Abstract
Background: Dye solutions for intraoperative staining of epiretinal membranes and the internal limiting membrane improve the visualisation of these thin structures and facilitate their removal. In the present study the authors investigated the effects of indocyanine green 0.05%, trypan blue 0.15%, and patent blue 0.48% on bovine retinal function.
Methods: Bovine retina preparations were perfused with a standard solution and the electroretinogram (ERG) was recorded repeatedly. After recording of stable ERG amplitudes the nutrient solution was substituted by one of the dye solutions. The duration of retinal exposure to a dye solution was varied between 10 seconds and 2 minutes. Thereupon, the preparation was reperfused with standard solution for at least 115 minutes. The percentage of b-wave reduction after exposition was calculated.
Results: Reductions of the b-wave amplitude were found for each dye solution tested. The effects after application of patent blue and indocyanine green were completely reversible within the recovery time for an exposure period of 60 and 30 seconds, respectively. The application of trypan blue lead to a loss of the b-wave when the retina was exposed for 15 seconds or longer. This effect was only partly reversible within the recovery time.
Conclusion: The ERG showed toxic effects of trypan blue after a short period of retinal exposure. The intraocular application of trypan blue should be limited to selected cases. However, intraocular application of indocyanine green and patent blue in a sufficient concentration and taking account of a short period of retinal exposure seems possible.
Keywords: retina, dye, indocyanine green, patent blue, trypan blue
In the past few years indocyanine green has been widely adopted for the staining of the internal limiting membrane (ILM) during macular hole surgery to improve its visualisation and to facilitate the removal of this diaphanous structure. More recently, clinical trials demonstrated that staining membranes with trypan blue facilitated surgery in patients with proliferative vitreoretinopathy (PVR), macular pucker, and macular hole.1–3 The application of dye solutions prevents the intraoperative creation of punctate chorioretinal lesions during grasping of the ILM and epiretinal membrane.4
Although these dye solutions proved effective for the application during macular surgery and in patients with PVR, how the dye affects the intraocular tissues especially the retina has not been investigated extensively. Furthermore, there has been growing clinical evidence that these dye solutions might not be as safe as first thought.5–8 The vital dye patent blue is used for staining the anterior lense capsule for circular curvilinear capsulorhexis in patients with mature cataracts.9 Lately, the application of patent blue for staining of the ILM during macular hole surgery has been proposed as an alternative to the standard procedures which include indocyanine green or trypan blue assisted peeling of the ILM.
The aim of our study was to evaluate the effects of dye solutions at the retinal level to find a safe intraocular administration regimen. We studied the effects of indocyanine green, trypan blue, and patent blue on the parameters of the electroretinogram (ERG) in the isolated perfused bovine retina using the isolated perfused vertebrate retina technique, an electrophysiological in vitro technique for examination of retinal toxicity.10–12
MATERIALS AND METHODS
The preparation was done under dim red light: freshly enucleated bovine eyes were opened equatorially and the vitreous humour was removed. From the posterior segment circular pieces were obtained using a 7 mm trephine. The retina was isolated from the underlying pigment epithilium and mounted on a mesh occupying the centre of the perfusing chamber. Recording of the ERG was done in the surrounding nutrient medium via two silver/silver chloride electrodes on either side of the retina. The chamber was installed in an electrically and optically isolated air thermostat. The perfusion velocity was controlled by a roller pump and set to 1 ml/min. The temperature was kept constant at 30°C. The perfusing medium was pre-equilibrated with oxygen and the oxygen level was monitored by a Clarke electrode. The ERG was elicited at intervals of 5 minutes using a single white flash for stimulation. The flash intensity was set to 6.3 millilux at the retinal surface using calibrated neutral density filters. The duration of light stimulation was 500 ms controlled by a timer operating a mechanical shutter system. The ERG was amplified and bandpass limited between 0.1 and 300 Hz. The signal was AD converted and stored using a PC based signal acquisition and analysis system.
