Abstract
Aims: To document loss of central field in patients with scars from toxoplasmic retinochoroiditis close to the disc after resolution of disease.
Methods: Patients with a clinical diagnosis of toxoplasmic retinochoroiditis were enrolled from four centres. Automated central visual field testing was performed when their disease had settled and retinal photographs of the lesions were taken. The type of central field defect (whether absolute or relative) and whether it broke out to the periphery were correlated with the size of the retinochoroidal scar and its proximity to the optic nerve head.
Results: 69 eyes were enrolled; 16 (26%) were discarded because of poor field performance. Of the 53 remaining eyes, 31 showed absolute defects and 20 relative defects. Scars within one disc diameter of the disc were more likely to be associated with absolute defects breaking out to the periphery.
Conclusion: The scarring induced by toxoplasmic retinochoroiditis is associated with considerable field loss when it occurs close to the optic nerve head. Current treatment is unlikely to ameliorate this situation. The degree of visual field loss should be an outcome measure for future trials of the efficacy of treatment trials for the disease.
Keywords: visual fields, toxoplasma, retinochoroiditis
Toxoplasmic retinochoroiditis classically presents as a focus of new retinitis adjacent to an old chorioretinal scar. After about 6 weeks, the focus of active retinitis slowly fades leaving a further, larger scar in its place, with resolution of other intraocular inflammatory signs.1 Whether this process of healing can be accelerated by the use of antibiotics and/or corticosteroids has not been established through formal clinical trials.2 Despite this, the main indications for treatment are the presence of fresh retinitis within the macular arcade or an active lesion lying within one disc diameter of the optic nerve head.3–5 In this latter situation, it has been hypothesised that the necrotising retinitis leads to full thickness damage, with involvement of both photoreceptors and second order neurons as well as the nerve fibre layer itself. If this were the case then the resulting scotoma would not only be absolute in the area of photoreceptor destruction, but would also break out to the periphery because of interruption of distal signalling. Similarly, the closer the damage is to the disc, the greater might be the resultant scotoma because of greater involvement of the nerve fibre layer.
Evidence supporting this hypothesis is sparse. Several textbooks on visual fields describe a typical scotoma with breakout to the periphery and this undoubtedly occurs in some cases.6 However, since there has been no systematic examination of the visual fields after attacks of disease have settled, we undertook a prospective study of patients presenting with new toxoplasmic retinochoroiditis and measured their fields by automated perimetry when their disease was clinically quiet.
METHODS
Study design
A prospective, cross sectional, observational study of consecutive patients presenting to uveitis clinics with a diagnosis of toxoplasmic retinochoroiditis. Patients were derived from clinics in the United Kingdom (St Thomas’s Hospital and Moorfields, London), Brazil (Federal University of Minas Gerais, Belo Horizonte), and Mauritius (Subramania Bharati Eye Hospital, Moka). The study received ethical permission from the local ethics committee of Moorfields Eye Hospital.
Inclusion criteria
An active or inactive retinochoroidal scar consistent with toxoplasmic retinochoroiditis was present in any position in the fundus. Wherever possible, anti-toxoplasma antibodies were detected by serological testing. Where active disease was present, this was managed appropriately and visual field testing was not undertaken until the lesion was considered resolved and the ocular media had cleared (less than 1+ vitreous cells or BINO score less than 1+7). Historical patients were recalled for this study.
Exclusion criteria
Patients who were unwilling or unable to perform visual field testing (see below) were excluded, as well as any patient with known ocular/neurological pathology giving rise to visual field loss.
Visual field testing
Visual field testing was undertaken in a number of ways depending on the clinic attended. In the United Kingdom, the central visual field was recorded using the Humphrey 24/2 FastPac strategy or if unavailable a 24/2 SITA fast or SITA standard strategy. The right eye was tested first and then the left eye. Wherever possible the test was repeated at least 4 weeks apart at a second visit to allow for the learning curve. In Brazil, the central field was recorded using the Octopus 1-2-3, CT1 dynamic standard and in Mauritius, the Kowa AP 3000 automated perimeter, precision strategy was used. Specifically excluded were patients who had more than 33% fixation losses, more than 20% false positive, or more than 20% false negative responses. Details of full ophthalmological investigation were prospectively recorded and dilated fundus photographs included for subsequent analysis.
Analysis of data
Field loss was assessed independently and scotomata were coded as absolute or relative; whether these broke out to the periphery was also determined. The mean defect was recorded where possible. Further analysis involved stratification of field loss and comparison with (a) the size of the scar, and (b) the position of the scar in relation to the optic disc derived from retinal photographs and drawings.
RESULTS
Fields were recorded and photographs taken in 69 eyes. Of these, 16 (23.2%) were excluded because of low quality of field. This subgroup was analysed to see if there were any differences between those excluded and those included. The excluded eyes did not differ significantly in terms of age, sex, race, visual acuity, type of field defect, size of lesion, or distance of lesion from the disc. The remaining cohort of 53 eyes (29 R, 24 L) comprised 26 male, 22 female, mean age 30.7 years (range 12–69). These were divided among four main ethnic groups—Afro-Caribbean 14 (26.4%), white 13 (24.5%), Asian Indian (Mauritian) 12 (22.6%), and Latin American 14 (26.4%).
