Abstract
The present study was designed to determine the short-term effects of alcohol consumption on hormonal responses and mood states in nulliparous women who have regular menstrual cycles. To this aim, we conducted a within-subjects design study in which eight women consumed a 0.4-g/kg dose of alcohol in orange juice during one test session (alcohol condition) and an equal volume of orange juice (control condition) during the other. Changes in plasma prolactin, oxytocin and cortisol levels, blood alcohol concentrations (BACs), and mood states were compared. BAC peaked at approximately 36.7 + 5.4 min after the consumption of the alcoholic beverage and decreased thereafter. Alcohol consumption significantly increased the area under the concentration–time curve (AUC) of prolactin (P < .01) and decreased the oxytocin AUC (P = .04) when compared to the control condition. Cortisol AUCs were not different across the two experimental conditions. Similar to that previously observed in lactating women, changes in prolactin and oxytocin paralleled changes in feelings of drunkenness. The magnitude and persistence of the alcohol-induced hormonal changes in nulliparous women were significantly less pronounced than those observed in lactating women, further highlighting the dynamics of the system under study during lactation.
Keywords: Alcohol, Prolactin, Oxytocin, Cortisol, Women, Mood
1. Introduction
The physiological effects and health consequences of drinking moderate amounts of alcohol vary across the life span and with reproductive state in women (Gill, 2000). In terms of general health, moderate alcohol consumption is associated with both positive (e.g., protection against cardiovascular disease) and negative (e.g., increased risk of breast cancer) outcomes in pre- and postmenopausal women (Dumitrescu & Cotarla, 2005; Singletary & Gapstur, 2001). Only in recent years has experimental research focused on the reproductive state of lactation. In the short term, alcohol has been shown to disrupt the two key hormones underlying milk production and ejection (Mennella et al., 2005). The alcohol-induced decrease in oxytocin and increase in prolactin levels were observed during and after periods of breast stimulation and were significantly related to lactational performance (e.g., milk yield) and mood states (e.g., feelings of drunkenness), respectively.
Whether alcohol has similar effects on prolactin and oxytocin in nonlactating, premenopausal women has received little attention. To our knowledge, there has been only one study in such women that looked at the short-term effects of alcohol consumption on oxytocin levels (Coiro et al., 1992). Here, alcohol consumption decreased oxytocin levels, a finding that is consistent with its efficacy in partially blocking uterine contractions during labor (Fuchs et al., 1982). Although there are more studies on alcohol’s effects on prolactin, the findings remain equivocal. Whereas some studies reported significant increases in prolactin (Carlson et al., 1985; Lex et al., 1991; Mendelson et al., 1987; Sarkola et al., 1999), others reported significant decreases (Becker et al., 1988; Volpi et al., 1994) during the immediate hours following alcohol consumption in premenopausal women.
Some of these discrepancies may be due to the methodologies used and the characteristics of the population studied. First, sufficient time must elapse between the needle prick and blood sampling since prolactin is very stress labile and rises during the first half hour following a needle prick (Grayson et al., 1997). Second, food intake should be controlled for since prolactin levels can be potentiated by certain gastrointestinal hormones and high blood glucose levels (Widstrom et al., 1984). Third, the use of birth control pills or hormone replacement therapy (Ginsburg et al., 1996) and women’s drinking habits (Mello et al., 1989) are important factors since they may modulate alcohol’s effect on hormonal responsivity. Fourth, the phase of the menstrual cycle needs to be taken into consideration (Gill, 1997) since some, but not all, studies have found variations in prolactin (Brzyski et al., 1997; Buckman et al., 1980; De Leon et al., 1992; Fujimoto et al., 1990; Landgren et al., 2004; Snowden et al., 1986) and oxytocin (Altemus et al., 2001; Sajonia et al., 2005; Shuvovski et al., 1989) across the menstrual cycle. When fluctuations were detected, prolactin levels slightly surged midcycle (Brzyski et al., 1997; Buckman et al., 1980; De Leon et al., 1992), whereas oxytocin levels were significantly lower during the luteal phase when compared to the follicular or ovulatory phase (Sajonia et al., 2005; Shuvovski et al., 1989). Furthermore, hormonal response to breast stimulation may differ during the different phases of the cycle (Leake et al., 1984). Fifth, because the levels of these hormones are low in nonlactating women, several studies have evaluated the effects of alcohol consumption on hormonal responses during breast stimulation (Coiro et al., 1992; Volpi et al., 1994). However, while some studies report significant increases in prolactin (Volpi et al., 1994) and oxytocin (Coiro et al., 1992) during breast pump-induced stimulation of the breasts in nonlactating women, others revealed that such methods failed to reliably produce such hormonal changes (Amico & Finley, 1986; Noel et al., 1974). Whether the studies differed in the intensity of the stimulation with the breast pump is not known since this information was not provided. Moreover, different degrees of discomfort during the breast stimulation procedure may affect prolactin secretion patterns since prolactin is released not only in response to stimulation of the mammary gland but also in response to stress and pain (Grayson et al., 1997).
The present study was designed to expand upon the recent findings in lactating women (Mennella et al., 2005) and determine the short-term effects of drinking a moderate dose of alcohol on prolactin and oxytocin responses as well as mood states in nulliparous women. To allow for comparisons, nulliparous women were tested during one (i.e., follicular) phase of the menstrual cycle and the methodologies that controlled for time of day, prior food intake, as well as breast stimulation were identical to those used in lactating women. Cortisol levels were monitored to ensure that alterations in hormonal responses were not related to the stress of the procedures.