The retina was superfused with a serum free standard medium (NaCl 120, KCl 2, MgCl2 0.1, CaCl2 0.15, NaH2PO4 1.5, Na2HPO4 13.5, and glucose 5 (mmol/l)) and stimulated repeatedly until stable amplitudes were reached. Then the standard medium was substituted by one of the dye solutions. For each dye tested the duration of retinal exposure to the particular dye solution was varied between 10 seconds and 2 minutes (10, 15, 30, 60, 120 seconds). After application of the dye solution the preparation was reperfused with standard solution for at least 115 minutes. The b-wave amplitude was measured from the trough of the a-wave to the peak of the b-wave. The percentage of b-wave reduction after exposition was calculated. Changes of the b-wave amplitude were carefully monitored and the reversibility of the dye effects on the b-wave after reperfusion with the standard perfusate was measured.
The dye solutions applied were prepared as follows: 50 mg of commercially available ICG (Pulsion, Medical AG, Munich, Germany) were dissolved with 10 ml sterile distilled water, which is disposable within the manufacturer’s kit. One ml of this preparation was then diluted in 9 ml of BSS Plus (Alcon Surgical, Fort Worth, TX, USA) resulting in a final concentration of 0.05%. A sterile solution of trypan blue dissolved in a sodium chloride phosphate buffer was used for the trypan blue experiments (MembraneBlue, DORC International b v, Zuidland, the Netherlands). For use during vitreoretinal operations the manufacturer supplies trypan blue in a concentration of 0.15% which was also the concentration used in our study. Patent blue was applied in a phosphate buffered solution at a concentration of 0.48% as supplied by the manufacturer for surgery of the posterior segment (Blueron, Fluoron, Heidelberg, Germany).
Each dye solution and each exposure time was tested on six different isolated retina preparations. Before initiation of the superfusion with a dye solution a period of at least 60 minutes presenting stable amplitudes was required. For statistical analysis the t test was used; p<0.05 was regarded as statistically significant.
RESULTS
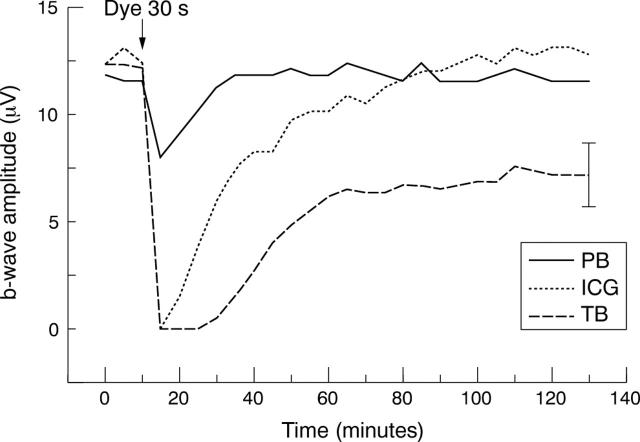
Stable ERG amplitudes were reached within 2 hours superfusion of the retinal preparations. Environmental parameters such as pH, osmotic pressure, temperature, and pO2 of the perfusion medium remained unchanged during the perfusion with the dye solution. Reductions of the b-wave amplitude were found for each dye solution tested. The application of indocyanine green for 10 seconds was found to reduce the b-wave amplitude by 69.4% (SD 27.7%) (p = 0.02). After application of indocyanine green for 15 seconds or longer a temporary loss of the b-wave was seen. The effects after application of indocyanine green were completely reversible within the recovery time when the exposure period did not exceed 30 seconds (figs 1–3).
Figure 1.
Average of representative test series (n = 6). Black arrow marks dye application for 30 seconds ICG, indocyanine green (dot); TB, trypan blue (dash); PB, patent blue (solid). One representative standard deviation given.
