Abstract
Aim: To assess intraocular pressure (IOP) changes of the contralateral eyes of eyes undergoing trabeculectomy with mitomycin C (MMC).
Methods: Non-comparative retrospective study of 24 consecutive patients who underwent trabeculectomy with MMC that led to more than 45% reduction in IOP. In the contralateral eyes, IOP before surgery was compared with IOP 1 day and 1 month after surgery. 11 fellow eyes were under topical hypotensive therapy while 13 contralateral eyes were not (12 contralateral eyes had previous filtering surgery and one had normal tension glaucoma). No patients had systemic ocular hypotensive therapy.
Results: Mean IOP in all contralateral eyes decreased from 15.5 (SD 5.5) mm Hg to 12.5 (3.8) mm Hg (p<0.01), and 13.0 (4.7) mm Hg (p<0.001) 1 day and 1 month after surgery, respectively. In the 11 fellow eyes under topical ocular hypotensive therapy mean IOP was reduced from 19.5 (4.0) mm Hg to 13.5 (2.2) mm Hg (p<0.01), and 16.5 (2.8) mm Hg (p<0.05) 1 day and 1 month after surgery, respectively. In the 13 fellow eyes not under topical ocular hypotensive therapy mean IOP was reduced from 12.1 (4.2) mm Hg to 11.6 (4.7) mm Hg (p not significant) and 9.8 (3.8) mm Hg (p0.01) 1 day and 1 month after surgery, respectively.
Conclusions: In the present population, a month after trabeculectomy, mean IOP in the contralateral eyes decreased independently of whether these contralateral eyes were undergoing topical ocular hypotensive therapy or not.
Keywords: intraocular pressure, mitomycin C, trabeculectomy
In 1924 Weekers,1 to describe changes of intraocular pressure (IOP) in contralateral eyes, coined the term “ophthalmotonic consensual reaction.” Since then many studies have reported how an fall in IOP in one eye occurs in the other eye too; such an effect seems to occur after ocular compression, tonography, trauma, cauterisation of the sclera, paracentesis, laser trabeculoplasty as well as fistulising surgery.2–6
In contrast with many previously published reports,2–6 it has recently been reported7 that after trabeculectomy, the IOP or the aqueous humour inflow was increased in the contralateral eyes. The purpose of this study was to assess IOP changes in the contralateral eyes, 1 day and 1 month after trabeculectomy.
PATIENTS AND METHODS
The medical charts of 24 consecutive patients who underwent trabeculectomy with mitomycin C (MMC) performed by the same surgeon (IOH) between 1995 and 2000 at the University Eye Clinic, Basel, were retrospectively reviewed. Inclusion criteria for this study were IOP reduction in the operated eye of more than 45%. No patients were undergoing systemic hypotensive therapy. In these patients the contralateral eyes were divided into two groups, a first group of 11 fellow eyes that were undergoing topical ocular hypotensive therapy and a second group of 13 fellow eyes that were not. Among these 13 fellow eyes that had no topical ocular hypotensive therapy, 12 had previously had a trabeculectomy with MMC and one had normal tension glaucoma that did not need ocular hypotensive treatment.
The IOP in each fellow eye was recorded 1 day before surgery, as well as 1 day and 1 month after surgery. Data are presented as a means (SD). Statistical analysis of the data was performed using paired Student’s t test with a p value lower or equal to 0.05 considered to be statistically significant.
RESULTS
Demographic details of the present population and the type of glaucoma affecting each eye are shown in the tables 1 and 2, respectively. The treatment of fellow eye before and during this study is presented in table 3.
Table 1.
Demographic data of 24 patients
| Characteristics | No |
| Eyes | 24 |
| Patients | 24 |
| Sex | |
| Female | 11 (46%) |
| Male | 13 (54%) |
| Race | |
| White | 24 (100%) |
| Age | |
| Average (SD) | 72.0 (11.8) (43–90) |
| Eye | |
| Right | 9 (38%) |
| Left | 15 (52%) |
Table 2.
