Linezolid is a new oxazolidinone antibiotic with activity against many important pathogens including methicillin resistant Staphylococcus and penicillin resistant Streptococcus.1 We report a case of toxic optic neuropathy from chronic treatment with linezolid. Correct diagnosis and discontinuation of the drug resulted in significant recovery of vision.
Case report
A 56 year old man presented with bilateral, progressive decline in visual acuity for 6–8 months. Medical history included chronic diabetes mellitus, below the knee amputation of the right leg, hypertension, sinusitis with nasal allergies, and asthma. Several years earlier he had fractured his left ankle and developed osteomyelitis from methicillin resistant Staphylococcus. He had received linezolid 600 mg by mouth twice a day for 12 months, then once daily for 44 months. Other medications included rosiglitazone, metoprolol, rifampin, furosemide, lisinopril, amlodipine, insulin, and vitamin B complex/folic acid for assistance with wound healing. He was a non-smoker and consumed less than one unit of alcohol per week.
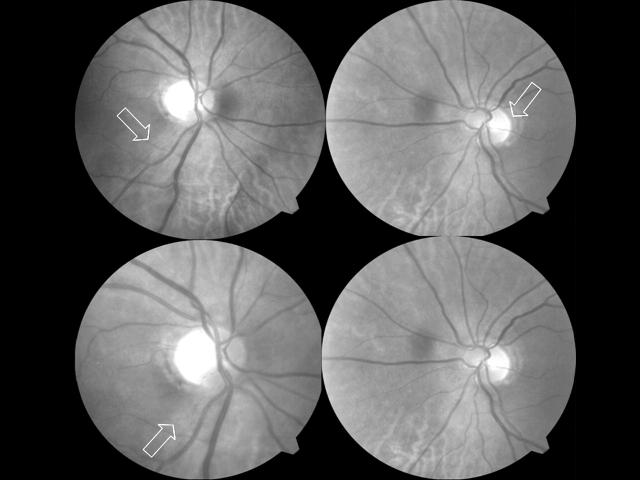
Best corrected visual acuities were 20/400 in both eyes with eccentric fixation. Ishihara colour plates were 1/8 right eye and 3/8 left eye. No relative afferent pupillary defect was present. Intraocular pressures were 16 mm Hg in both eyes. 1+ nuclear sclerosis was present in both eyes. Fundus examination revealed temporal optic nerve pallor with a corresponding temporal nerve fibre layer defect more evident in the right eye (fig 1) and a normal macula in both eyes. Humphrey visual field testing (full field 120 point screen) revealed central scotomas in both eyes (fig 2). Fluorescein angiography revealed a normal macula without staining of the peripapillary region in both eyes (not shown). Optical coherence tomography (Stratus OCT, Carl Zeiss Ophthalmic Systems Inc, Humphrey Division, Dublin, CA, USA) of the fovea revealed a central foveal thickness of 205 (SD 5) μm right eye and 216 (4) μm left eye and a normal macular volume (7.28 mm3 right eye; 6.94 mm3 left eye). Retinal nerve fibre layer thickness analysis by OCT revealed a normal 360° average measurement (79.55 μm right eye, 80.57 μm left eye) with no significant change in thickness detected in the temporal quadrant (64 μm right eye and 58 μm left eye). Full field scotopic and photopic electroretinography demonstrated a normal amplitude and latency in both eyes, as expected given the small central scotoma.
Figure 1.
Top, photograph suggests subtle temporal pallor to the optic nerve in the right eye with a nerve fibre layer defect temporally (arrow, upper left); left eye appears normal (arrow, upper right) with no evident nerve fibre layer defect. Bottom, 3 months after discontinuing linezolid there is temporal pallor and nerve fibre layer defects in the right eye (lower left), again with no definite changes in the left eye (lower right).
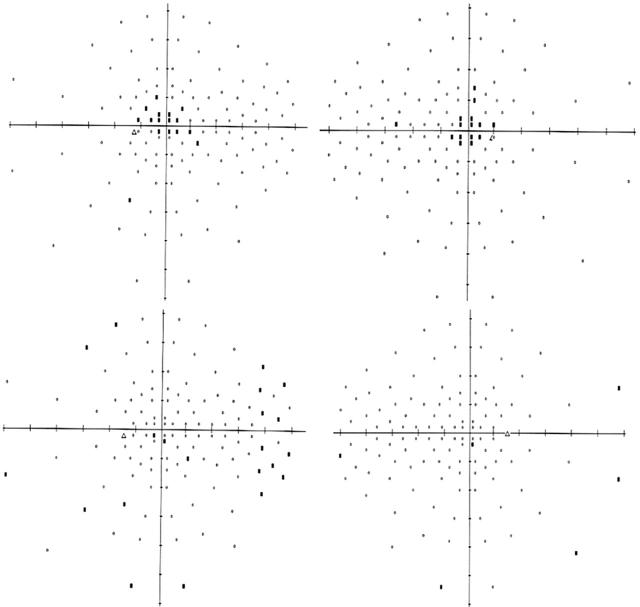
Figure 2.