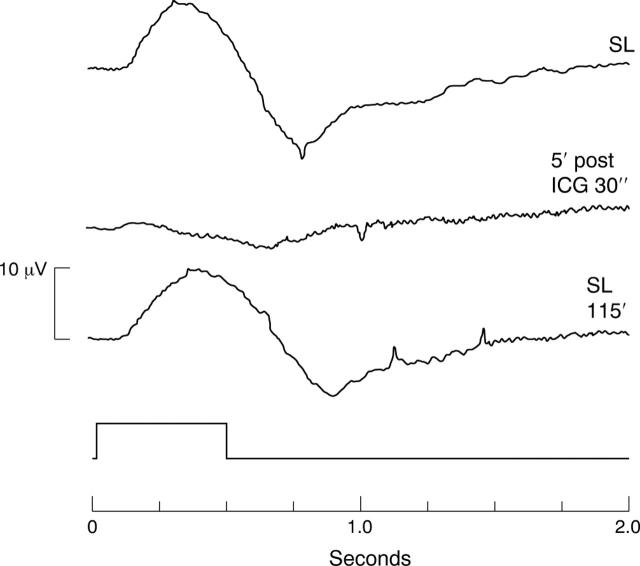
Figure 3.
ERG recordings under control conditions. SL, standard solution; 5′ post CL 30″, ERG 5 minutes after exposure to control solution (sterile water and BSS plus) for 30 seconds; SL 115′, ERG after reperfusion for 115 minutes.
The application of trypan blue leads to a loss of the b-wave when the retina was exposed for 10 seconds or longer. Following 10 seconds of retinal exposure the initial b-wave response level was reached within 85 minutes. The effect after application of trypan blue for 15 seconds was only partly reversible within the recovery time.
Patent blue was found to have a minor effect on the b-wave of the ERG compared with the effect of indocyanine green and trypan blue, respectively. A loss of the b-wave was only seen for an exposure period of 120 seconds. After application of patent blue for 60 seconds the b-wave reduction approached a mean value of 64.2% (SD 7.3) (p<0.001). The effects of patent blue on the ERG even for an exposure period of 120 seconds were completely reversible within the recovery time (p = 0.15). The percentages of b-wave amplitude reduction after reperfusion for 115 minutes are given in table 1.
Table 1.
Persistance of ERG effects after dye application
| Time (seconds) | Indocyanine green 0.05% | Trypan blue 0.15% | Patent blue 0.48% | |||
| PBR (SD) (%) | p Value | PBR (SD) (%) | p Value | PBR (SD) (%) | p Value | |
| 10 | −7.7 (22.0) | 0.53 | −3.7 (4.2) | 0.18 | −6.7 (7.8) | 0.18 |
| 15 | −2.1 (17.2) | 0.82 | 22.3 (9.6) | 0.01 | 5.0 (15.2) | 0.56 |
| 30 | −2.5 (5.0) | 0.39 | 40.6 (13.0) | 0.001 | −1.4 (8.0) | 0.74 |
| 60 | 48.2 (14.1) | 0.01 | 67.4 (13.7) | 0.001 | 5.0 (6.2) | 0.2 |
| 120 | 93.7 (12.5) | 0.001 | 97.1 (6.4) | 0.001 | 21.9 (22.1) | 0.14 |
PBR, persistent b-wave reduction after 115 minutes of reperfusion with the standard medium; SD, standard deviation. Statistical significance (t test) for differences between test (dye/time) series and control.
Thus, the reversibility of the dye effects proved time dependent within a narrow range. The time course of b-wave recovery correlated with the duration of retinal exposure. The kinetics of b-wave recovery were markedly slower after a longer period of dye application. A time response function of the dye effects on the b-wave amplitude could be calculated. Thus, a calculated exposure period of 41 seconds (trypan blue) and 62 seconds (indocyanine green) leads to a 50% reduction of the b-wave amplitude.
