Abstract
Background/aims: Advanced glycation end products (AGEs) are considered to act as mediators of both age related pathologies and diabetic complications. It was recently reported that glyceraldehyde derived AGE (AGE-2) has a strong biological effect on various diseases. The aim of this study was to investigate the serum AGE-2 levels in Vogt-Koyanagi-Harada (VKH) disease.
Methods: Sera were obtained from 31 patients with active VKH. 20 of these 31 patients were treated with systemic corticosteroids. As controls, 33 healthy volunteers were also examined. The serum AGE-2 levels were determined with a competitive enzyme linked immunosorbent assay using AGE-2 polyclonal antibody.
Results: The mean AGE-2 level in the sera of patients with VKH disease was 4.91 (SD 2.23) U/ml, which was significantly lower than that of the healthy control subjects (8.32 (2.94), p<0.001). The average serum AGE-2 level significantly increased to 13.49 (2.17) U/ml after the patients were treated with systemic corticosteroids (p<0.001).
Conclusions: These results suggest that AGE-2 may be involved in the onset of VKH disease.
Keywords: Vogt-Koyanagi-Harada disease, advanced glycation end products, melanocyte
Vogt-Koyanagi-Harada (VKH) disease is a bilateral, granulomatous panuveitis often associated with such extraocular organs as the auditory system, meninges, and the skin.1 The results of earlier studies indicated that VKH disease is a cell mediated autoimmune disorder which acts against melanocytes.2,3 As a result the melanocytes of every organ could be affected by this disease. As a genetic factor, both our colleagues and others have previously reported VKH disease to be closely related to some HLA haplotypes such as HLA-DRB1*0405, DRB1*0410, DQA1*03 and DQB1*04.4–6 However, some patients develop VKH disease even without demonstrating such HLA haplotypes. Since HLA typing does not greatly contribute to the diagnosis, today such patients are commonly treated with systemic corticosteroids.
Advanced glycation end products (AGEs) and Maillard reaction products are permanently modified protein derivatives which form in the presence of reducing sugars, such as glucose and fructose by non-enzymatic glycation, oxidation reactions, and further irreversible rearrangements in vivo and in heat treated food.7 AGEs are thus suggested to have a role in the diabetic complications of retinopathy and nephropathy, as well as the age related pathologies such as Alzheimer’s disease, a decreased skin elasticity, male erectile dysfunction, pulmonary fibrosis, and atherosclerosis.7 Although only a few scientists have so far focused on Maillard chemistry over the past five decades after its discovery, food scientists recently recognised that non-enzymatic glycation has an important role in food flavour, aroma, and nutritional bioavailability.7 The Maillard reaction has so far only been partially characterised, and the structures of some AGEs generated in vivo have never even been determined. Six distinct AGE classes (designated as AGE-1 to AGE-6) have been reported in the serum of diabetic patients.8,9,10,11 Recent studies have demonstrated that the receptor for AGE (RAGE) is expressed in human melanoma cells, and glyceraldehyde derived AGE (AGE-2) and glycolaldehyde derived AGE (AGE-3), but not other subtypes of AGEs, can thus upregulate the growth and migration of human melanoma cells.12 More recently, it was indicated that AGEs should be classified as non-toxic (N-carboxymethyllysine, pentosidine, etc) and toxic (AGE-2, AGE-3, etc); the latter are considered to be important in the pathophysiological processes of AGE formation.13 In this study, we hypothesised that if AGE-RAGE interactions can enhance the growth of melanoma cells, then low levels of AGE-2 might be detected in the sera of patients with acute VKH disease, an autoimmune disease against systemic melanocytes. Even if the AGEs are related to immunological tolerance to melanocytes, the serum levels of AGEs should increase again after the treatment of VKH disease.
PATIENTS AND METHODS
Thirty one patients with acute VKH who visited the uveitis survey clinic of Hokkaido University Hospital between 2000 and 2003 were examined. Each patient met the diagnostic criteria for VKH disease.14 They consisted of 13 men and 18 women, and their mean age was 42.7 (SD 15.2) years old (range 15–74 years old) at their first visits. None of them had previously received systemic corticosteroid agents. Thirty three age matched healthy volunteers served as controls. Although some of the patients received topical corticosteroid therapy, no subjects were treated with either systemic corticosteroids or immunosuppressive agents. After the diagnosis of VKH disease, all 31 patients received high dose corticosteroid therapy, starting with prednisolone 200 mg. Among the VKH patients there were three with diabetes mellitus without diabetic complications. No other systemic diseases were detected.