Type of glaucoma affecting each eye
| Type of glaucoma | No (%) eyes |
| Primary open angle | 18 (75) |
| Pseudoexfoliation | 5 (21) |
| Normal tension | 1 (4) |
| Total | 24 eyes |
Table 3.
Treatment of contralateral eyes
| Treatment | No of eyes (%) |
| Local hypotensive drops | 11 (45.8) |
| β blockers | 2 (8.3) |
| Dorzolamide | 1 (4.2) |
| Latanoprost | 2 (8.3) |
| β blockers + pilocarpine | 1 (4.2) |
| β blockers + dorzolamide | 2 (8.3) |
| β blockers + pilocarpine + dorzolamide | 1 (4.2) |
| β blockers + pilocarpine + latanoprost | 1 (4.2) |
| β blockers + pilocarpine + dorzolamide + latanoprost | 1 (4.2) |
| No local hypotensive drops | 13 (54.2) |
| Without glaucoma surgery | 1 (4.2) |
| Glaucoma surgery | 12 (50.0) |
Average of time after surgery 13.1 (24.4) months.
Mean IOP in the 24 fellow eyes was decreased after surgery from 15.5 (5.5) mm Hg to 12.5 (3.8) mm Hg (p<0.01) and 13.0 (4.7) mm Hg (p<0.001) 1 day, and 1 month after surgery, respectively (fig 1).
Figure 1.
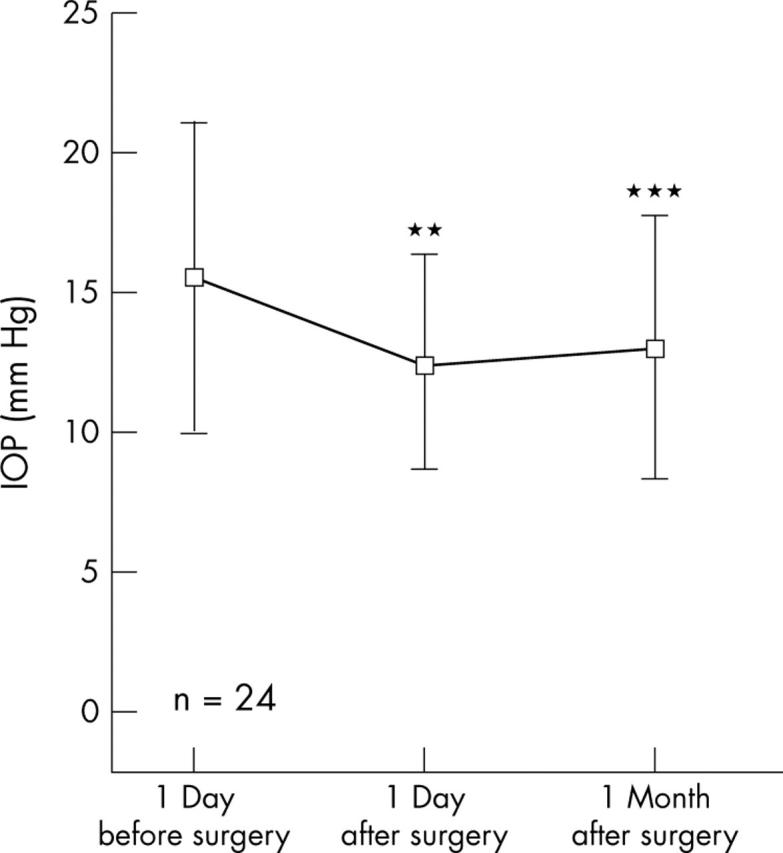
Mean IOP in the 24 fellow eyes.
