Abstract
Aim: Colourless solutions of mitomycin C (MMC) and 5-fluorouracil (5-FU) are widely used during trabeculectomy to inhibit postoperative scarring. The poor visibility of these agents on the eye has several drawbacks including the inability to accurately assess the area of treatment. This study examined the utility of using trypan blue dye to colour antimetabolites used during trabeculectomy and the effect of trypan blue on antimetabolite cytotoxicity in vitro.
Methods: For in vitro experiments, MMC (0.4 mg/ml) and 5-FU (25 mg/ml) were reconstituted with or without trypan blue. A lactate dehydrogenase release assay was used to measure drug induced cell death and viable cell number 7 days after treatment. For clinical assessment, trypan blue 0.1% was added to MMC and 5-FU to final concentrations of between 0.01% and 0.05%. The mixture was applied to Tenon’s capsule and sclera via pre-wet or into dry 5×8 mm sponges (MMC and 5-FU) for 3 minutes or by direct subconjunctival injection after completion of surgery (5-FU). Twenty two consecutive patients undergoing trabeculectomy either with or without trypan blue were followed for 2 years postoperatively.
Results: The addition of 0.05% trypan blue to MMC or 5-FU did not alter MMC induced cell death or the number of viable fibroblast in vitro. In vivo, trypan blue clearly delineated the antimetabolite treatment area and facilitated control of excess antimetabolite at the wound margins as well as sponge removal. With direct subconjunctival injection, total staining area varied for a given volume with location of the needle tip. Any leakage from the injection site could be easily seen. No adverse effects attributable to trypan blue were found in 2 years of follow up.
Conclusions: Trypan blue permits delineation of antimetabolite/tissue interactions without affecting cytoxicity for the assays investigated. Trypan blue can be used to visualise antimetabolite soaked sponges, estimate treatment area, and show areas of unintended tissue contact during trabeculectomy. The addition of trypan blue to antimetabolites has potential benefits in clinical, research, and teaching aspects of ocular surgery and therapy.
Keywords: glaucoma, trabeculectomy, antimetabolite, mitomycin, fluorouracil, trypan blue, Sydney, university
Topical antimetabolites such as mitomycin C (MMC) and 5-fluorouracil (5-FU) are widely used to reduce postoperative subconjunctival scarring after glaucoma filtration surgery. However the poor visibility of these drugs poses a fundamental difficulty in their safe and effective use. Fluorouracil is colourless and MMC has only a slightly bluish hue at concentrations above 0.2 mg/ml. As a consequence it is not possible to delineate the area of subconjunctival tissues exposed to these agents.
The area of antimetabolite treatment during trabeculectomy may play an important role in the risk of bleb related complications such as endophthalmitis.1 Larger MMC treatment areas have been associated with reduced bleb related complications.2 However MMC is toxic to both corneal and vascular endothelium3,4 and has been reported to induce limbal stem cell failure after topical use.5 MMC is usually delivered via a sponge. Fluorouracil can be delivered in the same way or as a subconjunctival injection. In either case the extent of treatment cannot be accurately measured but can only be inferred by the area of sponge used or tissue visibly raised by subconjunctival injection. As a result there is no consensus as to what represents a large treatment area. Using larger volumes of antimetabolite in an effort to increase treatment area increases the chances of unintended exposure to surrounding tissues.
A consequence of the poor visibility of 5-FU in ocular tissues is unrecognised contamination of the tear film after subconjunctival injection. As a result corneal epitheliopathy is relatively common and may limit its use in affected patients who require further antiscarring therapy.6–8 Inadvertent intracameral passage of 5-FU after a subconjunctival injection is not easily seen and may occur more frequently than is currently recognised.9 The ability to visualise these toxic agents would provide a significant advantage with respect to avoiding non-intentional exposure to ocular tissues.
Trypan blue is a vital dye used in laboratory based cellular research to differentiate viable cells, which exclude dye, from non-viable cells that are permissive to trypan blue. Reports of its clinical use in ophthalmology date back more than three decades.10 Trypan blue is used to stain the anterior capsule during cataract surgery (0.1% concentration)11 and delineate epiretinal membranes during peeling (0.06% concentration).12,13 In the USA trypan blue recently received Food and Drug Administration (FDA) approval to stain the anterior lens capsule during cataract surgery.
Although high concentrations of trypan blue on the retina may be toxic, currently used intraocular doses do not appear to be harmful.12–16 Trypan blue therefore is a potential candidate for improving visibility of MMC and 5-FU. A concern of reconstituting MMC and 5-FU with trypan blue is that this may affect the cytotoxic action of these agents.