After obtaining informed consent in accordance with the tenets of the Declaration of Helsinki, sera were collected from 31 VKH patients with acute panuveitis (mean 42.7 (SD 15.2) years old) and 33 (14 men and 19 women) healthy volunteers (37.1 (11.5)). We also collected the sera from seven men and 13 women (46.4 (12.8) years old) of 31 VKH patients 4 weeks after being treated with systemic corticosteroids. None of the VKH patients demonstrated either ocular or systemic inflammation at the time.
The serum levels of AGE-2 were measured with a competitive enzyme linked immunosorbent assay (ELISA), as described previously.9 The results are expressed in AGE-2 units (U)/ml of the serum, with 1 U defined as the amount of AGE-2 antibody reactive materials that caused competition in ELISA to the same extent as did 1 μg of AGE-2-BSA standard. The same set of experiments was repeated three times and the results were averaged. Moreover, we also studied the fasting plasma glucose levels of 23 out of 31 VKH patients both at the acute stage before systemic corticosteroid therapy and at the convalescent stage under systemic medication to consider the effects of the fasting plasma glucose levels on the serum level of AGE-2. A statistical analysis was performed using Mann-Whitney U test or paired t test.
RESULTS
The mean serum levels of AGE-2 were measured as 4.91 (SD 2.23) U/ml in acute VKH patients and 8.32 (2.94) U/ml in healthy control subjects. The average level of AGE-2 in the sera of acute stage VKH patients was significantly lower than that of the healthy control subjects (p<0.001 by the Mann-Whitney U test) (fig 1). In addition, in 20 out of the 31 VKH patients, the average serum level of AGE-2 was 4.66 (1.81) U/ml at the acute stage and 13.49 (2.17) U/ml at the treated stage. The serum AGE-2 levels upregulated in all 20 VKH patients, following treatment with systemic corticosteroids and serum AGE-2 level of treated stage, was significantly higher than that at the acute stage (p<0.001 by paired t test) (fig 2).
Figure 1.
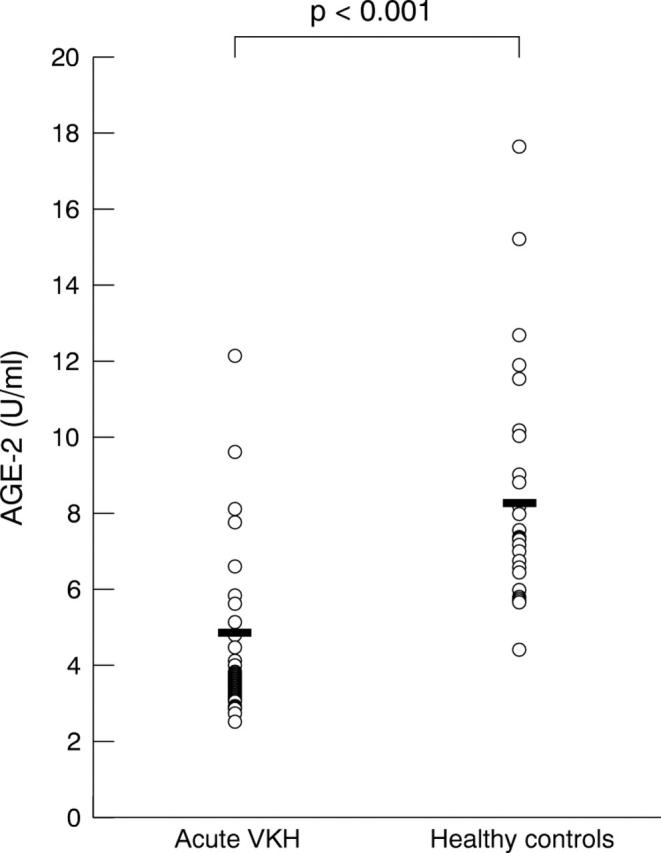
Serum levels of AGE-2 in patients with acute VKH and healthy controls. The average level of AGE-2 in the sera of acute stage VKH patients (4.91 (2.23)) was significantly lower than that of the healthy control subjects (8.32 (2.94)) (p<0.001). Statistical analysis was performed using Mann-Whitney U test.
Figure 2.
