SUMMARY
Cargo partitioning into intralumenal vesicles (ILVs) of multivesicular endosomes underlies such cellular processes as growth factor down-regulation, viral budding, and biogenesis of lysosome-related organelles including melanosomes. Here we show that the melanosomal protein, Pmel17, is sorted into ILVs by a novel mechanism that is conserved in non-pigment cells and is dependent upon lumenal determinants. ILV targeting of Pmel17 is unaffected by mutagenesis of cytoplasmic lysine and cysteine residues or replacement of the cytoplasmic domain, indicating independence of ubiquitylation, and unlike ILV targeting of ubiquitylated cargo, is insensitive to functional inhibition of Hrs and ESCRT complexes. Chimeric protein and deletion analyses indicate that two N-terminal lumenal sub-domains are necessary and sufficient for ILV targeting. Pmel17 fibril formation, which occurs during melanosome maturation in melanocytes, requires a third lumenal sub-domain and proteolytic processing that itself requires ILV localization. These results establish a novel Hrs- and perhaps ESCRT-independent pathway of ILV sorting by lumenal determinants and a requirement for ILV sorting in fibril formation.
Keywords: melanosome, endosome, multivesicular body, fibril formation, amyloid
Abbreviations: EGFR, EGF receptor; ESCRT, endosomal sorting complex required for transport; HPM, Hrs-positive membranes; IEM, immunoelectron microscopy; IFM, immunofluorescence microscopy; ILV, intralumenal vesicle; LBPA, lysobisphosphatidic acid; LRO, lysosome-related organelle; MVB, multivesicular body; PC, proprotein convertase; PKD, polycystic kidney disease-1 domain; Tf, transferrin; Ubq, ubiquitin; WT, wild-type
INTRODUCTION
The endocytic pathway is composed of distinct organelles and subdomains, each with a unique function in processing and partitioning endocytic cargo (Gruenberg, 2001; Gruenberg and Stenmark, 2004; Maxfield and McGraw, 2004). Maturation from early to late endosomes requires removal of cargo bound for the cell surface or the biosynthetic pathway and targeting of other cargo to the endosome limiting membrane or lumen. Within vacuolar regions of early endosomes, selected protein and lipid cargoes partition into membrane domains that invaginate to form intralumenal vesicles (ILVs), which accumulate during maturation into multivesicular late endosomes (multivesicular bodies or MVBs; Gruenberg and Stenmark, 2004; Katzmann et al., 2002). Protein sorting into ILVs is critical for such processes as growth factor down-regulation (Raiborg et al., 2003; Urbé et al., 2003), antigen presentation (Zwart et al., 2005), exosome formation for cell-cell communication (Février and Raposo, 2004), formation of lysosome-related organelles (LROs; Dell’Angelica et al., 2000), and viral budding (Morita and Sundquist, 2004). Delineating the molecular mechanisms that regulate this sorting step may provide targets for therapeutic manipulation.
Among the best characterized ILV cargoes is the EGF receptor (EGFR). Upon ligand binding, EGFR is endocytosed and targeted to ILVs within early endosomes; this terminates signaling by sequestering the receptor from the cytoplasm and facilitating degradation in lysosomes (Gruenberg and Stenmark, 2004). EGFR sorting to ILVs requires conjugation and recognition of ubiquitin (Ubq) by cytoplasmic proteins, such as Hrs and STAM, within clathrin-coated limiting membrane domains of vacuolar endosomes (Bache et al., 2003b; Raiborg et al., 2002; Urbé et al., 2003). Ubiquitylated receptors are thought to be serially transferred from Hrs/STAM and incorporated into forming ILVs by downstream endosomal sorting complexes required for transport (ESCRT)-1, -2 and –3 in concert with Alix/AIP-4 and an AAA-ATPase (hVps4), the latter required for recycling ESCRT complexes (Babst, 2005; Bache et al., 2003a; Katzmann et al., 2003). The “ESCRT pathway” was first identified in yeast and is required for delivery of long-lived enzymes, such as carboxypeptidase S, to the vacuole lumen (Katzmann et al., 2001). Whether this pathway participates in ILV delivery of long-lived proteins in mammalian cells has yet to be established.