DISCUSSION
The purpose of this study was to evaluate safe intraocular administration regimen of the commercially available dye solutions for vitreoretinal surgery. As many types of retinal cells and synapses are involved in generation of the b-wave, a reduced b-wave indicates significant dysfunction of retinal neurons. Our experiments revealed irreversible effects of trypan blue 0.15% on the ERG already after a short period of retinal exposure.
Indocyanine green 0.05% and patent blue 0.48% showed completely reversible effects after retinal exposure of up to 30 and 120 seconds, respectively. Undoubtedly, indocyanine green and trypan blue significantly facilitate the removal of the ILM and epiretinal membrane. However, potential toxic effects which may result from the intraoperative contact with neuroretinal cells need to be excluded before various application techniques are claimed to be safe.
Originally, trypan blue had been widely used for staining the anterior lens capsule to facilitate the creation of a curvilinear capsulorhexis during surgery in mature cataract.13 For cataract surgery trypan blue is usually applied in a concentration of 0.06% (Vision Blue, DORC International b v, Zuidland, the Netherlands). Based on the clinical observation that trypan blue is highly effective for staining of the anterior lens capsule with no clinical reports on side effects of this dye during or after anterior segment surgery, the application of trypan blue for vitreoretinal surgery has been suggested. As far as we know only one study evaluating the effects of trypan blue on retinal function had been published.14 In addition, only a few morphological studies have been performed to assess the potential changes of cellular morphology after exposure to trypan blue. Sethi and Charteris examined (sub)retinal tissue specimens of four cat eyes after exposure to trypan blue 0.15%. They found subtle alterations in retinal cell biology as a result of the surgical trauma with no evidence of cytotoxicity of the dye.15 However, van Dooren et al found even morphological changes of corneal endothelial cells and corneal fibroblasts after exposure to trypan blue 0.1% for 5 minutes which underlines its cytotoxic potential.16
Veckeneer et al evaluated the ocular toxicity of trypan blue 0.06% and 0.2% 4 weeks after intravitreal injection into rabbit eyes.14 For both concentrations tested they found no significant reduction of the ERG amplitudes which was defined as an amplitude reduction exceeding 30% compared with the preinjection recordings. The report of Veckeneer et al is in contrast to our results which revealed toxic effects of trypan blue after a short period of retinal exposure. This apparent contradiction may be explained by differences in methodology. As Veckeneer et al performed intravitreal injections of trypan blue it can be assumed that the dye was diluted inside the vitreous cavity of the rabbit eyes. Taking into account a vitreous volume of approximately 3 ml the retinal surface was exposed to a trypan blue concentration which was 30-fold lower compared with the concentration of the injected solution. The experimental set up used in our study allowed for studying the effects of dye solutions in precisely known concentrations. Significant changes of the light responses were seen after exposure to trypan blue 0.15% for 15 seconds. Li et al reported on the use of trypan blue 0.06% for staining the ILM and epiretinal membrane during vitrectomy in 14 consecutive patients with macular hole or macular pucker.3 Trypan blue was injected after fluid/air exchange. The posterior pole was exposed for a period of 2 minutes before the removal of the dye. No intraoperative or postoperative complications were observed in this series with a mean follow up of 4.4 months.
Under the presented in vitro conditions, the commercially available trypan blue solution at a concentration of 0.15% (MembraneBlue) induced persistent retinal side effects after retinal exposure of 15 seconds or longer. These findings suggest that there could be a narrow time range for the epiretinal administration of trypan blue 0.15%.