Humphrey full field 120. Top left and right, left eye and right eye, respectively, demonstrating central scotomas at presentation. Bottom left and right, left eye and right eye, respectively, showing improvement in the central scotomas 3 months after discontinuation of linezolid.
The patient was diagnosed with bilateral optic neuropathy; chronic use of linezolid was suspected as the cause. Linezolid was discontinued and the patient noted subjective visual improvement within several weeks. Three months later his vision improved to 20/40 in both eyes with resolution of the central scotomas (fig 2). There were 8/15 and 13/15 central fixation losses in the right and left eye respectively initially; this improved to 4/15 right eye and 2/16 left eye 3 months after discontinuing therapy.
Comment
Toxic optic neuropathy has been associated with numerous compounds, including exposure to ethylene glycol, methanol, isoniazid, ethambutol, and fluoroquinolone, and deficiency of vitamin B12, folate, and thiamine.2,3 Linezolid was approved by the FDA, based on studies employing 28 days of administration.1 There have been five cases of optic neuropathy associated with prolonged use of linezolid3–6 and more than 20 cases of peripheral neuropathy including one individual who developed both sequelae.6–9 In previous optic neuropathy cases the duration of treatment ranged from 5–10 months at a dose of 600 mg once or twice per day.3–6 All cases were bilateral with initial vision decreased from 20/60 to counting fingers both eyes.3–6 Discontinuation of therapy resulted in improvement of the optic neuropathy with significant improvement in visual acuity within 1–8 months in all patients, although residual deficits in central acuity or visual evoked response may persist.3–6
Oxazolidinones inhibit bacterial protein synthesis by binding to the 70S ribosomal initiation complex.10 In nutritional optic neuropathies, paracentral scotomas develop from disruption in mitochondrial function in retinal ganglion cells,8 which are more susceptible to mitochondrial disruption.2 Mitochondrial dysfunction is the cause of Leber’s hereditary optic neuropathy, chloramphenicol induced bone marrow suppression, and optic neuropathy due to ethambutol and a variety of antibiotics.2,11,12 It is likely that the development of linezolid associated optic neuropathy, manifest by the development of central scotomas and temporal optic nerve pathology, may be the result of a similar mechanism.
It is important for ophthalmologists to perform a complete review of systems and elicit a history of prescription and non-prescription medication use. Awareness of the potential for linezolid induced optic neuropathy is important since drug withdrawal can lead to visual recovery.
References
- 1.Birmingham MC, Rayner CR, Meagher AK, et al. Linezolid for the treatment of multidrug-resistant, gram-positive infections: experience from a compassionate-use program. Clin Infect Dis 2003;36:159–68. [DOI] [PubMed] [Google Scholar]
- 2.Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res 2004;23:53–89. [DOI] [PubMed] [Google Scholar]
- 3.Lee E, Burger S, Shah J, et al. Linezolid-associated toxic optic neuropathy: a report of 2 cases. Clin Infect Dis 2003;37:1389–91. [DOI] [PubMed] [Google Scholar]
- 4.Frippiat F, Bergiers C, Michel C, et al. Severe bilateral optic neuritis associated with prolonged linezolid therapy. J Antimicrob Chemother 2004;53:1114–15. [DOI] [PubMed] [Google Scholar]
- 5.Lewis KE, Ebden P, Wooster SL, et al. Multi-system infection with Nocardia farcinica—therapy with linezolid and minocycline. J Infect 2003;46:199–202. [DOI] [PubMed] [Google Scholar]
- 6.Corallo CE, Paull AE. Linezolid-induced neuropathy. Med J Aust 2002;177:332. [DOI] [PubMed] [Google Scholar]
- 7.Bressler AM, Zimmer SM, Gilmore JL, et al. Peripheral neuropathy associated with prolonged use of linezolid. Lancet Infect Dis 2004;4:528–31. [DOI] [PubMed] [Google Scholar]
- 8.Rho JP, Sia IG, Crum BA, et al. Linezolid-associated peripheral neuropathy. Mayo Clin Proc 2004;79:927–30. [DOI] [PubMed] [Google Scholar]
- 9.Legout L, Senneville E, Gomel JJ, et al. Linezolid-induced neuropathy. Clin Infect Dis 2004;38:767–8. [DOI] [PubMed] [Google Scholar]
- 10.Shinabarger DL, Marotti KR, Murray RW, et al. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob Agents Chemother 1997;41:2132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skinnider LF, Ghadially FN. Chloramphenicol-induced mitochondrial and ultrastructural changes in hemopoietic cells. Arch Pathol Lab Med 1976;100:601–5. [PubMed] [Google Scholar]
- 12.Bernstein WB, Trotta RF, Rector JT, et al. Mechanisms for linezolid-induced anemia and thrombocytopenia. Ann Pharmacother 2003;37:517–20. [DOI] [PubMed] [Google Scholar]