2. Methods
2.1. Subjects
Eight, nonsmoking, healthy, nulliparous women who had regular menstrual cycles participated in the study. The women (four Caucasian, three African American, and one Hispanic) were, on average, 25.0 ± 1.2 years of age and their body mass indices were, on average, 24.9 ± 1.9 kg/m2. Three additional women began testing but were excluded because of procedural difficulties. During the initial screening, women were excluded if they were lifetime alcohol abstainers or on any medications including oral contraceptives since there is some suggestion that both basal and peak prolactin levels are altered in such women (Snowden et al., 1986). All procedures were approved by the Office of Regulatory Affairs at the University of Pennsylvania, and each woman gave informed written consent prior to testing.
The women reported that they drank, on average, 5.8 ± 2.0 alcoholic beverages during the 3 weeks preceding the first testing day (range = 0–15 drinks) and 2.1 ± 0.5 drinks per occasion (range = 0–4 drinks per occasion). The Michigan Alcohol Screening Test (MAST) was administered to each woman; all but one scored less than five on the MAST, whereas the remaining woman did not complete the test.
2.2. Procedures
A within-subjects design study was conducted. The design controlled for time of day and time since the women last ate since the episodic secretion of prolactin has a circadian rhythm and can be potentiated by feeding, and in turn, certain gastrointestinal hormones and high blood glucose levels (Bencker et al., 1990; Freeman et al., 2000; Fujimoto et al., 1990; Kok et al., 2006; Widstrom et al., 1984). Subjects were tested at the General Clinical Research Center (GCRC) at the University of Pennsylvania on 2 days separated by 1 week (±3 days). Because the phase of the menstrual cycle can modulate alcohol’s effect on hormonal responsivity (Gill, 1997), both test sessions occurred during the follicular phase of the menstrual cycle. The follicular phase was chosen since gonadal steroids are the lowest of all phases of the menstrual cycle (Stock et al., 1999) and therefore are more comparable to the low levels of these hormones during lactation (Stock et al., 1999). The test session in which alcohol was consumed occurred 10.6 ± 2.2 days, whereas the one in which they consumed the control, nonalcoholic drink occurred 8.9 ± 2.5 days, from the last menses [paired t (7df) = 0.42; P = .69].
Using methods established in our laboratory (Mennella et al., 2005), subjects arrived at the GCRC at 0800 h (±30 min) following an overnight fast and remained fasted during the entire testing procedures. An intravenous line was inserted into the antecubital vein of an arm. Because prolactin is very stress labile and rises during the first half hour following needle prick (Grayson et al., 1997), subjects acclimated in the private testing room for 45 min. Subjects were not allowed to watch television, sleep, or talk about food or infants throughout the entire testing sessions since these behaviors may affect the hormones under study. Instead, they were allowed to read magazines or novels or to converse on other topics.
After acclimatization, blood samples were obtained at fixed intervals (–40, –25, and –10 min) before drinking a 0.4-g/kg dose of alcohol in orange juice (15% vol/vol) on one testing day (alcohol condition) and an equal volume of orange juice on the other (control condition). The order of testing was randomized between subjects. During both conditions, 3 ml of alcohol was pipetted onto the surface of the cup to serve as a smell and taste mask. The beverage was aliquoted into two equal volumes, and each aliquot was consumed within consecutive 5-min periods (Table 1).
Table 1.
Schedule of events
| Time (min)
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −85 | −40 | −25 | −10 | 0 | 10 | 25 | 35–51 | 65 | 80 | 95 | 110 | 125 | 140 | ||
| Event | Insert line | Begin BVG | End BVG | Breast pump stimulation | |||||||||||
| Blood sampling | x | x | x | xxxxxxxxx | x | x | x | x | x | x | |||||
| BAC | x | x | x | x | x | x | x | x | x | x | |||||
| ARCI mood ratings | x | x | x | x | x | ||||||||||
Each subject was tested on 2 days separated by 1 week. During one test session, they consumed a 0.4-g/kg dose of an alcoholic beverage (BVG) at time 0, whereas on the other they consumed an equal volume of a control BVG. Approximately 35 min after drinking the BVG, the women pumped their breasts with an electric breast pump for 16 min (breast stimulation). The symbol x denotes occurrence of blood samples, determination of blood alcohol concentrations (BACs), and completion of Addiction Research Center Inventory (ARCI) questionnaires to evaluate various mood states prior to and after the consumption of the BVG.
As shown in Table 1, approximately one half hour after subjects started drinking the beverage, at the time when blood alcohol levels were peaking on the alcohol day (Mennella et al., 2005), breast stimulation was provided by an electric breast pump (Medela Model Classic™, Crystal Lake, IL). The vacuum regulator that controls the level of suction of the pump could range from minimum (1) to maximum (5). For all but one subject, the vacuum regulator was in the same position on both test days (mean = 2.7 ± 0.3).
Blood samples were again taken immediately before pumping (t = 35 min), and every 2 min for 16 min of stimulation of alternating breasts (37, 39, 41, 43, 45, 47, 49, and 51 min postconsumption of beverage) with an electric breast pump, and then every 15 min without breast stimulation for the next 90 min (65, 80, 95, 110, 125, and 140 min). Each sample collection involved the removal of 1 ml of blood to clear the catheter tubing, followed by a 5-ml collection into Vacutainer tubes containing EDTA. Samples were kept on ice for no longer than 1 h and were centrifuged, separated into aliquots, and stored at −70°C for later assay. Nurses were blind to the conditions of the experiment.
