Conjunctival imprint cytology (CIC) offers valuable clues to the diagnosis and study of the pathogenesis of conjunctival disorders.1–3 The technique involves the use of a membrane filter paper to pick up a layer of cells from the conjunctival surface.
This study was conducted to evaluate the results of CIC using a nylon filter paper compared to routinely used cellulose acetate paper. It involved 20 normal asymptomatic eyes of 10 participants. The participants had no ocular complaints and they were evaluated to rule out any conjunctival disease.
The procedure was explained to the participants and their consent was given.
CIC was done to assess the normal conjunctival cytology using Ultipor (nylon6, 6) and sartorius-type 111 (cellulose acetate paper).
The physical properties such as pore size and thickness of the two papers were matched.
Technique
Cellulose acetate and nylon membrane filters were cut into small triangles and squares respectively to make their identification easy after staining. The conjunctiva was anaesthetised by topical 4% xylocaine. The filter paper was applied to the bulbar conjunctiva with blunt forceps. Gentle pressure was applied for 3–5 seconds and the paper was removed in a peeling motion. It was fixed thereafter in 95% ethanol and stained with either haematoxylin and eosin (H&E) or periodic acid Schiff (PAS) and haematoxylin stains.
The filter papers after staining were cleared in acetone and xylene, mounted in DPX and viewed under the light microscope. The morphology of epithelial cells in H&E stain and number of goblet cells in PAS stain were noted.
Results
The participants involved in this study were in age group 22–37 years. A few initial slides were discarded because of overstaining. The time required to stain the filter papers compared to any other fixed tissue is lessened, and staining time is reduced to half with nylon paper compared with cellulose acetate paper.
Average time required for staining nylon and cellulose acetate paper was 20 minutes and 35 minutes, respectively, for PAS staining and with H&E stain it was 5 minutes and 10 minutes, respectively.
The specimens revealed sheets of small round epithelial cells in H&E stained nylon paper (fig 1A) and cellulose acetate paper (fig 1B).
Figure 1.
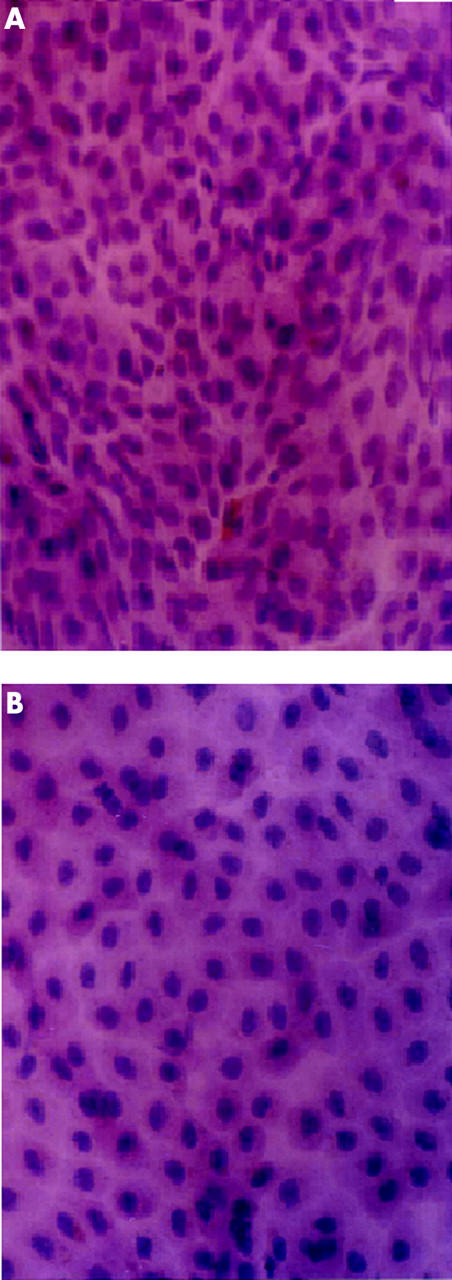
(A) Haematoxylin and eosin stained nylon paper at 40× magnification showing sheets of epithelial cells. (B) Haematoxylin and eosin stained cellulose acetate paper at 40× magnification revealing sheets of small round epithelial cells.
Additional plump, oval, deeply pink PAS positive goblet cells amidst PAS negative cohesive sheet of epithelial cells were seen in schiff stained specimens on nylon paper. (fig 2).
Figure 2.