Not all cargo is recruited to ILVs by the same mechanism. For example, Sna3p (Bilodeau et al., 2002) and Cvt17p (Epple et al., 2003) in yeast and human δ–opioid receptor (Hislop et al., 2004) do not require direct ubiquitylation and/or engagement by the Ubq interacting motifs of Hrs and STAM (or their yeast orthologues) to be incorporated into ILVs. Some of these cargoes may access ILVs by associating with ubiquitylated proteins and engaging Hrs (Hislop et al., 2004) or by binding downstream ESCRT components (Geminard et al., 2004; Strack et al., 2003; Stuchell et al., 2004; von Schwedler et al., 2003). However, other mechanisms may exist. For example, ILVs enriched in the lipid lysobisphosphatidic acid (LBPA) are found in distinct MVBs from those enriched in cholesterol (Möbius et al., 2003) or EGFR (White et al., 2005), and only the latter MVBs accumulate upon EGF stimulation in an annexin1-dependent manner (White et al., 2005). LBPA can induce ILV formation in acidified synthetic liposomes in the absence of protein, suggesting that lipid-driven and ESCRT-dependent pathways may coexist (Matsuo et al., 2004). To date, however, no mammalian protein cargo has been shown to follow an ESCRT-independent ILV pathway.
MVBs are intermediates not only for lysosomal degradation but also for the formation of LROs such as cytotoxic T cell granules and melanosomes (Raposo and Marks, 2002; Stinchcombe et al., 2004). Tissue-specific cargoes localize to ILVs of LROs, as exemplified by the melanosome constituent, Pmel17 (Pmel). In melanocytes, Pmel is incorporated into ILVs within vacuolar endosomes characterized by a bilayered coat (Raposo et al., 2001), morphologically similar to Hrs-coated endosomes (Sachse et al., 2002). During transit to/through endosomes, Pmel is cleaved within the lumenal domain by a proprotein convertase (PC), liberating a lumenal fragment with fibrillogenic activity (Berson et al., 2003). Segregation of the resulting amyloid-like fibrils (Fowler et al., 2006) from endosomes marks the biogenesis of the stage II melanosome, in which the fibrils form a “matrix” for melanin deposition as the melanosome matures (Marks and Seabra, 2001). Stage II melanosomes segregate from endosomes only in melanocytes; however, Pmel is enriched on ILVs and forms fibrils even upon ectopic expression in non-pigment cells (Berson et al., 2001), indicating that both properties reflect conserved mechanisms that are not limited to pigment cells. To understand the role of ILVs in fibril formation, we have dissected the molecular requirements for sorting of Pmel to ILVs. We reveal a novel mechanism for ILV localization that is conserved in non-pigment cells, dependent solely on lumenal determinants, independent of ubiquitylation, and resistant to inhibition of ESCRT pathway function. Moreover, we show that ILVs are requisite intermediates in the biogenesis of melanosome fibrils, at least in part due to a requirement for ILV sorting in Pmel proteolytic processing.
RESULTS
Sorting of Pmel onto ILVs is insensitive to interference with Hrs and ESCRT machinery
Pmel is sorted to ILVs in clathrin-coated early endosomes as an intermediate en route to fibrillar stage II melanosomes, such that approximately 10% of mature Pmel17 resides in multivesicular endosomes at steady state (Raposo et al., 2001). Immunoelectron microscopy (IEM) analyses of double-labeled ultrathin cryosections of human MNT-1 melanoma cells show extensive labeling for Hrs on Pmel-containing endosomes (Suppl. Fig. S1). Moreover, Pmel on the limiting membrane is found within Hrs-positive, clathrin-coated subdomains (Suppl. Fig. S1A), and many of the Pmel-containing internal membranes (small arrows, Suppl. Fig. S1A, B) are adjacent to Hrs-coated regions (large arrows). The Hrs-coated Pmel-positive endosomes are highly tubulated as observed by IEM (arrowheads, Suppl. Fig. S1B) and by electron microscopy analysis of whole mounted MNT-1 cells (arrowhead, Suppl. Fig. S1C). This pattern of localization mimics that of ubiquitylated cargo such as EGFR (Sachse et al., 2002), which requires Hrs for sorting to ILVs.
To probe the dependence of Pmel sorting on Hrs and downstream ESCRT machinery, we used dominant-negative and siRNA approaches. Overexpression of Hrs, a multifunctional adaptor protein, disrupts ILV localization and degradation of EGFR or a transferrin (Tf)-Ubq conjugate (Raiborg et al., 2002; Urbé et al., 2003). ESCRT-I function is similarly inhibited by overexpression of its subunit, Tsg101 (Demirov et al., 2002; Goila-Gaur et al., 2003), and the ESCRT pathway is more globally inhibited by overexpression of an ATP binding-defective form of hVps4b [Vps4(K173A); Bishop and Woodman, 2000)]. We compared the effects of overexpressing these transgenes in transfected 1011-mel human melanoma cells or of depleting Hrs in siRNA-transduced MNT-1 melanoma cells on the steady-state localization of Pmel and known Ubq-ESCRT cargo using immunofluorescence microscopy (IFM) and IEM.