At fixed intervals throughout each test day, blood alcohol concentrations (BACs) were estimated by having subjects breathe into an Alco-Sensor III (St. Louis, MO) (Mumenthaler et al., 2000). The Alco-Sensor III device provides readings of BAC levels by assuming a blood:breath ratio of 2,100:1, and the correlation between ethanol levels measured in blood samples and those measured in exhaled alcohol concentration breath samples has been shown to be better than 0.98 for both the absorption and elimination phases (Inns et al., 1979). In addition, subjects completed the Addiction Research Center Inventory (ARCI) to assess various measures of self-reported drug effects (Holdstock & de Wit, 1998). This questionnaire consists of a number of scales including the Morphine Benzedrine Group scale which measures drug-induced euphoria; the Pentobarbital-Chlorpromazine-Alcohol Group scale which measures sedation; the Lysergic scale which measures dysphoric and somatic effects; the Benzedrine Group and Amphetamine scales which measure stimulant-like effects; and the Drunk Scale which measures drunkenness.
2.3. Hormone assays
Plasma samples were measured in duplicate by double-antibody radioimmunoassays purchased from Phoenix Pharmaceuticals, Inc. (Belmont, CA) for oxytocin and from ICN Diagnostics (Costa Mesa, CA) for cortisol and by immunoradiometric assay purchased from ICN Diagnostics for prolactin. Assays were performed blind to the condition by the Diabetes Research Center of the University of Pennsylvania. Intra-assay variation was 2.8, 3.0, and 1.3% and interassay variation was 1.9, 8.9, and 10.2% for oxytocin, prolactin, and cortisol, respectively.
2.4. Statistical analyses
Separate repeated measures mixed analyses of variance were conducted to determine whether there were significant differences in prolactin, oxytocin, and cortisol levels as well as various mood states with experimental conditions (i.e., alcohol, control) and time as the within-subjects factors. When the ANOVA yielded significant effects, post hoc analyses were conducted.
Because there were no significant differences in the basal oxytocin [F (2, 14df) = 0.52; P = .60] and prolactin [F (2, 14df) = 0.01; P = .99] levels, we calculated changes in prolactin and oxytocin from the respective baseline values (mean of three baseline samples) for each subject. Because there was a significant effect of time on cortisol baseline samples [F (2, 14df) = 3.80; P = .05], the last sample (t = −10 min) was used as the baseline value for these calculations. The area under the curve (AUC) was calculated by using a point-to-point method (OriginLab® Corporation, Northampton, MA) from baseline until the end of the breast stimulation (AUC0–51min), as well as from baseline until the end of the test session (AUC0–140 min). The AUCs for each hormone and for each subject were calculated independently. Paired t-tests were conducted to compare the respective AUCs between experimental conditions. The critical value for significance was P < .05, and all P values represent two-tailed tests.
A stepwise forward regression analysis was also conducted to assess the value of BAC and hormonal changes (prolactin, oxytocin) as predictors of the subjective mood states, as determined by the ARCI. For this analysis, each variable is first ranked by its F value. The variable with the highest significant F value is entered into the logistic regression model. To avoid the loss of nearly significant variables, P < .10 was considered as significant by this stepwise analysis (see Perola et al., 1994).
3. Results
Breast stimulation did not produce significant increases in prolactin on the days women consumed the nonalcoholic beverage. However, there was a significant interaction between experimental condition and time of sampling for prolactin plasma levels [F (15, 105df) = 5.26; P < .0001]. As shown in Fig. 1A, prolactin levels were significantly higher both immediately before the beginning of the breast pump stimulation (at t = 35 min) as well as during breast stimulation on the days that women consumed alcohol (P < .001). The AUCs from the time the women drank the alcohol to the end of the breast stimulation [AUC0–51 min; paired t(7df) = 3.46; P = .01], during the breast stimulation period [AUC35–51 min; paired t(7df) = 3.21; P = .015], as well as throughout the entire session (AUC0–140 min) significantly increased during the alcohol session [paired t-test (7df) = 2.45; P = .04]. This enhanced response was observed in virtually all women tested on the day they consumed alcohol when compared to control beverage.
Fig. 1.