The aims of this study were to determine whether addition of trypan blue to MMC and 5-FU altered the cytotoxicity of these drugs on cultured human Tenon’s capsule fibroblasts and to assess the utility of these mixtures in glaucoma surgery.
METHODS
The Tenets of the Declaration of Helsinki were followed and institutional ethics committee approval was granted. Informed consent was obtained before surgery for all patients.
Laboratory studies
Primary human Tenon’s capsule fibroblasts were propagated from explanted subconjunctival Tenon’s capsule isolated during glaucoma filtration surgery. Explanted tissue was anchored onto the bottom of a six well plate (Becton Dickinson, San Jose, CA, USA) with a sterile cover slip and overlaid with RPMI culture medium (Sigma, Poole, UK). Culture media were supplemented with L-glutamine 2MM and penicillin 100 000 units/l (all Gibco, Uxbridge, UK) and fetal calf serum (FCS; 10% of final volume; Gibco). Once the monolayers had reached confluence (around 2 weeks), the fibroblasts were passaged and cultured in 175 cm2 tissue culture flasks.
For experimentation, fibroblasts were trypsinised, seeded into 96 well tissue culture plates, and incubated overnight to permit attachment. MMC and 5-FU were applied at 0.4 mg/ml and 25 mg/ml (final concentration) respectively. Trypan blue (VisionBlue DORC, Zuidland, Holland) was added to a final concentration of 0.05%. All antimetabolites were reconstituted in serum-free culture medium.
Tenon’s fibroblasts were seeded at a concentration of 5000 fibroblasts per well into 96 well plates (Becton Dickinson) and incubated overnight. The fibroblast monolayers were then washed to remove serum and covered with a single application of MMC as described previously.17 Unless otherwise stated, the treatment time for all experiments was 5 minutes. Control fibroblasts were treated with a 5 minute application of serum-free RPMI with or without 0.05% trypan blue. Following treatment the monolayers were washed immediately three times and incubated in serum-free RPMI.
A lactate dehydrogenase (LDH) release assay was used to quantify fibroblast death as previously described.18 LDH is a stable cytoplasmic enzyme present in all cells. It is rapidly released in dying cells after damage to the plasma membrane. Lactate dehydrogenase catalyses the reduction of a colourless tetrazolium salt to coloured formazan that absorbs a broad spectrum of light with maximum absorbance around 492 nm. Lactate dehydrogenase activity is present in serum. Experiments were therefore performed in serum-free RPMI.
Human Tenon’s capsule fibroblasts seeded into 96 well plates were treated with a single 5 minute application of MMC or 5-FU with/or without additional trypan blue 0.05%, washed in PBS and incubated in 400 μl phenol red-free RPMI. After 84 hours (7 days) of incubation, 100 μl of supernatant was extracted from each well and placed into separate wells of a new 96 well plate (Becton Dickinson). 100 μl of catalyst solution (at 37°C) was added to each well and incubated for 15 minutes. Absorbance was measured with a microtitre plate reader using a 490–492 nm filter. Background absorbance was measured with wells containing phenol red-free RPMI only.
A modification of the LDH release assay was used to measure viable fibroblast numbers. This assay was based on the assumption that fibroblasts attached to the plastic well are viable as apoptotic/dead fibroblasts detach from the monolayer.19 To measure adherent fibroblast number, monolayers were gently washed twice in serum-free culture medium to remove dead cells. The residual monolayer was then covered with 300 μl of phenol red-free RPMI and 100 μl of 2% Triton-X. After 5 minutes, 100 μl of supernatant was removed and added to 100 μl of catalyst solution in a new flat bottomed 96 well plate. Absorbance was measured with a microtitre plate reader using a 490–492 nm filter.
For cell culture data, mean absorbance values were compared between treatment groups using a non-paired two tailed Student’s t test. A p value of less than 0.05 was deemed to be statistically significant.
Clinical studies
For clinical studies we used a commercially available preparation of trypan blue 0.1% (VisionBlue DORC). Study subjects for the case series were part of a larger prospective study examining wound healing in trabeculectomy. Of 22 consecutive antimetabolite trabeculectomies (22 eyes of 18 patients) performed by the authors, 11 eyes had trypan blue mixed with antimetabolite. All cases had a minimum of 2 years’ follow up. In all cases, the use of antimetabolites was planned before recruitment into the study. At the time of surgery, trypan blue was added to MMC or 5-FU. For MMC, the final concentration of trypan blue ranged between 0.01% and 0.05%. The final MMC concentration was that planned before surgery (between 0.2 mg/ml and 0.4 mg/ml). For 5-FU, a 0.01% trypan blue solution was used with a final 5-FU concentration 45 mg/ml.