MART-1, a substrate for Ubq-dependent lysosomal sorting (Lévy, et al, 2005), localizes to fine puncta throughout the cytoplasm of control cells with some perinuclear accumulation, as previously shown (de Mazière et al., 2002) (Fig. 1a–c). Ubiquitylated proteins (X-Ubq) are observed evenly throughout the cytoplasm of control cells and often in the nucleus, with only rare cytoplasmic inclusions (Fig. 2a–c). As expected, expression of transgenes for 24 h or more block cargo degradation and sorting to ILVs, as shown by the accumulation of both MART-1 and X-Ubq in huge aggregates of Hrs-positive membranes (HPM) in cells overexpressing myc-tagged Hrs (arrows, Figs. 1d–f, 2d–f), or in enlarged ring structures in cells overexpressing HA-tagged Tsg101 (arrowheads, Figs. 1g–i, 2g–i) or V5-tagged Vps4(K173A) (arrows, Figs. 1j–l, 2j–l). EGFR sorting and degradation are also inhibited by myc-Hrs overexpression, as shown by EGFR-EYFP distribution to HPM (Suppl. Fig. S2a–f), although less consistently affected by overexpression of V5-Vps4(K173A) or HA-Tsg101 (Suppl. Fig. S2g–l show examples in which there was a clear effect; the lack of consistency may reflect a potential defect in the EGF signaling pathway in these melanoma cells, which do not express detectable endogenous EGFR). The extent of endosomal disruption by myc-Hrs overexpression is reflected by the nearly stoichiometric trapping of the late endosomal SNARE protein, syntaxin 7 (Suppl. Fig. S2m–o), and the recycling Tf receptor (not shown) in HPM. By contrast, Pmel distribution is completely unaffected by transgene overexpression in a high fraction of cells with disrupted MART-1, X-Ubq or EGFR-EYFP localization (Figs. 1, 2, Suppl. Fig. S2). Pmel is largely excluded from the aberrant HPM or ring-like structures, and is consistently found in disperse puncta characteristic of stage II melanosomes as in control cells. Only in cells with prolonged (48 rather than 24 h) and/or extraordinarily high levels of myc-Hrs or V5-Vps4(K173A) expression, likely causing indirect effects on endosomal transport (judged by missorting of the lysosomal limiting membrane protein LAMP-1), is Pmel distribution altered relative to controls; even in these cases, Pmel labeling is largely non-overlapping with that for MART-1, X-Ubq, or EGFR-EYFP (Suppl. Fig. S3), and in those structures that do overlap, Pmel labeling is often found surrounded by labeling for MART-1 or X-Ubq (arrow insets, Suppl. Fig. S3a–c). Consistently, siRNA-mediated depletion of Hrs from a second melanoma cell line, MNT-1, caused a dramatic loss of MART-1 labeling but had no effect on Pmel distribution (Fig. 3C). By comparison, Pmel is dramatically redistributed to large half-moon or ring-like structures by a 2 h treatment with vacuolin-1 (arrows, Suppl. Fig. S4), a drug that independently interferes with or reverses ILV formation (Cerny et al., 2004). Together, the data indicate that Pmel distribution is relatively insensitive to dominant negative interference with the Hrs/ESCRT pathway or to Hrs depletion.
Figure 1. Overexpression of Hrs, Tsg101, or Vps4(K173A) alters the distribution of MART-1 but not Pmel.

Representative images from IFM analysis of 1011-mel cells transfected 24 h prior to acetone fixation with myc-Rab27a as a control (a–c), myc-Hrs (d–f), HA-Tsg101 (g–i), or V5-hVps4b(K173A) (j–l) and labeled for the myc (a, d), HA (g) or V5 (j) epitope tag, MART-1 (b, e, h, k) or Pmel (c, f, i, l, using antibody NKI-beteb) and fluorochrome-conjugated secondary antibodies. Insets, 5X magnification of indicated regions as blue/green (d, g, j), green/red (e, h, k) and blue/red (f, i, l) merged images. Note that acetone fixation, required to preserve MART-1 immunogenicity, reduces cytoplasmic labeling for V5-hVps4b(K173A) relative to formaldehyde-fixation (Figs. 2, S2, S3). Note the accumulation of MART-1, but not Pmel, within large HPM in d and e (arrows) and the ring-like structures in g and h (arrowhead, insets). Bar 28 μm.