Recently there has been growing experimental and even clinical evidence that indocyanine green might not be as safe as originally thought. Haritoglou et al observed large visual field defects in patients after indocyanine green assisted ILM peeling whereas no scotomas were seen in patients that underwent conventional peeling of the ILM.17 Furthermore, Gandorfer et al found a severe damage to the inner retina after experimental macular surgery and application of indocyanine green 0.05% in human donor eyes. They presumed ICG induced a photodynamic effect at the vitreoretinal surface with disruption of Müller cells.5 The potential photodynamic properties of indocyanine green have been recently used to kill colonic cancer cells in vitro.18 However, even without the intraoperative use of indocyanine green, Eckardt et al found Müller cell processes with signs of necrosis or degeneration present in the ILM which was removed in macular hole surgery.19 Indocyanine green is a frequently used dye with a favourable safety profile after intravenous application for angiography.20 The concentrations of intraoperatively applied indocyanine green solutions vary from 0.05% to 0.5% depending on the type of membrane which is targeted.6,21,22
The issue on indocyanine green toxicity is still the subject of heavy debate. We found that indocyanine green 0.05% leads to a reversible effect on the ERG even after retinal exposure of up to 30 seconds. Thus, toxic effects on retinal function after intraoperative short term application of indocyanine green 0.05% are unlikely to occur. However, for the intraocular application of indocyanine green for staining the ILM we followed certain restrictions such as a short cumulative perioded of retinal exposure and a careful removal of the dye following the intraoperative application.
Patent blue is a vital dye which is frequently used for lymphatic mapping to identify sentinel lymph nodes in patients with cancer before lymph node biopsy. In ophthalmic surgery patent blue has been used to stain the anterior lens capsule during cataract surgery. In vitro studies preceding the clinical application in cataract surgery showed no cytotoxic effects of patent blue. The intraocular application during surgery inside the anterior segment proved to be effective and safe.9 Other than rare allergic reactions which were seen during or after lymphography no systemic adverse effects of patent blue are known.23
Therefore, with respect to a possible intraoperative use of patent blue in vitreoretinal surgery fewer side effects on retinal neurons are likely to occur. To date, only a few clinical experiences of intraoperative staining of the ILM and macular pucker membranes with patent blue exist. Therefore, correlation of potentially helpful patent blue concentrations with our findings remains difficult. Nevertheless, the intraoperative administration of patent blue may be a promising strategy aimed at a reduction of neuronal damage which might be induced by the commonly applied dye solutions of higher concentrations.
The ERG reflects photoreceptor and higher order neuron activity of the retina. Therefore, it is a useful index of retinal integrity, allowing the effects of potentially toxic drugs on retinal function to be monitored. In the described perfusion chamber, the bovine retina can be kept responsive to light stimulation for several hours. The experimental setup is useful, especially for studying the effects of chemicals in precisely known concentrations. In recent studies testing drug effects on retinal function using the isolated perfused vertebrate retina technique, striking similarities of the dye induced ERG changes between human and bovine retina preparations were observed. However, in some cases the effective concentrations were different from those reported from other species but markedly close to those therapeutically used in humans.11,12
Clinical and experimental changes of the ERG indicate an impairment of retinal function and allow careful conclusions concerning the clinical situation.24,25 We are aware that no definitive conclusions can be drawn based on a single in vitro study. The purpose of our study was the comparison of three dyes in clinical concentrations under the same experimental conditions. The experiments revealed significant differences between the applied dyes with respect to the effects on retinal function. From our point of view, the presented setting mimics the intraoperative situation after fluid/air exchange which avoids a diffuse spread over the retinal surface. Thus, the dye concentration which reaches the retina is close to the intraoperative situation in human macular surgery.
Further studies to evaluate the potential use of patent blue for intraoperative staining of the ILM and epiretinal membranes are necessary to clarify whether patent blue application facilitates their identification and removal. If patent blue offers the positive staining properties of trypan blue and indocyanine green it could become the ideal tool for the intraoperative staining of membranes in vitreoretinal surgery.