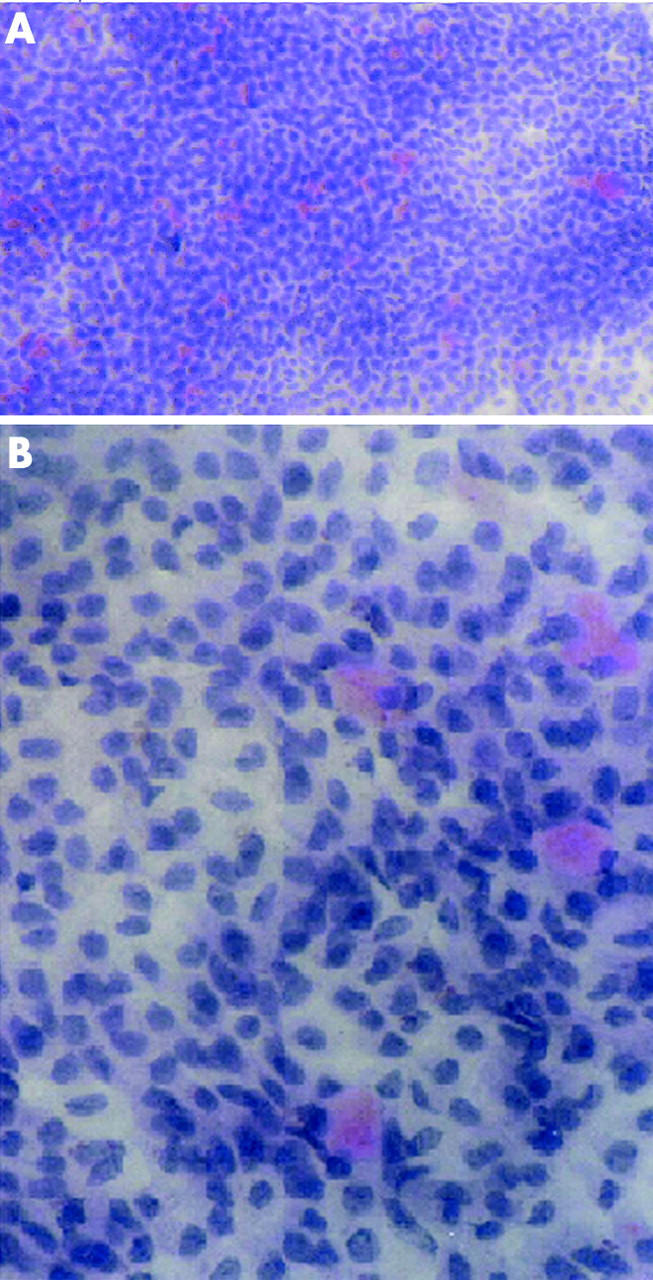
Periodic acid Schiff (PAS) stained nylon paper at 10× and 40× magnification showing plump, oval, deeply pink PAS positive goblet cells amidst PAS negative cohesive sheet of epithelial cells.
The cell layer varies from one to several cells thick with occasional gaps where no cells adhere to the membrane filter. Cellulose acetate paper revealed a single layered sheet but the Ultipor showed that there were multiple layers in most places.
Occasionally the cells were not picked up or they were clumped so as to be visible as layers. This was seen equally with both the filter papers.
Cells were collected on nylon paper even in presence of lacrimation during the procedure. The cell morphology of specimens collected on either of the filter papers was comparable.
Comment
CIC has been in use, as diagnostic tool since 1978, when Egbert first demonstrated its successful use with absorbent filter paper.4 Before this Thatcher used a plastic device to collect the epithelia.5 Since then membrane filters like cellulose acetate have been widely used for this technique.6,7
The filtration membrane is a thin, polymeric film made up of microscopic pores. They can be composed of variety of natural and synthetic materials like cellulose acetate and cellulose nitrate in the former category, and PTFE, PVDF, glass fibres, and nylon in latter.
In this study nylon and cellulose acetate were used for comparison of the results.
The nylon paper is more compatible with the organic solvents used in staining procedures. The adsorption is better with nylon then the cellulose acetate paper. Also there is a cost difference between the two, with cellulose acetate paper costing three times that of nylon.
The cytological features of epithelial as well as goblet cells were studied. The goblet cells are identified conclusively by the PAS positive cytoplasm or by their eccentrically placed nuclei and plump shape and large size. The epithelial cells are small and round with eosinophilic cytoplasm. The nuclei are large and basophilic.
Added benefit of nylon over cellulose acetate are:
Cost effective
Less staining time
Ability to collect cell even if lacrimation wets the paper
Comparable morphological results to cellulose acetate
Compatible with variety of solvents hence more stable
Deeper layers also picked, hence detailed evaluation of biopsy.
Acknowledgments
The authors acknowledge the assistance of Dr Krishna Mohan, Birla Institute of Science and Technology, for providing the filter papers.
References
- 1.Thygeson P . The cytology of conjunctival exudates. Am J Ophthalmol 1946;29:1499. [DOI] [PubMed] [Google Scholar]
- 2.Duszynski L . Cytology of the conjunctival sac. Amer J Ophthalmol 1954;37:576. [DOI] [PubMed] [Google Scholar]
- 3.Norn MS. Cytology of the conjunctival fluid. Acta Ophthalmol 1960;59 (suppl) :11. [DOI] [PubMed] [Google Scholar]
- 4.Egbert PR, Lauber S, Maurice DM. A simple conjunctival biopsy. Am J Ophthalmology 1977;84:798–801. [DOI] [PubMed] [Google Scholar]
- 5.Thatcher RW, Darougar S, Jones BR. Conjunctival impression cytology. Arch Ophthalmol 1977;95:678–81. [DOI] [PubMed] [Google Scholar]
- 6.Gadkari SS, Adrianwala SD, Prayag AS, et al. Conjunctival impression cytology—a study of normal conjunctiva. J Postgrad Med 1992;38:21–3. [PubMed] [Google Scholar]
- 7.Nelson JD, Havener VR, Cameron JD. Cellulose acetate impressions of the ocular surface. Dry eye states. Arch Ophthalmo1 1983;101:1869–72. [DOI] [PubMed] [Google Scholar]