Figure 2. Overexpression of Hrs, Tsg101, or Vps4(K173A) alters the distribution of X-Ubq, but not Pmel.

Experiment is the same as in Fig. 1, except that cells were fixed with formaldehyde and labeled for the epitope tag (a, d, g, j), X-Ubq (b, e, h, k) or Pmel (c, f, i, l). Note the accumulation of X-Ubq in HPM (e and arrows) and ring-like structures (h, k inset arrow) and the exclusion of Pmel from them (f, i, l). Insets, 5X magnification of indicated regions and merged channels as in Fig. 1. Bar, 20 μm.
Figure 3. Pmel, but not MART-1, is present on ILVs in Hrs-overexpressing or Hrs-depleted cells.
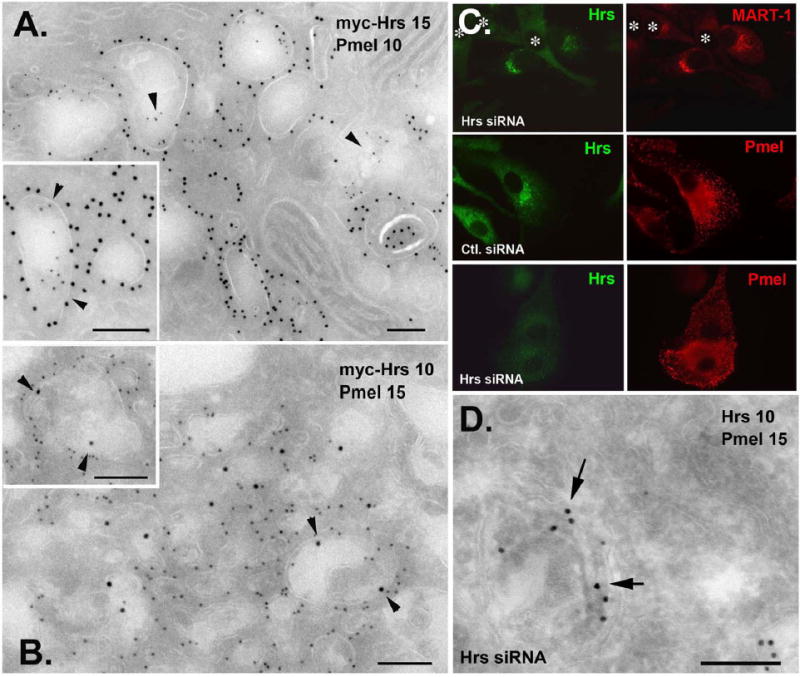
A. Ultrathin cryosections of FACS-sorted, myc-Hrs-overexpressing 1011-mel cells were immunogold labeled for myc (PAG15) and Pmel (PAG10). Pmel is localized on internal membranes of Hrs-positive compartments (arrowheads, inset). B. The same cells were immunogold labeled for myc (PAG10) and MART-1 (PAG15). Note the label for MART-1 on the limiting membrane of Hrs-positive compartments. The density of MART-1 label is similar to that of untransfected cells (see Suppl. Fig. 5A). C. and D. MNT-1 cells were transfected with Hrs or control siRNA, and analyzed by IFM (C) or IEM (D) with antibodies to Hrs and either MART-1 or Pmel as indicated. Transfected cells in C are indicated by *. Note the absence of label for MART-1 in Hrs-depleted cells (C, top). D, note labeling for Pmel (PAG15) over fibrils and ILVs in a Hrs-depleted cell. Bars, 200 nm.