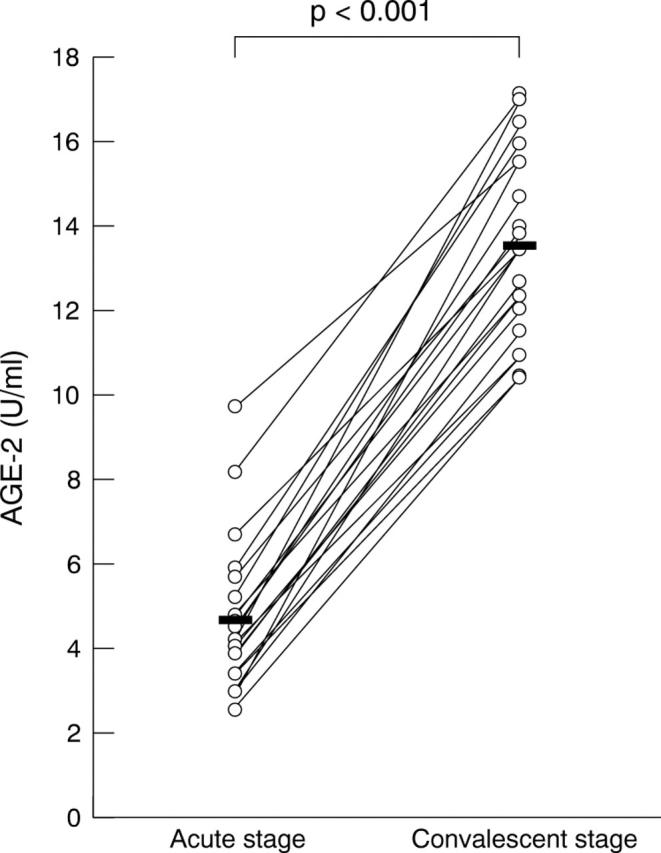
Serial observation of serum AGE-2 levels in VKH patients. Serum samples were collected 1 day before and 28 days after starting their systemic corticosteroid administration. The average level of AGE-2 was significantly elevated in patients treated with systemic corticosteroids (13.5 (2.17)) compared with that before treatment (4.66 (1.81)) (p<0.001). Statistical analysis was performed using paired t test.
Hyperglycaemia is a major cause for an increased serum AGE-2 level, because AGEs are the “end products” of glycation. To investigate whether the low levels of serum AGE-2 in VKH disease was associated with blood glucose levels, we next measured their fasting plasma glucose levels. The fasting plasma glucose levels of VKH patients were 109.8 (51.5) mg/dl at the acute stage and 81.5 (21.2) mg/dl at the convalescent stage. Although fasting plasma glucose level was significantly higher at the acute stage than at the convalescent stage in VKH patients (p<0.01 by paired t test), the levels were considered to be normal during the study period (fig 3).
Figure 3.
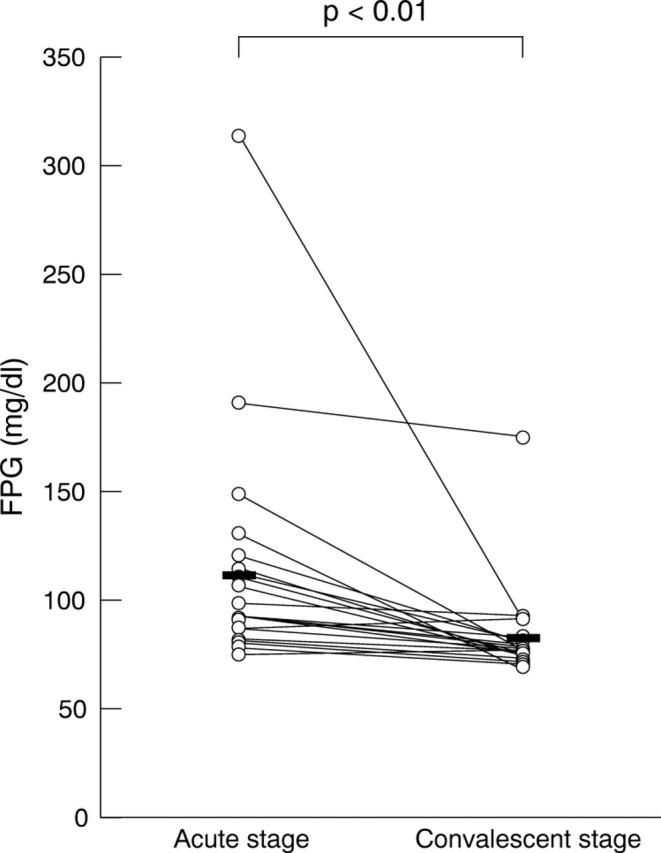
Serial observation of fasting plasma glucose levels in VKH patients. The samples were collected 1 day before and 28 days after starting their systemic corticosteroid administration. The average level of fasting plasma glucose was significantly decreased in patients treated with systemic corticosteroids (81.5 (21.2)) compared with that before treatment (109.8 (51.5)) (p<0.01). Statistical analysis was performed using paired t test.
