arch Ophthalmol 1998;116:785–9. [DOI] [PubMed] [Google Scholar]
- 12.Su EN, Alder VA, Yu DY, et al. Continued progression of retinopathy despite spontaneous recovery to normoglycemia in a long-term study of streptozotocin-induced diabetes in rats. Graefes Arch Clin Exp Ophthalmol 2000;238:163–73. [DOI] [PubMed] [Google Scholar]
- 13.Okamoto N, Tobe T, Hackett SF, et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol 1997;151:281–91. [PMC free article] [PubMed] [Google Scholar]
- 14.Ohno-Matsui K, Hirose A, Yamamoto S, et al. Inducible expression of vascular endothelial growth factor in adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol 2002;160:711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi K, Saishin Y, Silva RL, et al. Intraocular expression of endostatin reduces VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment. Faseb J 2003;17:896–8. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi K, Saishin Y, Mori K, et al. Topical nepafenac inhibits ocular neovascularization. Invest Ophthalmol Vis Sci 2003;44:409–15. [DOI] [PubMed] [Google Scholar]
- 17.Oshima Y, Deering T, Oshima S, et al. Angiopoietin-2 enhances retinal vessel sensitivity to vascular endothelial growth factor. J Cell Physiol 2004;199:412–17. [DOI] [PubMed] [Google Scholar]
- 18.Nambu H, Nambu R, Oshima Y, et al. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther 2004;11:865–73. [DOI] [PubMed] [Google Scholar]
- 19.Saishin Y, Takahashi K, Seo MS, et al. The kinase inhibitor PKC412 suppresses epiretinal membrane formation and retinal detachment in mice with proliferative retinopathies. Invest Ophthalmol Vis Sci 2003;44:3656–62. [DOI] [PubMed] [Google Scholar]
- 20.Nie Z, Chen S, Kumar R, et al. RER, an evolutionarily conserved sequence upstream of the rhodopsin gene, has enhancer activity. J Biol Chem 1996;271:2667–75. [DOI] [PubMed] [Google Scholar]
- 21.Fortini ME, Rubin GM. Analysis of cis-acting requirements of the Rh3 and Rh4 genes reveals a bipartite organization to rhodopsin promoters in Drosophila melanogaster. Genes Dev 1990;4:444–63. [DOI] [PubMed] [Google Scholar]
- 22.Zack DJ, Bennett J, Wang Y, et al. Unusual topography of bovine rhodopsin promoter-lacZ fusion gene expression in transgenic mouse retinas. Neuron 1991;6:187–99. [DOI] [PubMed] [Google Scholar]
- 23.May LA, Lai CM, Rakoczy PE. In vitro comparison studies of truncated rhodopsin promoter fragments from various species in human cell lines. Clin Experiment Ophthalmol 2003;31:445–50. [DOI] [PubMed] [Google Scholar]
- 24.Chavand O, Spilsbury K, Rakoczy PE. Addition of a c-myc epitope tag within the VEGF protein does not affect in vitro biological activity. Biochem Cell Biol 2001;79:107–12. [PubMed] [Google Scholar]
- 25.Shen WY, Garrett KL, da Cruz L, et al. Dynamics of phosphorothioate oligonucleotides in normal and laser photocoagulated retina. Br J Ophthalmol 1999;83:852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diabetic Retinopathy Study. Report Number 6. Design, methods, and baseline results. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Invest Ophthalmol Vis Sci 1981;21:1–226. [PubMed] [Google Scholar]
- 27.Hamanaka H, Katoh-Fukui Y, Suzuki K, et al. Altered cholesterol metabolism in human apolipoprotein E4 knock-in mice. Hum Mol Genet 2000;9:353–61. [DOI] [PubMed] [Google Scholar]
- 28.Tolentino MJ, McLeod DS, Taomoto M, et al. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol 2002;133:373–85. [DOI] [PubMed] [Google Scholar]
- 29.Lu M, Perez VL, Ma N, et al. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci 1999;40:1808–12. [PubMed] [Google Scholar]
- 30.Robison WG Jr, Laver NM, Lou MF. The role of aldose reductase in diabetic retinopathy: prevention and intervention studies. In: NN O, Chader GJ, eds. Progress in retinal and eye research. Oxford: Pergammon, 1995:593–640.
- 31.Amin RH, Frank RN, Kennedy A, et al. Vascular endothelial growth factor is present in glial cells of the retina and optic nerve of human subjects with nonproliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 1997;38:36–47. [PubMed] [Google Scholar]
- 32.Sone H, Kawakami Y, Okuda Y, et al. Ocular vascular endothelial growth factor levels in diabetic rats are elevated before observable retinal proliferative changes. Diabetologia 1997;40:726–30. [DOI] [PubMed] [Google Scholar]
- 33.Schwesinger C, Yee C, Rohan RM, et al. Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am J Pathol 2001;158:1161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oshima Y, Oshima S, Nambu H, et al. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J Cell Physiol 2004;201:393–400. [DOI] [PubMed] [Google Scholar]
- 35.Spilsbury K, Garrett KL, Shen WY, et al. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol 2000;157:135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang F, Rendahl KG, Manning WC, et al. AAV-mediated expression of vascular endothelial growth factor induces choroidal neovascularization in rat. Invest Ophthalmol Vis Sci 2003;44:781–90. [DOI] [PubMed] [Google Scholar]
- 37.Reynaud X, Dorey CK. Extraretinal neovascularization induced by hypoxic episodes in the neonatal rat. Invest Ophthalmol Vis Sci 1994;35:3169–77. [PubMed] [Google Scholar]
- 38.Rakoczy PE, Brankov M, Fonceca A, et al. Enhanced recombinant adeno-associated virus-mediated vascular endothelial growth factor expression in the adult mouse retina: a potential model for diabetic retinopathy. Diabetes 2003;52:857–63. [DOI] [PubMed] [Google Scholar]
- 39.Mori K, Duh E, Gehlbach P, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol 2001;188:253–63. [DOI] [PubMed] [Google Scholar]