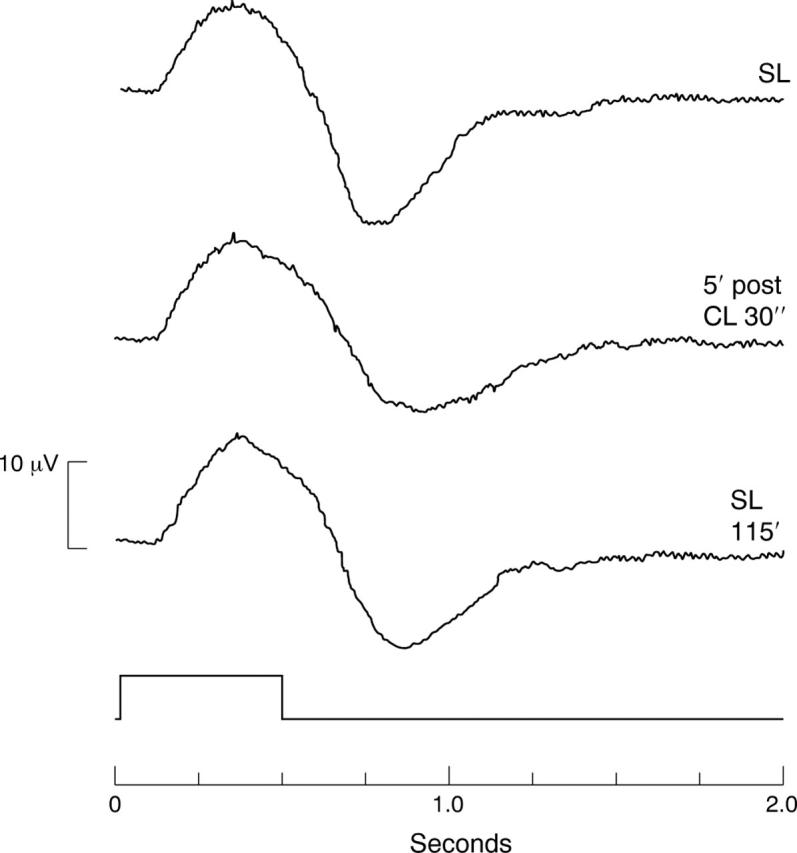
Figure 2.
Effects of indocyanine green on the ERG of the bovine retina. SL, standard solution; 5′ post ICG 30″, ERG 5 minutes after exposure to indocyanine green for 30 seconds; SL 115′, ERG after reperfusion for 115 minutes.
Abbreviations
ERG, electroretinogram
ILM, internal limiting membrane
PVR, proliferative vitreoretinopathy
REFERENCES
- 1.Feron EJ, Veckeneer M, Parys-Van Ginderdeuren R, et al. Trypan blue staining of epiretinal membranes in proliferative vitreoretinopathy. Arch Ophthalmol 2002;120:141–4. [DOI] [PubMed] [Google Scholar]
- 2.Stalmans P , Feron E, Parys-Van Ginderdeuren R, et al. Double vital-staining using trypan blue and infracyanine green in macular pucker surgery. Br J Ophthalmol 2003;87:713–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li K , Wong D, Hiscott P, et al. Trypan blue staining of internal limiting membrane during vitrectomy: visual results and histopathological findings. Br J Ophthalmol 2003;87:216–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karacorlu M , Karacorlu S, Ozdemir H. Iatrogenic punctate chorioretinopathy after internal limiting membrane peeling. Am J Ophthalmol 2003;135:178–82. [DOI] [PubMed] [Google Scholar]
- 5.Gandorfer A , Haritoglou C, Gandorfer AC, et al. Retinal damage from indocyanine green in experimental macular surgery. Invest Ophthalmol Vis Sci 2003;44:316–23. [DOI] [PubMed] [Google Scholar]
- 6.Horiguchi M , Nagata S, Yamamoto N, et al. Kinetics of indocyanine green dye after intraocular surgeries using indocyanine green staining. Arch Ophthalmol 2003;121:327–31. [DOI] [PubMed] [Google Scholar]
- 7.Haritoglou C , Gass CA, Schaumberger M, et al. Macular changes after peeling of the internal limiting membrane in macular hole surgery. Am J Ophthalmol 2001;132:363–8. [DOI] [PubMed] [Google Scholar]
- 8.Engelbrecht NE, Freeman J, Sternberg P Jr, et al. Retinal pigment epithelial changes after macular hole surgery with indocyanine green-assisted internal limiting membrane peeling. Am J Opthalmol 2002;133:89–94. [DOI] [PubMed] [Google Scholar]
- 9.Lohmann CP, Kobuch K, Winkler von Mohrenfels C, et al. Patent-Blau als Adjuvanz bei der Kapsulorhexis. Klin Monatsbl Augenheilkd 2003;220 (Suppl 1) :S4. [Google Scholar]
- 10.Sickel W . Respiratory and electrical responses to light stimulation in the retina of the frog. Science 1965;148:648–51. [DOI] [PubMed] [Google Scholar]
- 11.Lüke C , Walter P, Bartz-Schmidt KU, et al. Effects of antiviral agents on retinal function in vertebrate retina. In: Green K, ed. Advances in ocular toxicology. New York: Plenum Press, 1997:107–12.