DISCUSSION
The formation of advanced glycation end products (AGEs) is considered to be a key pathophysiological event with links to a range of important human diseases, and AGEs may act as mediators, not only of diabetic complications but also of widespread age related pathologies.7 AGEs have also been implicated in the development of some neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis.10 Besides these diseases, AGEs have also been regarded as an important pathological event in diabetic and age related ocular diseases.7,15
AGEs have been characterised as belonging to distinct classes such as AGE-1 to AGE-6, and their different cell mediated responses have recently been reported.8,9,16 In the present study, we focused on the serum AGE-2 levels of acute VKH patients, because AGE-2 showed a direct toxic effect in vitro.16
In this report, we demonstrated quite low levels of AGE-2 in the sera of patients with VKH disease at the exacerbation stage, and the levels also increased during the course of recovery. VKH disease is attributed to the destruction of melanocytes through immunological mechanisms, and antibodies and T cells specific for melanoma cells are also frequently detected, as well as normal melanocytes in VKH patients.2,3,17 Moreover, some patients have been reported to develop VKH disease following surgical treatment for malignant melanomas,18,19 while in some other patients with melanoma, vitiligo has been reported to develop either spontaneously or following immunotherapy.20 The laboratory data of VKH patients have also sometimes been described to be completely different from those with malignant melanoma. Though it is possible that AGE-RAGE interactions are involved in the onset of VKH disease besides malignant melanoma, further studies are needed to clarify their mechanisms of action. Experimental autoimmune anterior uveitis (EAAU) is an animal model for human endogenous uveitis induced by melanin associated proteins.21 If the AGE-RAGE interactions contribute to the destruction and/or proliferation of melanocytes in vivo, a study to modulate EAAU by varying the AGE-2 concentrations may thus help to explain the present results.
As reported elsewhere, most patients with VKH disease demonstrate glucose intolerance at onset of the disease; thereafter, such glucose tolerance tended to recover after treatment with systemic corticosteroids.22 It is thus reasonable that we detected high levels of fasting plasma glucose at the acute phase of the disease, and its decrease was accompanied by an improvement of VKH disease following systemic corticosteroid administration. Moreover, glucose intolerance has been reported to also be seen in active rheumatoid arthritis but it improved after corticosteroid therapy.23 In cases of rheumatoid arthritis, glucose intolerance has been suggested to be related to insulin resistance.23 However, it remains unclear as to how AGEs are involved in insulin resistance. Further studies on the interaction between AGEs and insulin resistance will be needed in the near future.
Although the biochemical mechanisms still remain unclear at the onset of some autoimmune diseases, this is the first report to show the dramatic decrease in the serum AGE-2 levels at the exacerbation stage of VKH, and the increase in those levels after recovery. It is possible that the AGE-2 associated mechanisms might therefore be involved in the onset of VKH disease.
Acknowledgments
This study was supported in part by a grant for research on sensory and communicative disorders from the Ministry of Health, Labor, and Welfare Japan, and by grants in aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Japan.