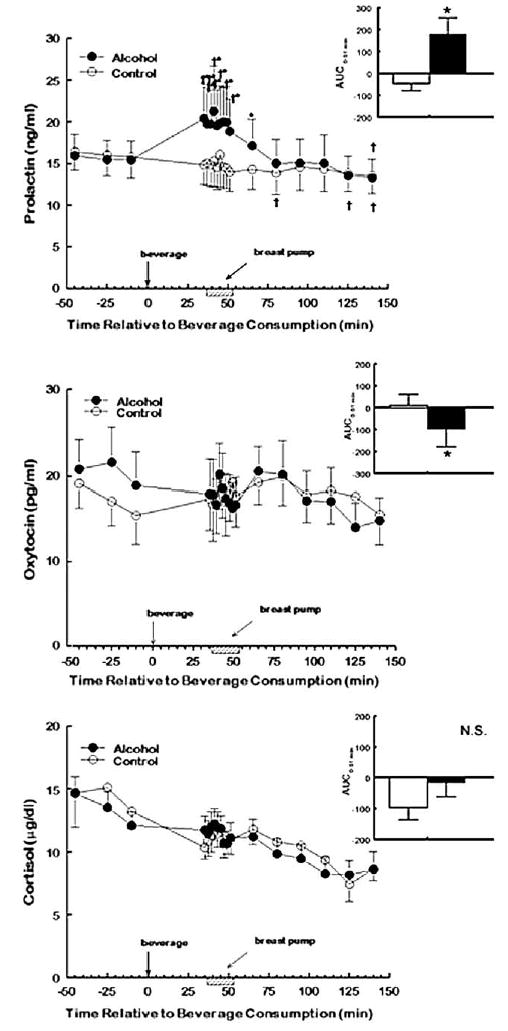
Mean (±S.E.M.) plasma prolactin (ng/ml; top panel), oxytocin (pg/ml; middle panel), and cortisol (μg/dl; bottom panel) levels in eight nulliparous women at baseline and at varying times following the consumption of a 0.4-g/kg dose of alcohol in orange juice on one test day (closed circles) and orange juice alone on the other (open circles). Women received breast stimulation with a breast pump (hatched bars) 35–51 min after the consumption of the beverage (time point = 0). The inset in each panel depicts mean (±S.E.M.)area under the concentration–time curve (AUC) on alcohol (black bars) and control (open bars) days. * denotes values that were significantly different from control session (AUC data) or significantly different from similar time points during the control (plasma levels; P < .05). † denotes values within each test session that are significantly different from their respective baseline values (P < .05). Conversions: oxytocin in picograms per milliliter × 0.80 = oxytocin in picomoles per liter; prolactin in nanograms per milliliter × 43.5 = prolactin in picomoles per liter; cortisol in micrograms per deciliter × 27.6 = cortisol in nanomoles per liter.
Breast stimulation also did not produce significant changes in oxytocin levels on the days women consumed the nonalcoholic, control beverage. There was no significant effect of experimental condition [F (1, 7df) = 0.14; P = .72] or time of sampling [F (15, 105df) = 1.36; P = .18] or a significant interaction between condition and time [F (15, 105df) = 0.63; P = .84] for oxytocin levels. In contrast to that observed for prolactin, Fig. 1B shows that there was a significant decrease in oxytocin from the time the women drank the alcohol to the end of the breast stimulation [AUC0–51 min; paired t-test (7df) = −2.53; P = .04] when compared to control day; this decreased oxytocin response was observed in all but one of the women tested. There was also a tendency for oxytocin to decrease during the breast stimulation period [AUC35–51; paired t (7df) = −2.18; P = .06] and throughout the 140 min post-consumption of the alcoholic beverage [AUC0–140 min; paired t(7df) = −2.15; P = .07].
Time of sampling was the only significant factor affecting cortisol levels [F (15, 105df) = 7.96; P < .001]. As shown in Fig. 1C, cortisol levels steadily decreased on both testing days, thus suggesting that the procedures were not stressful to the subjects.
BAC peaked at approximately 36.7 ± 5.4 min after the consumption of the alcoholic beverage and decreased thereafter. The peak ethanol levels ranged from 0.35 to 0.73 g/l. Paralleling the changing concentrations of blood alcohol levels were changes in the women’s mood states. As shown in Fig. 2, alcohol consumption significantly increased the women’s ratings of drunkenness, as determined by the ARCI, during the immediate hours following consumption of the alcoholic beverage [F (3, 21df) = 5.42; P = .006].
Fig. 2.
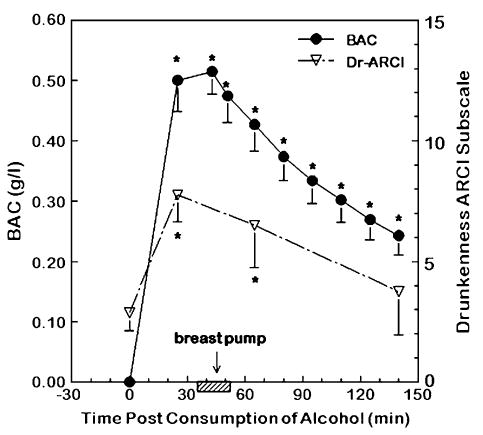
Mean (±S.E.M.) blood alcohol concentration (BAC; closed circles) and raw scores of the Addiction Research Center Inventory (ARCI) drunkenness scale (Dr-ARCI) scale which reflects levels of drunkenness (open triangles). To convert values for BAC to millimoles per liter, multiply by 0.217.
* denotes values that are significantly different from their respective baseline values (P < .05).
Individual variations in the subjective effects of alcohol were significantly related with the alcohol-induced changes in prolactin and oxytocin. As shown in Table 2, the greater the increase in prolactin and decrease in oxytocin at 65 min post-alcohol consumption, the more drunk the women felt [r(7df) = 0.71, P = .047 and r(7df) = −0.83, P = .01, respectively]. Although alcohol consumption significantly affected both oxytocin and prolactin levels, there was no correlation between these two hormones [r (7df) = −0.38; P = .36]. This lack of a correlation was also confirmed by Spearman’s rho (P = .35) analyses. Although there was a tendency for blood alcohol levels at t = 65 min to be related to feelings of drunkenness [r (7df) = 0.61; P = .11], stepwise regression analyses which included oxytocin, prolactin, and BAC levels revealed that the association between feelings of drunkenness was better explained by changes in these hormones than by BAC (Table 2).