The antimetabolite mixtures were used in the same way had the trypan blue not been present. MMC was applied to Tenon’s capsule and sclera via sponges cut from dry instrument wipe. In all cases 180 mm2 of sponge was used. This comprised four sponges measuring 5×8 mm, placed in the superotemporal and superonasal subconjunctival spaces and one 2.5×8 mm sponge, placed with its front edge over the posterior outline of a 4×4 mm square scleral flap. MMC was added to preplaced dry sponges via a fine syringe or applied presoaked. Treatment time was 3 minutes for all cases. After removal of the sponges, the treatment area was irrigated with 30 ml of balanced salt solution (BSS). The scleral trapdoor was closed with two 10-0 nylon adjustable sutures, which were manipulated as required postoperatively. The 5-FU solution was either applied via presoaked sponges using the MMC technique or by subconjunctival injection.
In a separate series of 10 eyes we added trypan blue to 5-FU for postoperative subconjunctival injections and observed its immediate effect.
RESULTS
In vitro studies
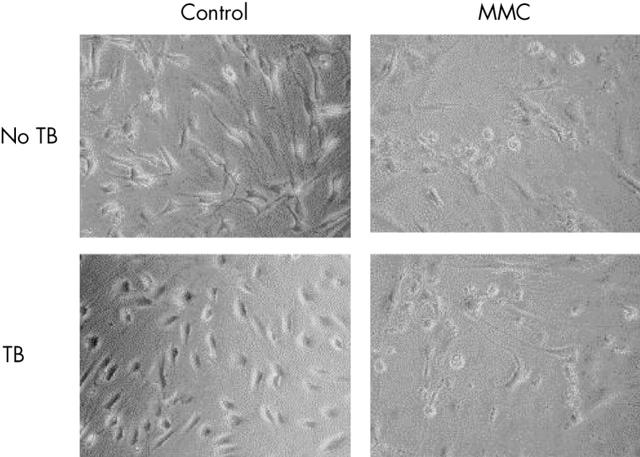
Single 5 minute applications of MMC (0.4 mg/ml) induced cell death in Tenon’s fibroblasts with a significant increase in LDH release compared with controls (p<0.01). Phase contrast microscopy revealed cell rounding and detachment from the cell culture plate suggestive of apoptosis as described previously18 (fig 1). Fluorouracil (25 mg/ml) treatment did not lead to a significant increase in LDH release (p = 0.82) indicating that 5-FU did not induce significant cell death. In addition, no morphological evidence of cell death was observed by day 7 with phase contrast microscopy in the control or 5-FU treated fibroblasts. Addition of trypan blue 0.05% did not alter spontaneous LDH release for any of the treatment groups (MMC p = 0.32, 5-FU p = 0.18, RPMI p = 0.12) (fig 2).
Figure 1.
Phase contrast micrographs showing control (left column) and mitomycin C (MMC) (0.4 mg/ml for 5 minutes) treated human Tenon’s fibroblasts 7 days after treatment. Evidence of cell rounding and detachment is visible after MMC treatment. The addition of trypan blue (TB) had no effect on attached fibroblast number for either treatment group.
Figure 2.