To confirm Pmel localization to ILVs in cells with disrupted Hrs/ESCRT function, we analyzed transfected cells by IEM. The HPM of FACS-sorted myc-Hrs-overexpressing cells are membrane-bound structures with vacuolar and tubular areas still bearing ILVs and other internal membranes (Fig. 3A, B; Suppl. Fig. S5B, C). The ILVs are less numerous and less highly contrasted by uranyl acetate staining than those in controls, perhaps reflecting partial inhibition of ILV formation and depletion of protein cargo from those ILVs that do form (Suppl. Fig. S5C). MART-1, which is normally localized extensively to ILVs within Hrs-coated and uncoated MVBs (Suppl. Fig. S5A and de Mazière et al., 2002), is observed only on the limiting membrane of HPM (Fig. 3B and inset). By contrast, Pmel is observed on both the limiting membrane and on ILVs (Fig. 3A inset arrowheads) as in untreated cells. Consistent with the transient accumulation of Pmel in MVBs in untreated cells (Raposo et al., 2001), Pmel in Hrs-overexpressing cells is also observed on structures with intralumenal striations characteristic of stage II melanosomes, as well as unusual multivesicular endosomes that lack labeling for Hrs (Suppl. Fig. S5B). Consistent with the lack of an effect on Pmel transport kinetics, immunoblotting of whole cell lysates of FACS-sorted myc-Hrs-overexpressing cells shows comparable steady-state levels of Pmel processing intermediates relative to controls (Suppl. Fig. S6). Similarly, in MNT-1 cells depleted of Hrs with siRNA, labeling for Pmel was observed over ILVs and intralumenal striations (Fig. 3D); consistent with IFM analyses, labeling for MART-1 was not detected, likely reflecting enhanced degradation. Thus, despite expected effects on MART-1, neither Hrs overexpression nor Hrs depletion prevents Pmel from partitioning to ILVs or progressing to fibril formation. In cells expressing HA-Tsg101, an extensive tubular membrane network accumulates, similar to that observed in Tsg101-depleted cells (Doyotte et al., 2005, although stacked cisternae were not observed); some of these membranes harbor poorly contrasted internal membranes, many of which are immunogold labeled for Pmel (Suppl. Fig. S7). These data indicate that formation of ILVs and sorting of Pmel to them continues upon disruption of Hrs/ESCRT function sufficient to block transport of known ubiquitylated substrates, and suggest that Pmel may be sorted to ILVs independent of the Hrs/ESCRT machinery.
ILV targeting of Pmel requires a lumenal determinant but not ubiquitylation
To define the cis-acting determinant(s) for Pmel sorting to ILVs, we analyzed wild-type (WT) or variant Pmel (Figs. 4A, 5A) expressed in HeLa, a non-pigmented cell type. HeLa cells do not express endogenous Pmel and accumulate ectopically expressed protein in ILVs and induced fibrils of late endosomes (Berson et al., 2001), negating confounding effects of endogenous Pmel on transgene behavior/detection and of the segregation of stage II melanosomes from endosomes. Accumulation in ILVs is assayed by IFM using antibodies to Pmel and to LAMP-1, a limiting membrane resident of MVBs and lysosomes; the resulting “jelly donut” appearance of LAMP-1 surrounding internal Pmel labeling faithfully reports ILV localization (Berson et al., 2001; see Fig. 5B).
Figure 4. Lumenal determinants direct ILV localization of Pmel.
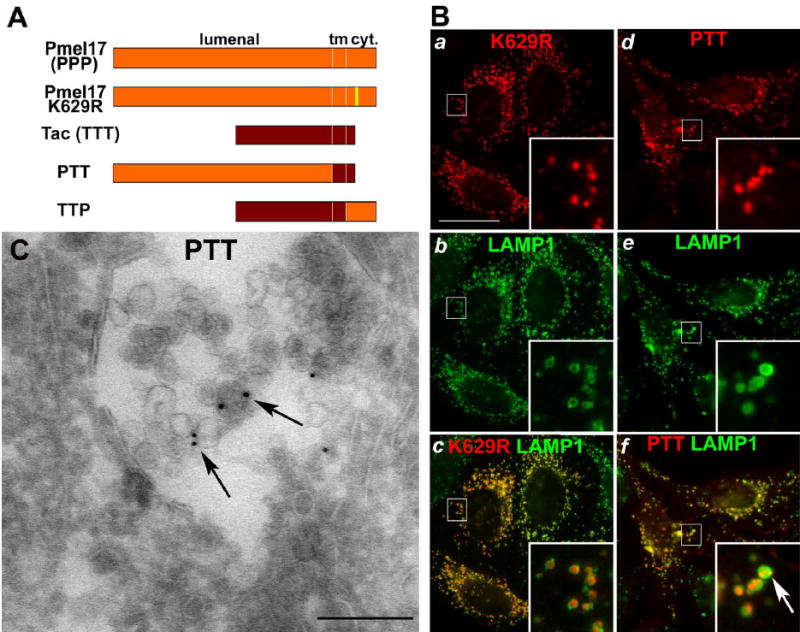
A. Topological domain structure of WT Pmel, the K629R mutant, Tac, and PTT and TTP chimerae. Pmel (orange) and Tac (maroon) lumenal, transmembrane and cytoplasmic domains are indicated; yellow, the cytoplasmic K629R substitution. B. IFM analysis of HeLa cells expressing Pmel K629R (a–c) or PTT (d–f) and labeled for the Pmel lumenal domain (with HMB-50; a, d), LAMP-1 (b, e), or both (c, f). Insets, 4X magnification of indicated regions. Note labeling for both K629R and PTT (red) within LAMP-1-positive (green) structures. Bar, 22 μm. C. Ultrathin cryosections of HeLa cells expressing PTT were immunogold labeled for Pmel (HMB-45 – PAG10). Note the labeling on ILVs of MVBs (arrows). Bar, 200 nm.