Table 2.
Correlations between feelings of drunkenness as measured by the Addiction Research Center Inventory (ARCI) (Dr-ARCI score), blood alcohol concentrations (BACs), and changes in prolactin (Delta prolactin) and oxytocin (Delta oxytocin) from the respective baseline values (mean of three baselines samples) at 65 min after alcohol consumption (t65)
| Drunkenness at t65 | BAC at t65 | Delta prolactin at t65 | Delta oxytocin at t65 | |
|---|---|---|---|---|
| Drunkenness at t65 | 1.00 | 0.61, P = .108 | 0.71, P = .047 | −0.83 P = .010 |
| BAC at t65 | 1.00 | 0.47, P = .243 | 0.34, P = .405 | |
| Delta prolactin at t65 | 1.00 | −0.38, P = .356 | ||
| Delta oxytocin at t65 | 1.00 |
Bold text indicates significant correlations.
4. Discussion
Alcohol consumption significantly elevated prolactin and decreased oxytocin levels, but did not alter cortisol levels, in healthy nulliparous, premenopausal women. Cortisol levels steadily decreased on both testing days, suggesting that the procedures were not stressful to the subjects. That the levels of prolactin were significantly increased before the start of breast pump stimulation, which occurred 35 min after the consumption of the alcoholic beverage when BAC levels were peaking, suggests that such increases were alcohol induced, a finding which is consistent with previous research (Lex et al., 1991; Mendelson et al., 1987; Sarkola et al., 1999). Nevertheless, although the methods were identical to those used in our previous study on lactating women (Mennella et al., 2005), the hormonal response observed in nulliparous women was less robust than that observed in lactating women. The diminutive response could be due in part to inability of the breast stimulation procedures to alter prolactin and oxytocin levels, a finding which is also consistent with previous research (Amico & Finley, 1986; Noel et al., 1974).
Alcohol-induced changes in prolactin and oxytocin were significantly related to the subjective effects of alcohol. That is, the greater the increase in prolactin and decrease in oxytocin, the more drunk the women felt. Although both hormones are linked to metabolic and psychological adaptations that occur during pregnancy and the postpartum period to prepare women for childbirth and lactation, variations in these hormones can induce changes in moods, emotions, and behavior (Carter et al., 2001; Ferreira et al., 1998; Sobrinho, 2003). In the present study, changes in prolactin and oxytocin levels were related to feelings of drunkenness. The notion that at least some of the subjective effects of alcohol were mediated by prolactin is suggested by several findings. First, those nonalcoholic subjects who have lower prolactin response to alcohol are less likely to feel intoxicated (Schuckit et al., 1983, 1987). Second, it has been hypothesized that the pronounced elevation in prolactin following the ingestion of other drugs of abuse such as 3,4-methylenedioxymethamphetamine (“Ecstasy”) mediates the drug-induced mood states of euphoria and relaxation (Passie et al., 2005). It is important to emphasize that associations between drug-induced hormonal responses and mood may be due to its effect on common neural circuits (e.g., limbic system) rather than the direct action of prolactin on the brain circuitry underlying certain mood states (Mendelson et al., 2003).
Epidemiological studies conducted during the past few decades have consistently revealed that moderate alcohol consumption is associated with increased risk for developing breast cancer in both pre- and postmenopausal women (Dumitrescu & Cotarla, 2005; Singletary & Gapstur, 2001). One of the many hypothesized mechanisms underlying this association is the effect of alcohol on the hormonal milieu (Hamajima et al., 2002; Singletary & Gapstur, 2001). Recently, interest has focused on prolactin, a well-known mitogen and differentiating agent of the mammary gland. Although its role in mammary tumor formation in women is still unknown (Wennbo & Tornell, 2000), prolactin has been shown to be involved in mammary cancer development in animal models (Vonderhaar, 1998), perhaps because it protects the cancer cells against apoptosis (Perks et al., 2004). In addition, it has been hypothesized that oxytocin may protect against breast cancer (Murrell, 1995). Taken together with the present findings, experimental research has revealed that moderate alcohol consumption can increase prolactin and decrease oxytocin levels in pre- (Carlson et al., 1985; Lex et al., 1991; Mendelson et al., 1987; Sarkola et al., 1999) and postmenopausal (Ginsburg et al., 1996) women. A hypothesized hormonal mechanism that alcohol-induced changes in prolactin and oxytocin levels underlie the alcohol–breast cancer relationship merits further investigation.
Acknowledgments
This work was supported by NIH Grants R01AA09523 and NIDDK19525 and Diabetes Research Center Grant RR00040. We would like to acknowledge the insights of Dr. Karen Teff in designing aspects of this protocol during the early stages of the investigation and the expert technical assistance of Ms. A. Lorraine Norfleet, MHA, BSN, BS, RN and the nurses at the GCRC of the Hospital of the University of Pennsylvania, Dr. Heather Collins at the Diabetes Research Center, University of Pennsylvania, and Ms. Janice Kennedy, BS.