In the 11 fellow eyes under topical ocular hypotensive therapy, after surgery mean IOP was reduced from 19.5 (4.0) mm Hg to 13.5 (2.2) mm Hg (p<0.01) and 16.5 (2.8) mm Hg (p<0.05) 1 day and 1 month after surgery, respectively (fig 2). In the 13 fellow eyes not under topical ocular hypotensive therapy, after surgery, mean IOP was reduced from 12.1 (4.2) mm Hg to 11.6 (4.7) mm Hg (p not significant) and 9.8 (3.8) mm Hg (p<0.01) 1 day and 1 month after surgery, respectively (fig 3).
Figure 2.
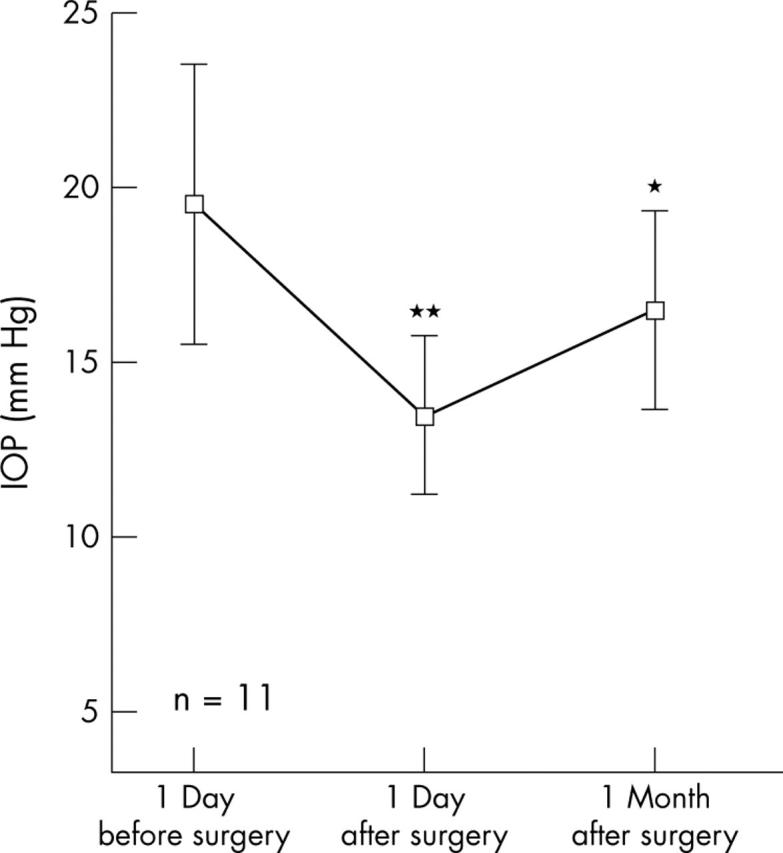
In the 11 fellow eyes under topical ocular hypotensive therapy, IOP reduction was 4 mm Hg after 1 day (p<0.01) and 3 mm Hg after 1 month (p<0.05) after surgery.
Figure 3.