Figure 5. Distinct lumenal subdomains are required for sorting to ILVs.

A. Schematic of the Pmel lumenal sub-domains and corresponding single domain deletion constructs, ΔNTR, ΔPKD, ΔRPT and ΔKLD. Line, dibasic PC cleavage site (CS); numbers, residues at the borders of domains and deletions. B. IFM analysis of HeLa cells expressing WT Pmel (a–c), ΔNTR (d–f), ΔPKD (g–i), ΔRPT (j–l) and ΔKLD (m–o), labeled for Pmel (a, d, g, j, m), LAMP-1 (b, e, h, k, n) or both (c, f, i, l, o). Insets, 4X magnification of indicated regions. WT, ΔRPT and ΔKLD were detected with HMB-50 anti-Pmel together with mAb H4A3 anti-LAMP-1 and isotype-specific secondary antibodies; ΔNTR and ΔPKD lose reactivity with HMB-50 and were thus detected with HMB-45 relative to rabbit anti-LAMP-1. Note the LAMP-1-ringed donut structures with WT Pmel, ΔRPT and ΔKLD but not ΔNTR and ΔPKD. Bar, 22 μm.
To test whether Pmel ILV sorting required direct ubiquitylation, we rendered the single cytoplasmic lysine (K629) ubiquitylation-incompetent by mutagenesis to arginine (Fig. 4A). IFM analysis of transiently transfected HeLa cells reveals this K629R mutant within LAMP-1-ringed structures as seen for WT Pmel (Fig. 4Ba–c and c inset), indicating efficient localization to MVBs. A K629R variant with both cytoplasmic cysteines (C652 and C654) mutagenized to serine showed an identical pattern (data not shown), negating a requirement for cysteinyl ubiquitylation (Cadwell and Coscoy, 2005). Moreover, ubiquitylated Pmel could not be detected at steady state by immunoprecipitation and immunoblotting (data not shown). These data indicate that Pmel ubiquitylation is not necessary for sorting to ILVs. To assess whether other cytoplasmic determinants direct Pmel to ILVs, we analyzed cells expressing a chimera (PTT) consisting of the Pmel lumenal domain and the transmembrane and cytoplasmic domains of an irrelevant plasma membrane protein, Tac (Fig. 4A). PTT is expressed more at the cell surface than WT Pmel (Fig. 4Bd–f) due to loss of an internalization signal (not shown), but is surprisingly enriched in the interior of multivesicular late endosomes revealed as LAMP-1-positive rings by IFM (Fig. 4Bf inset, arrow) or directly by IEM (arrows, Fig. 4C). By contrast, TTP – containing Tac lumenal and transmembrane domains and Pmel cytoplasmic domain - is largely excluded from ILVs (data not shown). Together, these data show that a lumenal determinant specifies endosomal sorting of Pmel to ILVs.
To further delimit the ILV sorting determinant, we analyzed Pmel mutants with targeted deletions in each of four lumenal subdomains (Fig. 5A; Theos et al., 2005b): an N-terminal region (NTR), a polycystic kidney disease protein-1-like repeat domain (PKD), a domain of imperfect direct repeats (RPT), and a “Kringle-like” domain (KLD). All mutants retain the dibasic proprotein convertase (PC) cleavage site that separates RPT and KLD and is crucial for subsequent fibrillogenesis but not for invagination onto ILVs (Berson et al., 2003). Mutants lacking the RPT (ΔRPT) or KLD (ΔKLD) are predominantly found within LAMP-1-ringed organelles by IFM (Fig. 5Bj–o) and in ILV-laden MVBs by IEM (Fig. 6C, D), as most often seen for WT Pmel (Fig. 5Ba–c) (Berson et al., 2001). As with WT Pmel, cells expressing ΔRPT have higher numbers of MVBs and more ILVs within them than untransfected cells, suggesting that the cargo itself induced ILV formation (this is not observed for ΔKLD, perhaps because of lower expression). Thus, neither RPT nor KLD is required for targeting to ILVs.