Of the 53 eyes in the study, 39 (73.6%) had a Snellen acuity of better than 6/12; 11 (20.8%) fell between 6/12 and 6/60, and three (5.7%) had an acuity of worse than 6/60; 51 eyes had a demonstrable central field defect. Absolute defects were seen in 31 eyes (60.8%), of which 18 (58.1%) showed breakout to the periphery (figs 1 and 2), the remaining 13 (41.9%) remaining confined to the lesion. Relative field defects were detected in 20 (39.2%) eyes. Neither the size of the scar nor its distance from the disc correlated with the type of field defect present.
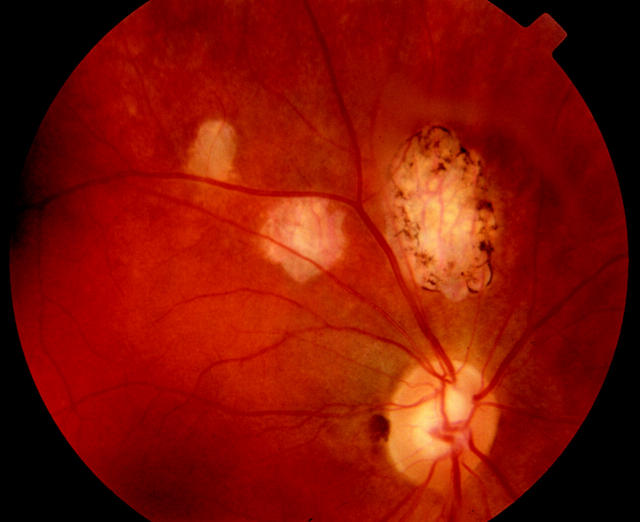
Figure 1.
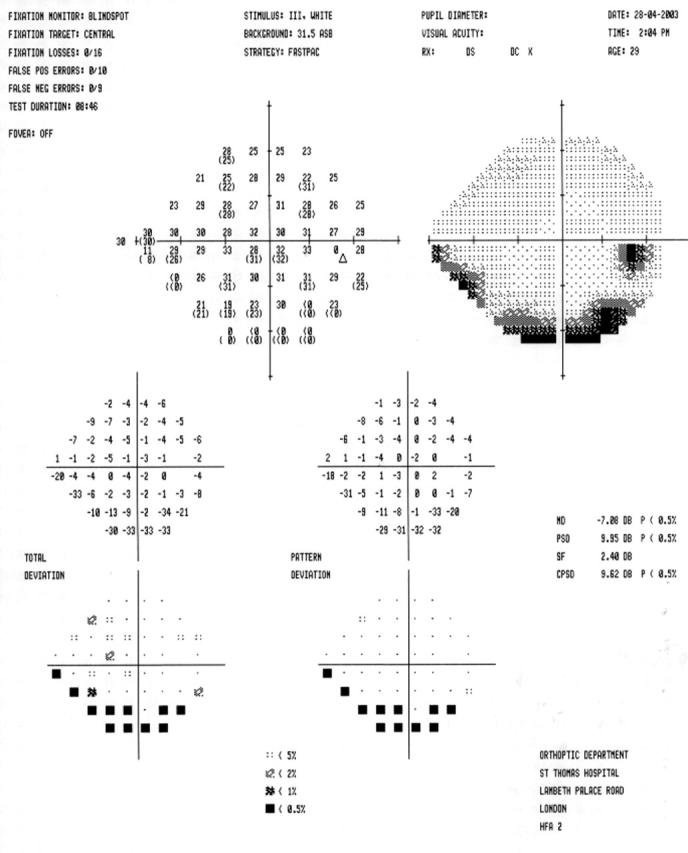
A FASTPAC Humphrey 24/2 visual field of the right eye.
Figure 2.
Colour fundus photograph of the right eye showing the area of retina with old toxoplasma scars. In this case there was an absolute defect with breakout to the periphery.
The characteristics of the field defect in relation to size of lesion are shown in table 1. It may be seen that there is no difference in whether the defect was relative or absolute in relation to the size of the lesion. For all patients where the mean defect was recorded (40), 22 had an absolute scotoma and 18 a relative one. For absolute scotomata, the average mean defect was −8.08 dB (−1.80 to −18.61, SD 4.26). For relative scotomata the average mean defect was −3.96 dB (−1.10 to −9.20, SD 2.22) (p = 0.0007).
Table 1.
Characteristics of field defect by size of lesion
| Size type of defect | 2 DD and below | >2 DD |
| Absolute | 21 | 10 |
| Relative | 12 | 8 |
| Nil | 1 | 1 |
DD, disc diameter.
An analysis of field defects by position of lesion in relation to the optic disc is shown in table 2. Lesions within one disc diameter of the optic disc were far more likely to be associated with an absolute defect with breakout to the periphery than lesions further away.
Table 2.