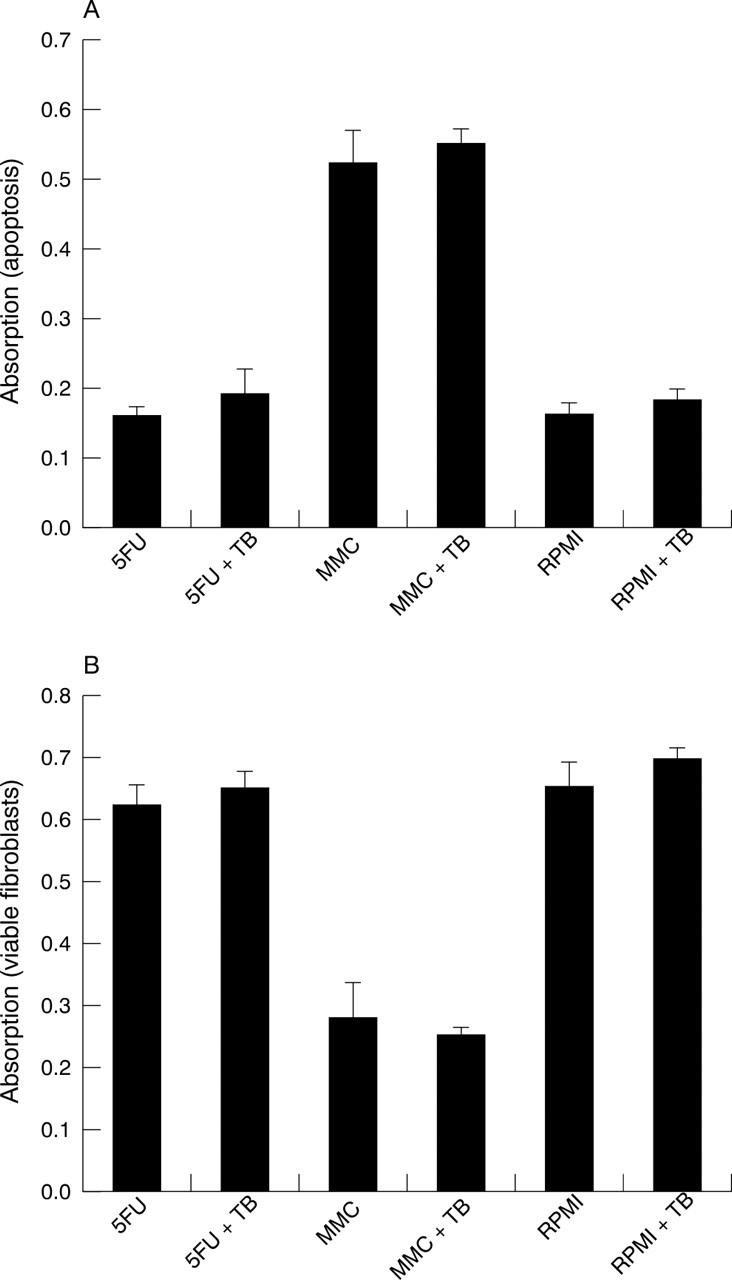
Lactate dehydrogenase release assay showing (A) fibroblast apoptosis and (B) viable fibroblast number 7 days after 5 minute applications of 5-fluorouracil (25 mg/ml) (5-FU), mitomycin C (0.4 mg/ml) (MMC), or RPMI culture medium (control) with or without trypan blue (TB). Addition of trypan blue had no effect on MMC induced apoptosis or viable cell number for any treatment group. Data shown are mean (standard deviation) values derived from a representative of two separate experiments performed in quadruplicate.
Viable fibroblast number was significantly reduced in the MMC treated fibroblasts compared with controls (p<0.01). Fluorouracil caused a small, reduction in viable fibroblast number compared with controls. This reduction reached statistical significance in only one of the two replicate experiments (p = 0.04). No difference in viable fibroblast number was observed in the second replicate experiment. Addition of trypan blue had no effect on viable fibroblast number within any of the treatment groups (MMC p = 0.35, 5-FU p = 0.24, RPMI p = 0.12) (fig 2).
Clinical studies
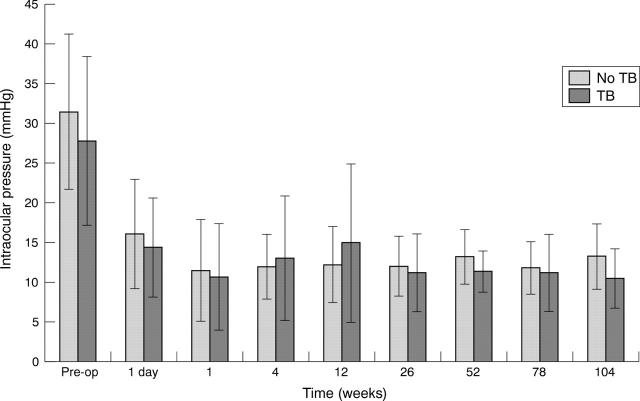
Addition of trypan blue at the concentrations studied made the antimetabolites clearly visible. Figures 3A and B show the effect of adding MMC with 0.01% trypan blue to preplaced dry sponges (case 13). Unexpectedly there was relatively little absorption of the MMC into the posterior sponges. This allowed the MMC to run between the sponges up to the limbus. During sponge removal, the diluted MMC stained the entire scleral flap (fig 3B). Examination of the sponges revealed that blood and serum had been absorbed into the sponge before instillation of MMC. Figures 3C and D show the effect of using presoaked sponges with the same MMC/ trypan blue concentration. The treatment area now corresponded better to the area of sponge placement. After sponge removal there was residual staining of treated tissue with trypan blue. Although capillary action pulled some MMC to the limbus (where it was easily seen and removed with a dry Wekcell sponge), the area in front of the sponges did not stain after treatment. Although in this case series there were no lost or retained sponges, the blue colouration of the trypan blue stained sponges greatly aided their visualisation during removal. Their visibility under the conjunctiva allowed a measurement of total treatment area to be made from a video still taken during treatment (fig 3D). Trypan blue was minimally visible on the first postoperative day in a minority of patients. No trypan blue could be seen on subsequent visits in any patients. The two case series were similar in type of glaucoma, initial IOP, IOP at 2 years, IOP reduction, complications, and postoperative interventions (table 1). Figure 4 shows the IOP profile of the two groups. Case 13, in which dry sponges were initially used, had a wound edge leak in the early postoperative period.