Figure 6. Ultrastructural analysis of Pmel deletion mutants.

Ultrathin cryosections of HeLa cells expressing ΔPKD (A,B), ΔRPT (C) and ΔKLD (D) were immunogold labeled with HMB-45 (A, B, D) or HMB-50 (C) and PAG-10. A and inset. ΔPKD labeling on tubular endosomal membranes. B. ΔPKD also labels the limiting membrane of vacuolar endosomes (arrowheads) and tubules emanating from them (arrow), as well as some internal membranes. Labeling for ΔRPT (C) and ΔKLD (D) decorates predominantly the internal membranes of MVBs and less on the limiting membrane. Bars, 200 nm.
By contrast, deletions within both domains upstream of RPT have a dramatic effect on Pmel localization (Fig. 5Bd–i). By IFM, ΔNTR and ΔPKD are largely excluded from LAMP-1-positive late endosomes/lysosomes (Fig. 5Bf, i), even after cells are treated with inhibitors of lysosomal degradation (data not shown). Rather, these mutants (Suppl. Fig. S8d–i), but not WT Pmel (Suppl. Fig. S8a–c), ΔRPT, or ΔKLD (data not shown), localize extensively to early sorting and recycling endosomes marked by internalized Alexa594-Tf. By IEM, ΔPKD accumulates primarily on the limiting membrane and tubular extensions of multivesicular endosomes (arrowheads and arrow, respectively, Fig. 6B) and on tubular endosomes near the Golgi apparatus (Fig. 6A); ΔPKD is depleted, although not excluded, from ILVs relative to WT, ΔRPT or ΔKLD (compare Fig. 6A, B with Fig. 6C, D). ΔNTR is similarly distributed by IEM (data not shown). Thus, efficient ILV sorting requires a determinant within the N-terminal domains of Pmel, and failure to sort to the lumen of early endosomes results in default recycling.
Interestingly, RPT is dispensable for ILV targeting but required for downstream fibril formation. A large fraction of HeLa cells transduced with WT Pmel or ΔKLD harbor fibrils within MVBs observed by direct EM of ultrathin Epon sections or as linear arrays of immunogold labeling by IEM (arrows, Suppl. Fig. S9A; Berson et al., 2001; Berson et al., 2003). By contrast, fibrils are never observed among cells expressing similar or higher levels of ΔRPT within MVBs. Rather, ΔRPT accumulates not only in MVBs but also in lysosomes, identified as electron-dense structures with whorls of internal membranes (arrow, Suppl. Fig. S9C), from which WT Pmel is normally excluded. This mimics the behavior of Pmel lacking a PC cleavage site, which also fails to make fibrils (Suppl. Fig. S9B; Berson et al., 2003). Fibrils are also not observed in cells expressing ΔNTR or ΔPKD, although these mutants are typically expressed within endosomes at lower levels.
ILV or MVB localization is a prerequisite for PC cleavage and subsequent fibril formation
Pmel in pigmented cells is ultimately proteolytically matured by PC cleavage, and its lumenal Mα fragments polymerize to form the intralumenal amyloid-like fibrils of premelanosomes (Berson et al., 2001; Berson et al., 2003; Fowler et al., 2006). What function, then, is served by sorting to ILVs? Cleavage is not required for ILV sorting (Berson et al., 2003); to test whether ILV sorting is required for cleavage, we exploited the conservation of Pmel maturation in HeLa cells and the sorting defects of ΔPKD and ΔNTR. Transiently transfected HeLa cells were subject to a metabolic pulse/chase, and then Pmel isoforms were immunoprecipitated from Triton X-100- lysates and analyzed by SDS-PAGE. After the pulse, WT and mutant Pmel appear as single chain precursors (P1; Mr ~100,000) with core N-linked oligosaccharides (Fig. 7A). After a 1 h chase, WT Pmel is largely matured first to a transient Golgi-modified full-length precursor (P2; Mr ~120,000), and then rapidly to large (Mr ~90,000) Mα and small (Mr ~28,000) Mβ fragments that accumulate in cell lysates (Berson et al., 2001 and Fig. 7A). In cells expressing ILV-localized ΔRPT and ΔKLD, the diagnostic Mβ appears with similar kinetics, indicating efficient proteolytic processing (note the smaller size of ΔRPT Mα and ΔKLD Mβ fragments). By contrast, whereas localization-defective ΔNTR and ΔPKD mature to P2 forms with normal kinetics, P2 accumulates and neither Mα nor Mβ appear during the chase (Fig. 7A, arrowheads). Mα and Mβ absence is due to their failure to form and not to rapid lysosomal degradation, since inhibitors of lysosomal degradation do not protect any cleavage products during the chase (Suppl. Fig. S10) or expose “latent” lysosomal localization by IFM (data not shown). Thus, ILV localization of Pmel correlates with susceptibility to PC cleavage.