Analysis of field defects by position of lesion in relation to the optic disc
| Absolute | Relative | Nil | ||
| With breakout | No breakout | |||
| Within 1 DD | 9 (75.0%) | 1 (8.3%) | 2 (16.7%) | 0 (0%) |
| Outside 1 DD | 9 (22.0%) | 12 (29.3%) | 18 (43.9%) | 2 (4.9%) |
DD, disc diameter.
For lesions within one disc diameter of the disc, the average mean defect, where recorded was −8.44 dB (−4.36 to −18.61, SD 4.26). For lesions outside one disc diameter from the disc, the mean defect, where recorded was −5.39 dB (−1.10 to −13.42, SD 3.67: p = 0.03).
DISCUSSION
Visual field loss arising as a result of toxoplasma retinochoroiditis, particularly when the focus of inflammation is within one disc diameter of the optic disc is poorly documented in the literature. Despite this, lesions occurring in this position are an indication for systemic treatment,4,5 although the benefit of this treatment has never been formally evaluated in terms of subsequent loss of field. Anecdotal evidence suggests that where such field loss occurs, it is likely to be absolute since all layers of the retina are involved in the inflammation induced by toxoplasmic retinochoroiditis.
In this study we have shown that absolute field defects were seen in 31 eyes. In approximately half of these there was breakout to the periphery, but in the other half the field defect remained localised to correspond to the area of the scar. There was no difference in the size of the scar with respect to whether defects were absolute or relative; however, absolute defects occurred when the scar was close to the optic nerve head. As might be expected the average mean defect was more for absolute compared to relative defects. Almost all (9/10) scars within one disc diameter of the optic nerve head gave rise to absolute defects with breakout.
Formal testing of the visual fields following attacks of toxoplasma retinochoroiditis has rarely been reported before. In a retrospective consecutive case series, Schlaegel reported the Goldmann field findings in 60 eyes8; 35% showed a field defect within 5° of fixation, with 27% being paracentral (from 6° to 13°), and 38% being peripheral. Analysis of whether the field defect was absolute or relative or whether it broke out to the periphery was not reported, and many of the eyes had active uveitis at the time of inclusion.
The present study may include biases because of patient selection. For instance, not every patient had two field tests because of the constraints of time/location. This may have contributed to the large number (25%) of fields that had to be rejected, although analysis of the rejected patients showed no difference in the location of scars or their size compared to the included fields. Secondly, there may have been selection bias since patients with more visually disabling disease tend to remain in clinics. Finally, there may also have been recall bias in the patients selected. Despite the prospective nature of this study, patients could be included from historical data sets, and patients with less disabling disease may have been lost to follow up.
The results of this study suggest that toxoplasmic retinochoroidal scars close to the optic nerve head are associated with absolute field defects with breakout to the periphery. For lesions further away from the disc, the prevalence of absolute defects with breakout reduced and lesions were more likely to produce relative defects. This may imply less destructive disease in this location (that is, the disease process may have only affected the outer retinal layers), but it is more likely that this is because of the larger receptor field for distal ganglion cells.
In summary, our study has confirmed that retinochoroidal scars lying within one disc diameter of the optic nerve head are likely to cause absolute defects with breakout to the periphery and should still be considered an absolute indication for treatment. Furthermore, visual field testing should form a routine part of the clinical assessment of patients when disease has settled and should certainly be included as a tool for the measurement of treatment efficacy in future placebo controlled studies, particularly where such loss may have functional consequences for the patient.
Acknowledgments
Supported in part by the Iris Fund for the Prevention of Blindness. The authors would like to thank Professor F Orefice (Brazil) and Dr I Gaya (Mauritius) for allowing us to study their patients.
Competing interests: none declared
REFERENCES
- 1.Rothova A. Ocular manifestations of toxoplasmosis. Curr Opin Ophthalmol 2003;14:384–8. [DOI] [PubMed] [Google Scholar]
- 2.Stanford MR, See SE, Jones LV, Gilbert RE. Antibiotics for toxoplasmic retinochoroiditis. An evidence-based systematic review. Ophthalmology 2003;110:926–32. [DOI] [PubMed] [Google Scholar]
- 3.Holland GN, O’Connor GR, Belfort R, et al. Toxoplasmosis. In: Pepose JS, Holland GN, Wilhelmus KR, eds. Ocular infection and immunity. St Louis: Mosby Year Book, 1996:1210.
- 4.Engstrom R, Holland GN, Nussenblatt RB, et al. Current practices in the management of ocular toxoplasmosis. Am J Ophthalmol 1991;111:601–10. [DOI] [PubMed] [Google Scholar]
- 5.Holland GN, Kewis KG. An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol 2002;134:102–14. [DOI] [PubMed] [Google Scholar]
- 6.Lachenmayr BJ, Vivell PMO, eds. Perimetry and its clinical correlates. Stuttgart: Georg Thienne Verlag, 1993:113–15.
- 7.Nussenblatt RB, Palestine A, Chan CC, et al. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 1985;92:467–71. [DOI] [PubMed] [Google Scholar]
- 8.Schlaegel TF, Weber JC. The macula in ocular toxoplasmosis. Arch Ophthalmol 1984;102:697–8. [DOI] [PubMed] [Google Scholar]