Figure 3.
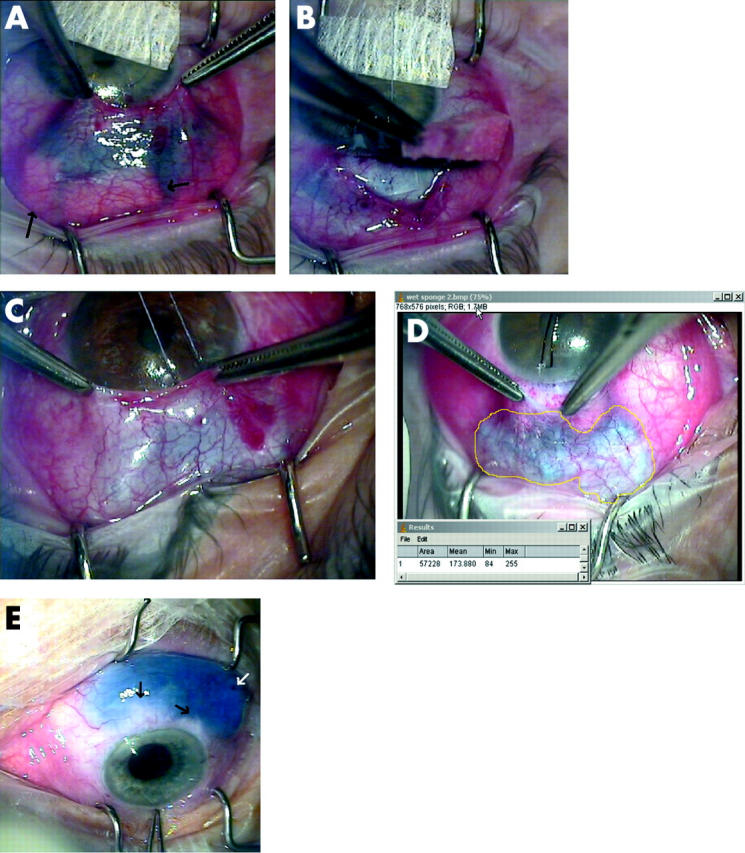
(A) Effect of adding mitomycin C (MMC) with 0.01% trypan blue to preplaced dry sponges during trabeculectomy. Arrows show posterior sponges with no trypan blue staining indicating no uptake of MMC. (B) One of the posterior sponges is removed, confirming no MMC uptake. Trypan blue can be seen staining the anterior operative field up to the limbus. (C) Effect of using sponges presoaked in MMC with 0.01% trypan blue seen through thick (C) and thin (D) tenon’s capsule. The treatment area now corresponds better to the area of sponge placement. (D) The area of treatment has been measured (area in pixels using Image J, (NIH, Bethesda, MD, USA). Note no inadvertent staining with trypan blue around the limbus. (E) Subconjunctival injection of 45 mg/ml 5-FU with 0.01% trypan blue after cataract surgery on an eye with a 680 day old trabeculectomy bleb. Black arrows show subconjunctival fibrous bands. White arrow shows point of conjunctival entry with ooze of blood but no trypan blue.
Table 1.