Abbreviations
AGEs, advanced glycation end products
EAAU, experimental autoimmune anterior uveitis
ELISA, enzyme linked immunosorbent assay
RAGE, receptor for AGE
VKH, Vogt-Koyanagi-Harada
REFERENCES
- 1.Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol 1995;39:265–92. [DOI] [PubMed] [Google Scholar]
- 2.Norose K, Yano A. Melanoma specific Th1 cytotoxic T lymphocyte lines in Vogt-Koyanagi-Harada disease. Br J Ophthalmol 1996;80:1002–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugita S, Sagawa K, Mochizuki M, et al. Melanocyte lysis by cytotoxic T lymphocytes recognizing the MART-1 melanoma antigen in HLA-A2 patients with Vogt-Koyanagi-Harada disease. Int Immunol 1996;8:799–803. [DOI] [PubMed] [Google Scholar]
- 4.Islam SM, Numaga J, Fujino Y, et al. HLA class II genes in Vogt-Koyanagi-Harada disease. Invest Ophthalmol Vis Sci 1994;35:3890–6. [PubMed] [Google Scholar]
- 5.Shindo Y, Inoko H, Yamamoto T, et al. HLA-DRB1 typing of Vogt-Koyanagi-Harada’s disease by PCR-RFLP and the strong association with DRB1*0405 and DRB1*0410. Br J Ophthalmol 1994;78:223–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shindo Y, Ohno S, Yamamoto T, et al. Complete association of the HLA-DRB1*04 and -DQB1*04 alleles with Vogt-Koyanagi-Harada’s disease. Hum Immunol 1994;39:169–76. [DOI] [PubMed] [Google Scholar]
- 7.Stitt AW. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol 2001;85:746–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi M, Makita Z, Yanagisawa K, et al. Detection of noncarboxymethyllysine and carboxymethyllysine advanced glycation end products (AGE) in serum of diabetic patients. Mol Med 1999;5:393–405. [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi M, Makita Z, Bucala R, et al. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol Med 2000;6:114–25. [PMC free article] [PubMed] [Google Scholar]
- 10.Takeuchi M, Yanase Y, Matsuura N, et al. Immunological detection of a novel advanced glycation end-product. Mol Med 2001;7:783–91. [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi M, Makita Z. Alternative routes for the formation of immunochemically distinct advanced glycation end-products in vivo. Curr Mol Med 2001;1:305–15. [DOI] [PubMed] [Google Scholar]
- 12.Abe R, Shimizu T, Sugawara H, et al. Regulation of human melanoma growth and metastasis by AGE-AGE receptor interactions. J Invest Dermatol 2004;122:461–7. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi M, Yamagishi S. TAGE (toxic AGEs) hypothesis in various chronic diseases. Med Hypotheses 2004;63:449–52. [DOI] [PubMed] [Google Scholar]
- 14.Read RW, Holland GN, Rao NA, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol 2001;131:647–52. [DOI] [PubMed] [Google Scholar]
- 15.Howes KA, Liu Y, Dunaief JL, et al. Receptor for advanced glycation end products and age-related macular degeneration. Invest Ophthalmol Vis Sci 2004;45:3713–20. [DOI] [PubMed] [Google Scholar]
- 16.Takeuchi M, Bucala R, Suzuki T, et al. Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J Neuropathol Exp Neurol 2000;59:1094–105. [DOI] [PubMed] [Google Scholar]
- 17.Kiniwa Y, Fujita T, Akada M, et al. Tumor antigens isolated from a patient with vitiligo and T-cell-infiltrated melanoma. Cancer Res 2001;61:7900–7. [PubMed] [Google Scholar]
- 18.Sober AJ, Haynes HA. Uveitis, poliosis, hypomelanosis, and alopecia in a patient with malignant melanoma. Arch Dermatol 1978;114:439–41. [PubMed] [Google Scholar]
- 19.Aisenbrey S, Luke C, Ayertey HD, et al. Vogt-Koyanagi-Harada syndrome associated with cutaneous malignant melanoma: an 11-year follow-up. Graefes Arch Clin Exp Ophthalmol 2003;241:996–9. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami Y, Suzuki Y, Shofuda T, et al. T cell immune responses against melanoma and melanocytes in cancer and autoimmunity. Pigment Cell Res 2000;13 (Suppl 8) :163–9. [DOI] [PubMed] [Google Scholar]
- 21.Broekhuyse RM, Kuhlmann ED, Winkens HJ, et al. Experimental autoimmune anterior uveitis (EAAU), a new form of experimental uveitis. I. Induction by a detergent-insoluble, intrinsic protein fraction of the retinal pigment epithelium. Exp Eye Res 1991;52:465–74. [DOI] [PubMed] [Google Scholar]
- 22.Yawata N, Nakamura S, Kijima M, et al. High incidence of glucose intolerance in Vogt-Koyanagi-Harada disease. Br J Ophthalmol 1999;83:39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svenson KL, Pollare T, Lithell H, et al. Impaired glucose handling in active rheumatoid arthritis: relationship to peripheral insulin resistance. Metabolism 1988;37:125–30. [DOI] [PubMed] [Google Scholar]