- 12.Walter P , Lüke C, Sickel W. Antibiotics and light responses in superfused bovine retina. Cel Mol Neurobiol 1999;19:87–92. [DOI] [PubMed] [Google Scholar]
- 13.Melles GR, De Waard PW, Pameyer JH, et al. Trypan blue capsule staining to visualize the capsulorhexis in cataract surgery. J Cataract Refract Surg 1999;25:7–9. [DOI] [PubMed] [Google Scholar]
- 14.Veckeneer M , Van Overdam K, Monzer J, et al. Ocular toxicity study of trypan blue injected into the vitreous cavity of rabbit eyes. Graefe’s Arch Clin Exp Ophthalmol 2001;239:698–704. [DOI] [PubMed] [Google Scholar]
- 15.DORC. Scientific Enclosure - Membrane Blue. 2003;3–4.
- 16.Van Dooren BT, Beekhuis WH, Pels E. Biocompatibility of trypan blue with human corneal cells. Arch Ophthalmol 2004;122:736–42. [DOI] [PubMed] [Google Scholar]
- 17.Haritoglou C , Gandorfer A, Gass CA, et al. The effect of indocyanine-green on functional outcome of macular pucker surgery. Am J Ophthalmol 2003;135:328–37. [DOI] [PubMed] [Google Scholar]
- 18.Baumler W , Abels C, Karrer S, et al. Photo-oxidative killing of human colonic cancer cells using indocyanine green and infrared light. Br J Cancer 1999;80:360–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burk SE, Da Mata AP, Snyder ME, et al. Indocyanine green-assisted peeling of the retinal internal limiting membrane. Ophthalmology 2000;107:2010–14. [DOI] [PubMed] [Google Scholar]
- 20.Hope-Ross M , Yanuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994;101:529–33. [DOI] [PubMed] [Google Scholar]
- 21.Da Mata AP, Burk SE, Riemann CD, et al. Indocyanine green-assisted peeling of the retinal internal limiting membrane during vitrectomy surgery for macular hole repair. Ophthalmology 2001;108:1187–92. [DOI] [PubMed] [Google Scholar]
- 22.Haritoglou C , Gandorfer A, Gass CA, et al. Indocyanine green-assisted peeling of the internal limiting membrane in macular hole surgery affects visual outcome: a clinicopathological correlation. Am J Ophthalmol 2002;134:836–41. [DOI] [PubMed] [Google Scholar]
- 23.Meseg W , Melzer KJ. A case of patent blue allergy in foot lymphography. Z Urol Nephrol 1972;65:945–7. [PubMed] [Google Scholar]
- 24.Karpe G . Early diagnosis of siderosis retinae. Docum Ophthalmol 1948;2:277. [DOI] [PubMed] [Google Scholar]
- 25.Sickel W . Retinal metabolism in dark and light. In: Fuortes MGF, ed. Handbook of Sensory Physiology Volume VII, 2. Berlin, Heidelberg, New York: Springer, 1972:667–727.