Characteristics and outcomes of 22 consecutive trabeculectomies with or without intraoperative trypan blue
| Case/sex/age | Diagnosis* | Antimetabolite | Pre-op IOP | Pre-op meds | IOP at 2 years | Interventions† | Complications‡ | IOP reduction mmHg (%) |
| No trypan blue | ||||||||
| 1/M/69 | OAG | 5-FU 50 mg/ml | 32 | 4 | 15 | 5-FU, needle | Hyphaema, day 10 | 17 (53) |
| 2/M/58 | TG | 5-FU 50 mg/ml | 50 | 0 | 18 | 5-FU | Wound edge leak required resuture | 32 (64) |
| 3/M/72 | OAG | MMC 0.4 mg/ml | 44 | 2 | 11 | None | None | 33 (75) |
| 4/M/69 | OAG | 5-FU 50 mg/ml | 38 | 3 | 13 | 5-FU | Choroidal effusion, week 1 | 25 (66) |
| 5/F/69 | UG | 5-FU 50 mg/ml | 29 | 4 | 14 | 5-FU × 4, CE | None | 15 (52) |
| 6/F/57 | OAG | 5-FU 50 mg/ml | 30 | 3 | 11 | 5-FU | None | 19 (63) |
| 7/M/80 | OAG | 5-FU 50 mg/ml | 26 | 3 | 14 | 5-FU | None | 12 (46) |
| 8/F/70 | UG | MMC 0.2 mg/ml | 16 | 4 | 9 | 5-FU | None | 7 (44) |
| 9/M/76 | OAG | MMC 0.2 mg/ml | 22 | 3 | 18 | 5-FU, CE, 2 meds | None | 4 (18) |
| 10/M/64 | ACG | MMC 0.2 mg/ml | 34 | 4 | 5 | 5-FU | None | 29 (85) |
| 11/M/79 | OAG | 5-FU 50 mg/ml | 25 | 4 | 18 | 7 (28) | ||
| Mean | 31.45 | 3.09 | 13.27 | 18 (58) | ||||
| Trypan blue | ||||||||
| 12/F/71 | OAG | 5-FU 45 mg/ml | 20 | 5 | 10 | 5-FU×2 | None | 10 (50) |
| 13*/M/58 | OAG | 5-FU 45 mg/ml | 22 | 3 | 14 | 5-FU | Choroidal effusion, wound edge leak, week 1 | 8 (36) |
| 14/M/55 | ACG | MMC 0.2 mg/ml | 34 | 5 | 10 | None | None | 24 (71) |
| 15/M/80 | OAG | 5-FU 45 mg/ml | 28 | 1 | 8 | 5-FU×3, needle | None | 20 (71) |
| 16/F/85 | UG | MMC 0.2 mg/ml | 27 | 2 | 6 | None | None | 21 (78) |
| 17/M/55 | NVG | MMC 0.4 mg/ml | 36 | 3 | 8 | 5-FU | None | 28 (78) |
| 18/F/80 | OAG | 5-FU 45 mg/ml | 20 | 3 | 14 | 5-FU | None | 6 (30) |
| 19/M/56 | ACG | 5-FU 45 mg/ml | 25 | 2 | 17 | 5-FU needle 2 meds | Wound edge leak, month 3 | 8 (32) |
| 20/M/67 | NTG | 5-FU 45 mg/ml | 8 | 4 | 10 | 5-FU | None | −2 (−25) |
| 21/M/72 | OAG | 5-FU 45 mg/ml | 44 | 4 | 13 | CE | None | 31 (70) |
| 22/M/65 | OAG | 5-FU 45 mg/ml | 42 | 4 | 5 | 5-FU ×2 | Circulating hyphaema, day 1 | 37 (88) |
| Mean | 27.82 | 3.27 | 10.45 | 17 (62) |
Diagnosis: OAG, open angle glaucoma; ACG, angle closure glaucoma; UG, uveitis glaucoma; TG, traumatic glaucoma.
*Case 13: sponges were placed dry and MMC was added via a syringe (see text).
†Interventions: 5-FU, subconjunctival 5-FU injection; needle, micropuncture of internal bleb walls with 30 gauge needle combined with 5-FU injection; meds, topical glaucoma medications recommenced; CE, cataract extraction.
‡Complications: choroidal effusions and wound edge leaks were transient and did not affect vision or require re-operation except for case 2 where wound resuturing was performed. Case 1: hyphaema was associated with oral NSAIDs taken for intercurrent illness. Month 3 wound leak of case 18 (other eye of case 14) was secondary to wound contraction.
Figure 4.
Mean intraocular pressure (and standard deviation) in 22 consecutive trabeculectomies with or without intraoperative trypan blue.