References
- Altemus M, Roca C, Galliven E, Romanos C, Deuster P. Increased Vasopressin and adrenocorticotropin responses to stress in the midluteal phase of the menstrual cycle. J Clin Endocrinol Metab. 2001;86:2525–2530. doi: 10.1210/jcem.86.6.7596. [DOI] [PubMed] [Google Scholar]
- Amico JA, Finley BE. Breast stimulation in cycling women, pregnant women and a woman with induced lactation: pattern of release of oxytocin, prolactin and luteinizing hormone. Clin Endocrinol (Oxf) 1986;25:97–106. doi: 10.1111/j.1365-2265.1986.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Becker U, Gluud C, Bennett P, Micic S, Svenstrup B, Winkler K, Christensen NJ, Hardt F. Effect of alcohol and glucose infusion on pituitary-gonadal hormones in normal females. Drug Alcohol Depend. 1988;22:141–149. doi: 10.1016/0376-8716(88)90049-x. [DOI] [PubMed] [Google Scholar]
- Bencker G, Jaspers C, Hausler G, Reinwein D. Control of prolactin secretion. Klin Wochenschr. 1990;68:1157–1167. doi: 10.1007/BF01815271. [DOI] [PubMed] [Google Scholar]
- Brzyski RG, Viniegra A, Archer DF. Suppression of luteal phase, but not midcycle, prolactin levels by chronic follicular phase opiate antagonism. Fertil Steril. 1997;68:855–859. doi: 10.1016/s0015-0282(97)00357-9. [DOI] [PubMed] [Google Scholar]
- Buckman MT, Peake GT, Srivastava LS. Periovulatory enhancement of spontaneous prolactin secretion in normal women. Metabolism. 1980;29:753–757. doi: 10.1016/0026-0495(80)90198-5. [DOI] [PubMed] [Google Scholar]
- Carlson HE, Wasser HL, Reidelberger RD. Beer-induced prolactin secretion: a clinical and laboratory study of the role of salsolinol. J Clin Endocrinol Metabol. 1985;60:673–677. doi: 10.1210/jcem-60-4-673. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. Prog Brain Res. 2001;133:241–249. doi: 10.1016/s0079-6123(01)33018-2. [DOI] [PubMed] [Google Scholar]
- Coiro V, Alboni A, Gramellini D, Cigarini C, Bianconi L, Pignatti D, Volpi R, Chiodera P. Inhibition by ethanol of the oxytocin response to breast stimulation in normal women and the role of endogenous opioids. Acta Endocrinol (Copenh) 1992;126:213–216. doi: 10.1530/acta.0.1260213. [DOI] [PubMed] [Google Scholar]
- De Leon RG, Austin KL, Richards L, Guerrero F. Lipid and hormonal profile of Panamanian women during the menstrual cycle. Int J Gynecol Obstet. 1992;39:219–226. doi: 10.1016/0020-7292(92)90660-b. [DOI] [PubMed] [Google Scholar]
- Dumitrescu RG, Cotarla I. Understanding breast cancer risk—where do we stand in 2005? J Cell Mol Med. 2005;9:208–221. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MF, Sobrinho LG, Santos MA, Sousa MF, Uvnas-Moberg K. Rapid weight gain, at least in some women, is an expression of a neuroendocrine state characterized by reduced hypothalamic dopaminergic tone. Psychoneuroendocrinology. 1998;23:1005–1013. doi: 10.1016/s0306-4530(98)00063-8. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Husslein P, Sumulong L, Micha JP, Dawood MY, Fuchs F. Plasma levels of oxytocin and 13, 14-dihydro-15-keto prostaglandin F2 alpha in preterm labor and the effect of ethanol and ritodrine. Am J Obstet Gynecol. 1982;144:753–759. doi: 10.1016/0002-9378(82)90347-7. [DOI] [PubMed] [Google Scholar]
- Fujimoto VY, Clifton DK, Cohen NL, Soules MR. Variability of serum prolactin and progesterone levels in normal women: the relevance of single hormone measurements in the clinical setting. Obstet Gynecol. 1990;76:71–78. [PubMed] [Google Scholar]
- Gill J. Women, alcohol and the menstrual cycle. Alcohol Alcohol. 1997;32:435–441. doi: 10.1093/oxfordjournals.alcalc.a008278. [DOI] [PubMed] [Google Scholar]
- Gill J. The effects of moderate alcohol consumption on female hormone levels and reproductive function. Alcohol Alcohol. 2000;35:417–423. doi: 10.1093/alcalc/35.5.417. [DOI] [PubMed] [Google Scholar]
- Ginsburg ES, Mello NK, Mendelson JH, Barbieri RL, Teoh SK, Rothman M, Gao X, Sholar JW. Effects of alcohol ingestion on estrogens in postmenopausal women. JAMA. 1996;276:1747–1751. doi: 10.1001/jama.1996.03540210055034. [DOI] [PubMed] [Google Scholar]
- Grayson RH, Halperin JM, Sharma V, Schwartz ST, Koda VH, Newcorn JH. Changes in plasma prolactin and catecholamine metabolite levels following acute needle stick in children. Psychiatry Res. 1997;69:27–32. doi: 10.1016/s0165-1781(96)03048-x. [DOI] [PubMed] [Google Scholar]
- Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Jr, Coates RJ, Liff JM, Talamini R, Chantarakul N, Koetsawang S, Rachawat D, Morabia A, Schuman L, Stewart W, Szklo M, Bain C, Schofield F, Siskind V, Band P, Coldman AJ, Gallagher RP, Hislop TG, Yang P, Kolonel LM, Nomura AM, Hu J, Johnson KC, Mao Y, De Sanjose S, Lee N, Marchbanks P, Ory HW, Peterson HB, Wilson HG, Wingo PA, Ebeling K, Kunde D, Nishan P, Hopper JL, Colditz G, Gajalanski V, Martin N, Pardthaisong T, Silpisornkosol S, Theetranont C, Boosiri B, Chutivongse S, Jimakorn P, Virutamasen P, Wongsrichanalai C, Ewertz M, Adami HO, Bergkvist L, Magnusson C, Persson I, Chang-Claude J, Paul C, Skegg DC, Spears GF, Boyle P, Evstifeeva T, Daling JR, Hutchinson WB, Malone K, Noonan EA, Stanford JL, Thomas DB, Weiss NS, White E, Andrieu N, Bremond A, Clavel F, Gairard B, Lansac J, Piana L, Renaud R, Izquierdo A, Viladiu P, Cuevas HR, Ontiveros P, Palet A, Salazar SB, Aristizabel N, Cuadros A, Tryggvadottir L, Tulinius H, Bachelot A, Le MG, Peto J, Franceschi S, Lubin F, Modan B, Ron E, Wax Y, Friedman GD, Hiatt RA, Levi F, Bishop T, Kosmelj K, Primic-Zakelj M, Ravnihar B, Stare J, Beeson WL, Fraser G, Bullbrook RD, Cuzick J, Duffy SW, Fentiman IS, Hayward JL, Wang DY, McMichael AJ, McPherson K, Hanson RL, Leske MC, Mahoney MC, Nasca PC, Varma AO, Weinstein AL, Moller TR, Olsson H, Ranstam J, Goldbohm RA, van den Brandt PA, Apelo RA, Baens J, de la Cruz JR, Javier B, Lacaya LB, Ngelangel CA, La Vecchia C, Negri E, Marubini E, Ferraroni M, Gerber M, Richardson S, Segala C, Gatei D, Kenya P, Kungu A, Mati JG, Brinton LA, Hoover R, Schairer C, Spirtas R, Lee HP, Rookus MA, van Leeuwen FE, Schoenberg JA, McCredie M, Gammon MD, Clarke EA, Jones L, Neil A, Vessey M, Yeates D, Appleby P, Banks E, Beral V, Bull D, Crossley B, Goodill A, Green J, Hermon C, Key T, Langston N, Lewis C, Reeves G, Collins R, Doll R, Peto R, Mabuchi K, Preston D, Hannaford P, Kay C, Rosero-Bixby L, Gao YT, Jin F, Yuan JM, Wei HY, Yun T, Zhiheng C, Berry G, Cooper Booth J, Jelihovsky T, MacLennan R, Shearman R, Wang QS, Baines CJ, Miller AB, Wall C, Lund E, Stalsberg H, Shu XO, Zheng W, Katsouyanni K, Trichopoulou A, Trichopoulos D, Dabancens A, Martinez L, Molina R, Salas O, Alexander FE, Anderson K, Folsom AR, Hulka BS, Bernstein L, Enger S, Haile RW, PaganiniHill A, Pike MC, Ross RK, Ursin G, Yu MC, Longnecker MP, Newcomb P, Bergkvist L, Kalache A, Farley TM, Holck S, Meirik O. Alcohol, tobacco and breast cancer—collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Individual differences in the biphasic effects of ethanol. Alcohol Clin Exp Res. 1998;22:1903–1911. [PubMed] [Google Scholar]
- Inns P, Morrison PJ, Pajoumond K. Evaluation of fuel cell alcometer for forensic and pharmacokinetic purposes. Br J Clin Pharmacol. 1979;7:439P–440P. doi: 10.1111/j.1365-2125.1979.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Kok P, Roelfsema F, Langendonk JG, de Wit CC, Frolich M, Burggraaf J, Meinders AE, Pijl H. Increased circadian prolactin release is blunted after body weight loss in obese premenopausal women. Am J Physiol Endocrinol Metab. 2006;290:E218–E224. doi: 10.1152/ajpendo.00156.2005. [DOI] [PubMed] [Google Scholar]
- Landgren B, Collins A, Csemiczky G, Burger HG, Baksheev L, Robertson D. Menopause transition: annual changes in serum hormonal patterns over the menstrual cycle in women during a nine-year period prior to menopause. J Clin Endocrinol Metab. 2004;89:2763–2769. doi: 10.1210/jc.2003-030824. [DOI] [PubMed] [Google Scholar]
- Leake RD, Buster JE, Fisher DA. The oxytocin secretory response to breast stimulation in women during the menstrual cycle. Am J Obstet Gynecol. 1984;148:457–460. doi: 10.1016/0002-9378(84)90726-9. [DOI] [PubMed] [Google Scholar]
- Lex BW, Ellingboe JE, Teoh SK, Mendelson JH, Rhoades E. Prolactin and cortisol levels following acute alcohol challenges in women with and without a family history of alcoholism. Alcohol. 1991;8:383–387. doi: 10.1016/0741-8329(91)90618-7. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Teoh SK. Neuroendocrine consequences of alcohol abuse in women. Ann N Y Acad Sci. 1989;562:211–240. doi: 10.1111/j.1749-6632.1989.tb21020.x. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Cristofaro P, Ellingboe J, Skupny A, Palmieri SL, Benedikt R, Schiff I. Alcohol effects on naloxone-stimulated luteinizing hormone, prolactin and estradiol in women. J Stud Alcohol. 1987;48:287–294. doi: 10.15288/jsa.1987.48.287. [DOI] [PubMed] [Google Scholar]