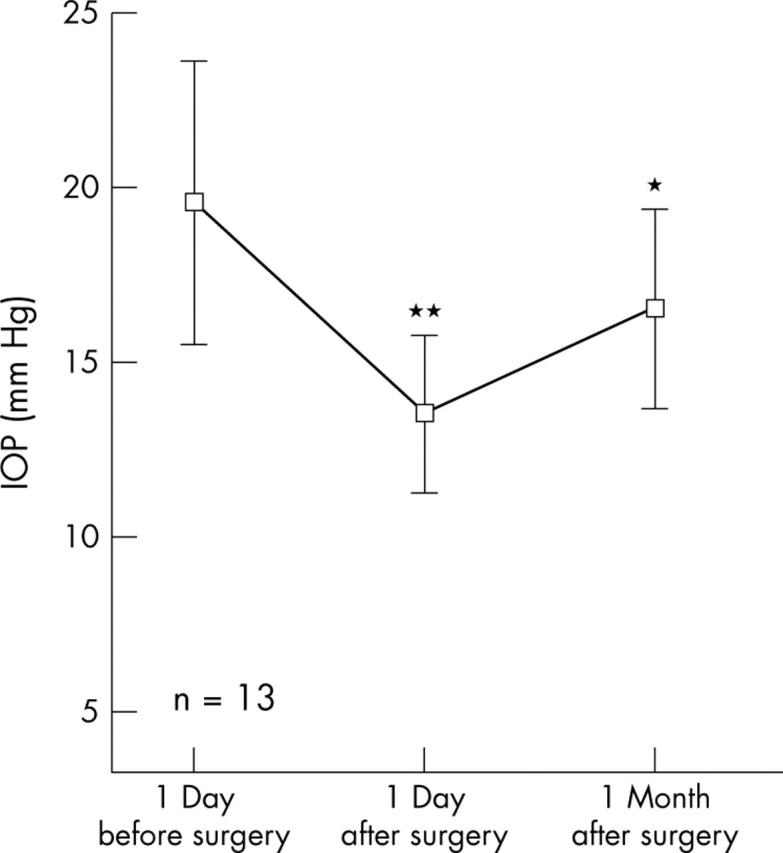
In the 13 fellow eyes not under topical ocular hypotensive therapy, IOP reduction was 0.5 mm Hg after 1 day (p, not significant) and 2.3 mm Hg after 1 month (p<0.01) after surgery.
DISCUSSION
This study indicates that 1 month after trabeculectomy with MMC the mean IOP significantly decreased in the contralateral eyes independently of whether they were undergoing topical hypotensive treatment or not. These findings seem to indicate that this is probably not because of improved compliance after surgery.
The IOP changes in contralateral eyes have been described by many investigators after ocular compression, tonography, trauma, cauterisation of the sclera, and paracentesis.3–5 Topical ocular hypotensive medications have been shown to cause a fall in IOP after unilateral application in the fellow, untreated eye. This has been described as an ophthalmotonic consensual reaction (OCR)1 and the potential sympathetic influences have been studied.8,9 However, this reduction had also been attributed to an effect caused by systemic absorption of the drug via the nasolacrimal pathways.10 It was observed that in eyes of patients with Horner’s syndrome OCR was less pronounced than in normal eyes.11 Cox and co-workers12 described an ablation of experimental animal eyes in which there was a rise in IOP in response to water drinking after unilateral optic nerve sections. They concluded that their results support the hypothesis of a supraoptic nuclear control mechanism of IOP.
Wilmer6 noted in experimental animals a fall in IOP in one eye after a fistulisation operation on the other eye. Also for short period of time the decrease in IOP after trabeculectomy was observed in human fellow eyes.
Diestelhorst and Krieglstein10 studied the effect of trabeculectomy on the aqueous humour flow of the unoperated fellow eye, using computerised anterior chamber fluorophotometry. Aqueous humour flow was measured in these eyes before surgery and on the fifth day after trabeculectomy. The average postoperative flow in the fellow eye increased from 2.56 μl/min to 2.9 μl/min (p<0.05%). They concluded that filtration surgery in one eye triggers a CNS mediated, reflective increase in aqueous flow to maintain physiological stability in the anterior chamber of the surgically treated eye. They argued that their results prove the existence of an ocular CNS reflex responsible for the regulation of IOP homeostasis, and that since this CNS reflex on aqueous humour dynamics affects both eyes, this would explain their clinical observation of IOP dysregulation in the unoperated fellow eye following unilateral trabeculectomy.
Regarding IOP in the unoperated fellow eyes, they reported no statistically significant change from the preoperative level. Statistical analysis did not show a significant correlation between IOP changes and the increase in aqueous humour flow observed in the unoperated fellow eyes. This seems to imply that the facility of outflow was sufficient to compensate for the increase in aqueous humour flow.