Addition of trypan blue to 5-FU given by subconjunctival injection showed that most of the 5-FU collected between the needle tip and point of conjunctival entry (data not shown). When the needle tip was passed only a short distance under the conjunctiva, trypan blue stained only locally and did not extend across the bleb. When the needle tip was advanced to its full extent the staining area was larger. Figure 3E shows 45 mg/ml 5FU with 0.05% trypan blue injected subconjunctivally after cataract surgery on an eye with a 680 day old trabeculectomy bleb. For this injection the needle tip was advanced about 75% across the stained area (approximately the back of the left-most black arrow). Both areas of subconjunctival fibrosis and any leakage from the entry site were easily visualised.
DISCUSSION
The use of adjunctive antimetabolites greatly increases the chance of success of trabeculectomy in eyes at high risk of postoperative bleb failure.20,21 However their use is also associated with complications such as corneal epitheliopathy, wound leak, and endophthalmitis.20–22 Although many factors play a part in the development of MMC and 5-FU complications, a substantial number are due to variations in local cellular toxicity. A number of modifications of surgical technique have been developed to reduce these variations.1 But ultimately most are hampered by the uncertainty of treatment area and dose of these drugs. Although a larger antimetabolite treatment area appears to improve trabeculectomy outcomes, the actual treatment area cannot currently be assessed. Long term studies with Baerveldt implants have shown that success does not continue to increase with increasing implant surface area, despite intermediate results suggesting this was the case.23,24 The same question can be answered in trabeculectomy only when a reliable technique to measure treatment area is available.
The corneal epitheliopathy from 5-FU results from inadvertent application. If 5-FU were coloured, leakage from the site of a 5-FU injection could be more easily recognised. This would allow the testing of new techniques to reduce leakage as well as facilitating prompt remediation of an acute leak.
The addition of trypan blue allows 5-FU and MMC to be visualised when applied topically. Both the contact area and any dilution of the drugs can be gauged from local tissue colour changes. Our initial experience using trypan blue indicated that application of antimetabolites to sponges introduced dry leads to treatment areas that were smaller and more anterior than intended. The use of trypan blue with subconjunctival 5-FU injections shows more precisely the location of the depot and allows leakage from the injection site to be assessed. It can also make subconjunctival fibrosis visible. Thus it may play a role in the more accurate division of subconjunctival fibrous bands during needle revision of trabeculectomy. In vitro data do not suggest that addition of trypan blue alters the cytotoxicity of MMC or 5-FU. However cell contraction assays were not performed.
Limitations of the clinical part of this study include the study design, which was a non-masked observational case series. The patient number was small although follow up was prospective and of reasonable length for a trabeculectomy study.
The addition of trypan blue to antimetabolites has potential benefits in clinical, research, and teaching aspects of ocular surgery and therapy. The technique is simple and the agent inexpensive and readily available throughout the world. It is particularly useful in trabeculectomy but is equally applicable to other ocular and non-ocular procedures where it is important to delineate and demarcate administration of these toxic drugs. However, before promoting its widespread use, further studies are needed to assess in vitro effects on other aspects of fibroblast function and the impact on clinical outcomes in a larger series.
Abbreviations
5-FU, 5-flourouracil
LDH, lactate dehydrogenase
MMC, mitomycin C
REFERENCES
- 1.Khaw PT. Advances in glaucoma surgery: evolution of antimetabolite adjunctive therapy. J Glaucoma 2001;10:S81–S84. [DOI] [PubMed] [Google Scholar]
- 2.Wells AP, Cordeiro MF, Bunce C, et al. Cystic bleb formation and related complications in limbus- versus fornix-based conjunctival flaps in pediatric and young adult trabeculectomy with mitomycin C. Ophthalmology 2003;110:2192–7. [DOI] [PubMed] [Google Scholar]