- Mendelson JA, Sholar MB, Mutschler NH, Jaszyna-Gasior M, Goletiani NV, Siegel AJ, Mello NK. Effects of intravenous cocaine and cigarette smoking on luteinizing hormone, testosterone, and prolactin in men. J Pharm Exp Ther. 2003;307:339–348. doi: 10.1124/jpet.103.052928. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, Teff KL. Acute alcohol consumption disrupts the hormonal milieu of lactating women. J Clin Endocrinol Metab. 2005;90:1979–1985. doi: 10.1210/jc.2004-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumenthaler MS, Taylor JL, Yesavage JA. Ethanol pharmacokinetics in white women: nonlinear model fitting versus zero-order elimination analyses. Alcohol Clin Exp Res. 2000;24:1353–1362. [PubMed] [Google Scholar]
- Murrell TG. The potential for oxytocin (OT) to prevent breast cancer: a hypothesis. Breast Cancer Res Treat. 1995;35:225–229. doi: 10.1007/BF00668213. [DOI] [PubMed] [Google Scholar]
- Noel GL, Suh HK, Frantz AG. Prolactin release during nursing and breast stimulation in postpartum and nonpostpartum subjects. J Clin Endocrinol Metab. 1974;38:413–423. doi: 10.1210/jcem-38-3-413. [DOI] [PubMed] [Google Scholar]
- Passie T, Hartmann U, Schneider U, Emrich HM, Krüger HC. Ecstasy (MDMA) mimics the post-orgasmic state: impairment of sexual drive and function during acute MDMA-effects may be due to increased prolactin secretion. Med Hypotheses. 2005;64:899–903. doi: 10.1016/j.mehy.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Perks CM, Keith AJ, Goodhew KL, Savage PB, Winters ZE, Holly JM. Prolactin acts as a potent survival factor for human breast cancer cell lines. Br J Cancer. 2004;91:305–311. doi: 10.1038/sj.bjc.6601947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perola M, Vuori E, Penttila A. Abuse of alcohol in sudden out-of-hospital deaths in Finland. Alcohol Clin Exp Res. 1994 Apr;18(2):255–260. doi: 10.1111/j.1530-0277.1994.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Sajonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, Fabbri F, Zanni G, Rgiatti P, Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47:164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Sarkola T, Makisalo H, Fukunaga T, Eriksson CJ. Acute effect of alcohol on estradiol, estrone, progesterone, prolactin, cortisol, and luteinizing hormone in premenopausal women. Alcohol Clin Exp Res. 1999;23:976–982. [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Serum prolactin levels in sons of alcoholics and control subjects. Am J Psychiatry. 1987;144:854–859. doi: 10.1176/ajp.144.7.854. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Parker DC, Rossman LR. Ethanol-related prolactin responses and risk for alcoholism. Biol Psychiatry. 1983;18:1153–1159. [PubMed] [Google Scholar]
- Shuvovski L, Healy DL, Findlay JK. Circulating immunoreactive oxytocin during the human menstrual cycle comes from the pituitary and is estradiol dependent. J Clin Endocrinol Metab. 1989;68:455–460. doi: 10.1210/jcem-68-2-455. [DOI] [PubMed] [Google Scholar]
- Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001;286:2143–2151. doi: 10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- Snowden EU, Khan-Dawood FS, Dawood MY. Opioid regulation of pituitary gonadotropins and prolactin in women using oral contraceptives. Am J Obstet Gynecol. 1986;154:440–444. doi: 10.1016/0002-9378(86)90687-3. [DOI] [PubMed] [Google Scholar]
- Sobrinho LG. Prolactin, psychological stress and environment in humans: adaptation and maladaptation. Pituitary. 2003;6:35–39. doi: 10.1023/a:1026229810876. [DOI] [PubMed] [Google Scholar]
- Stock SM, Sande EM, Bremme K. Leptin levels vary significantly during the menstrual cycle, pregnancy, and in vitro fertilization treatment: possible relation to estradiol. Fertil Steril. 1999;72:657–662. doi: 10.1016/s0015-0282(99)00321-0. [DOI] [PubMed] [Google Scholar]
- Volpi R, Chiodera P, Gramellini D, Cigarini C, Papadia C, Caffarri G, Rossi G, Coiro V. Endogenous opioid mediation of the inhibitory effect of ethanol on the prolactin response to breast stimulation in normal women. Life Sci. 1994;54:739–744. doi: 10.1016/0024-3205(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Vonderhaar BK. Prolactin: the forgotten hormone of human breast cancer. Pharmacol Ther. 1998;79:169–178. doi: 10.1016/s0163-7258(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Wennbo H, Tornell J. The role of prolactin and growth hormone in breast cancer. Oncogene. 2000;19:1072–1076. doi: 10.1038/sj.onc.1203349. [DOI] [PubMed] [Google Scholar]
- Widstrom AM, Winberg J, Werner S, Hamberger B, Eneroth P, Uvnas-Moberg K. Suckling in lactating women stimulates the secretion of insulin and prolactin without concomitant effects on gas-trin, growth hormone, calcitonin, vasopressin or catecholamines. Early Hum Dev. 1984;10:115–122. doi: 10.1016/0378-3782(84)90117-8. [DOI] [PubMed] [Google Scholar]