In contrast, a recent publication7 reported that the mean IOP significantly increased after trabeculectomy in the unoperated fellow eyes. The same study reported a preoperative IOP in the operated eye of 37.0 (SD 10.0) mm Hg. The authors report that they have not used any preoperative systemic ocular hypotensive therapy, and unfortunately they do not comment on how they were managing patients with such a high mean IOP preoperatively. As mentioned before, this report contrasts with previous literature reporting, for decades, on the tendency for a decrease in IOP in the contralateral eye.
A decrease of IOP in fellow eye after argon laser trabeculoplasty was also reported.2 Such reduction of IOP in fellow eye was theoretically explained by a better compliance of patients after intervention, when the patient becomes more conscientious about taking his medications. In this study such an explanation could probably be excluded because a significant IOP decrease 1 month after surgery was also observed in the eyes without any local hypotensive treatment. In other words, our study not only confirms previously published literature on the significant decrease in IOP in the contralateral eye but shows that there is a significant reduction irrespective of whether the patient was on topical ocular hypotensive medications or not. Our study results might lend more evidence to the theory of the existence of an ophthalmotonic consensual reaction. Further studies are needed to prove or disprove this mechanism, and to find ways to manipulate this reaction to the benefit of our patients.
Abbreviations
IOP, intraocular pressure
MMC, mitomycin C
REFERENCES
- 1.Weekers L. Modification expérimentales de l’ophtalmotonous. Reaction ophtalmotonique consenuelle. Arch Opthalmol (Paris) 1924;41:641–58. [Google Scholar]
- 2.Brooks AMV, West RH, Gillies. Argon laser trabeculoplasty five years on. Aust N Z J Ophthalmol 1988;16:343–51. [DOI] [PubMed] [Google Scholar]
- 3.Nagata N, Kurimoto S, Matsuka M. A study of consensual ophthalmotonic reaction. Acta Soc Ophthalmol Japan 1954;58:38–46. [Google Scholar]
- 4.Prijot EL, Stone HH. A study of consensual ophthalmotonic reaction. Am J Ophthalmol 1956;42:50–8. [DOI] [PubMed] [Google Scholar]
- 5.Stocker FW. On changes in intraocular pressure after application of the tonometer. Am J Ophthalmol 1958;45:192–6. [DOI] [PubMed] [Google Scholar]
- 6.Wilmer WE. Discussion on the results of operative treatment of glaucoma. Trans Ophthalmol Soc UK 1927;47:230–3. [Google Scholar]
- 7.Yarangümeli A, özlem Köz, Gülcan Kural. The effect of trabeculectomy on the intraocular pressure of the unoperated fellow eye. J Glaucoma 2003;12:108–13. [DOI] [PubMed] [Google Scholar]
- 8.Langham ME, Diggs EM. β-Adrenergic responses in the eyes of rabbites, primates and man. Exp Eye Res 1974;19:281–7. [DOI] [PubMed] [Google Scholar]
- 9.Radius RL, Diamond GR, Pollack IP, et al. A new drug for management of chronic simple glaucoma. Arch Ophthalmol 1978;96:1003–7. [DOI] [PubMed] [Google Scholar]
- 10.Diestelhorst M, Krieglstein G. The effect of trabeculkectomy on the aqueous humor flow of the unoperated fellow eye. Graefes Arch Clin Exp Ophthalmol 1991;229:274–6. [DOI] [PubMed] [Google Scholar]
- 11.Ten Tusscher MP, Beckers HJ, Vrensen GF, et al. Peripheral neural circuits regulating IOP? A review of its anatomical backbone. Doc Ophthalmol 1994;87:291–313. [DOI] [PubMed] [Google Scholar]
- 12.Cox CE, Fitzgerald CR, King RL. A preliminary report on the supraoptic nucleus and control of intraocular pressure. Invest Ophthalmol 1975;14:26–8. [PubMed] [Google Scholar]