- 3.Nuyts RM, Pels E, Greve EL. The effects of 5-fluorouracil and mitomycin C on the corneal endothelium. Curr Eye Res 1992;11:565–70. [DOI] [PubMed] [Google Scholar]
- 4.Smith S , D’Amore PA, Dreyer EB. Comparative toxicity of mitomycin C and 5-fluorouracil in vitro. Am J Ophthalmol 1994;118:332–7. [DOI] [PubMed] [Google Scholar]
- 5.Dudney BW, Malecha MA. Limbal stem cell deficiency following topical mitomycin C treatment of conjunctival-corneal intraepithelial neoplasia. Am J Ophthalmol 2004;137:950–1. [DOI] [PubMed] [Google Scholar]
- 6.Gressel MG, Parrish RK, Folberg R. 5-fluorouracil and glaucoma filtering surgery: I. An animal model. Ophthalmology 1984;91:378–83. [DOI] [PubMed] [Google Scholar]
- 7.Knapp A , Heuer DK, Stern GA, et al. Serious corneal complications of glaucoma filtering surgery with postoperative 5-fluorouracil. Am J Ophthalmol 1987;103:183–7. [DOI] [PubMed] [Google Scholar]
- 8.The Fluorouracil Filtering Surgery Study Group. Fluorouracil Filtering Surgery Study one-year follow-up. Am J Ophthalmol 1989;108:625–35. [DOI] [PubMed] [Google Scholar]
- 9.Libre PE. Transient, profound cataract associated with intracameral 5-fluorouracil. Am J Ophthalmol 2003;135:101–2. [DOI] [PubMed] [Google Scholar]
- 10.Norn MS. Pachometric study on the influence of corneal endothelial vital staining. Corneal thickness after cataract extraction studied by vital staining with trypan blue. Acta Ophthalmol (Copenh) 1973;51:679–86. [DOI] [PubMed] [Google Scholar]
- 11.Melles GR, de Waard PW, Pameyer JH, et al. Trypan blue capsule staining to visualize the capsulorhexis in cataract surgery. J Cataract Refract Surg 1999;25:7–9. [DOI] [PubMed] [Google Scholar]
- 12.Feron EJ, Veckeneer M, Parys-Van Ginderdeuren R, et al. Trypan blue staining of epiretinal membranes in proliferative vitreoretinopathy. Arch Ophthalmol 2002;120:141–4. [DOI] [PubMed] [Google Scholar]
- 13.Li K , Wong D, Hiscott P, et al. Trypan blue staining of internal limiting membrane and epiretinal membrane during vitrectomy: visual results and histopathological findings. Br J Ophthalmol 2003;87:216–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norn MS. Per operative trypan blue vital staining of corneal endothelium. Eight years’ follow up. Acta Ophthalmol (Copenh) 1980;58:550–5. [DOI] [PubMed] [Google Scholar]
- 15.Veckeneer M , Van Overdam K, Monzer J, et al. Ocular toxicity study of trypan blue injected into the vitreous cavity of rabbit eyes. Graefes Arch Clin Exp Ophthalmol 2001;239:698–704. [DOI] [PubMed] [Google Scholar]
- 16.Haritoglou C , Gandorfer A, Schaumberger M, et al. Trypan blue in macular pucker surgery: an evaluation of histology and functional outcome. Retina 2004;24:582–90. [DOI] [PubMed] [Google Scholar]
- 17.Khaw PT, Sherwood MB, MacKay SL, et al. Five-minute treatments with fluorouracil, floxuridine, and mitomycin have long-term effects on human Tenon’s capsule fibroblasts. Arch Ophthalmol 1992;110:1150–4. [DOI] [PubMed] [Google Scholar]
- 18.Crowston JG, Akbar AN, Constable PH, et al. Antimetabolite-induced apoptosis in Tenon’s capsule fibroblasts. Invest Ophthalmol Vis Sci 1998;39:449–54. [PubMed] [Google Scholar]
- 19.Crowston JG, Chang LH, Daniels JT, et al. T lymphocyte mediated lysis of mitomycin C treated Tenon’s capsule fibroblasts. Br J Ophthalmol 2004;88:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitazawa Y , Kawase K, Matsushita H, et al. Trabeculectomy with mitomycin. A comparative study with fluorouracil. Arch Ophthalmol 1991;109:1693–8. [DOI] [PubMed] [Google Scholar]
- 21.The Fluorouracil Filtering Surgery Study Group. Five-year follow-up of the Fluorouracil Filtering Surgery Study. Am J Ophthalmol 1996;121:349–66. [DOI] [PubMed] [Google Scholar]
- 22.Higginbotham EJ, Stevens RK, Musch DC, et al. Bleb-related endophthalmitis after trabeculectomy with mitomycin C. Ophthalmology 1996;103:650–6. [DOI] [PubMed] [Google Scholar]
- 23.Britt MT, LaBree LD, Lloyd MA, et al. Randomized clinical trial of the 350-mm2 versus the 500-mm2 Baerveldt implant: longer term results: is bigger better? Ophthalmology 1999;106:2312–18. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd MA, Baerveldt G, Fellenbaum PS, et al. Intermediate-term results of a randomized clinical trial of the 350- versus the 500-mm2 Baerveldt implant. Ophthalmology 1994;101:1456–63. [DOI] [PubMed] [Google Scholar]