Figure 7. Localization to ILVs is required for proprotein convertase processing.

A. HeLa cells expressing WT or mutant Pmel were metabolically pulse-labeled and chased as indicated. Pmel was immunoprecipitated from cell lysates with C-terminally-directed αPep13h antibody, fractionated by SDS-PAGE (ΔKLD, 15% acrylamide; others, 12% acrylamide), and analyzed by PhosphorImager analysis. Upper panels: regions surrounding P1, P2 and Mα (arrows; see text). Lower panels: regions surrounding Mβ. Right, migration of molecular weight markers. Note the appearance of Mβ by 1h of chase for WT, ΔRPT and ΔKLD (Mβ’), but not for ΔNTR or ΔPKD (arrowheads). The deletion in ΔKLD is within Mβ, resulting in faster migration. Representative of four independent experiments. B. Immunoblot analysis of whole cell lysate of HeLa cells cotransfected with the indicated Pmel mutant and either empty vector (−) or furin-HA (+). Blots were probed with αPep13h to Pmel (top, middle) or reprobed with anti-HA (bottom) to detect furin-HA. Only the relevant portions of the gels are shown. Top, immature P1 bands of ΔPKD and ΔKLD (P1A) and faster-migrating P1 bands of ΔNTR and ΔRPT (P1B). Middle, Mβ levels relative to P1 in cells expressing ΔNTR and ΔPKD, but not ΔRPT or ΔKLD, are increased by co-expression of furin-HA. Note the faster migrating Mβ’ of ΔKLD.
The processing defect of ΔNTR and ΔPKD could reflect either a requirement for ILV localization in Pmel cleavage or a conformational defect blocking PC access to the cleavage site. To distinguish these possibilities, we co-expressed each mutant in HeLa cells with an excess of the PC family member furin, which can restore Pmel cleavage in PC-deficient cells (Berson et al., 2003). Overexpression and consequent missorting of furin (Wolins et al., 1997) should restore ΔPKD and ΔNTR cleavage if the defect is due to mislocalization but not to misfolding. Lysates of transiently transfected HeLa cells were subjected to SDS-PAGE and probed for Mβ cleavage products by immunoblotting (Fig. 7B). In the absence of excess furin, the ratio of Mβ to full-length P1 precursor is low in both ΔNTR and ΔPKD compared with ΔRPT and ΔKLD, consistent with the data in Fig. 7A. When furin is co-expressed, the Mβ to P1 ratio remains the same for ΔRPT and ΔKLD, but is markedly increased for both ΔNTR and ΔPKD (Fig. 7B). This demonstrates that processing of ΔNTR and ΔPKD is rescued by furin overexpression, and thus that they are conformationally susceptible to PC cleavage but normally sequestered from the active PC. Since cleavage is a prerequisite for fibril formation, the data show that localization to ILVs, or to endosomal domains in which ILVs form, is required for fibril formation.
DISCUSSION
We show here that the melanosomal protein, Pmel, is sorted to endosomal ILVs by a novel pathway in both melanocytic and non-pigment cells. Relative to that taken by well studied ligand-activated receptors, this pathway is insensitive to inhibition of the ESCRT machinery and is dependent solely on lumenal determinants. Moreover, regulated cleavage of Pmel, a prerequisite for fibril formation and biogenesis of melanosomes, requires localization to ILVs. Our results have important implications both for general mechanisms of MVB formation in all cells and for the use of this pathway in tissue-specific and/or pathogenic processes of organelle biogenesis and amyloidogenesis.
Ubq- and ESCRT-independent MVB sorting?
Ubq-independent MVB sorting of cargo such as Sna3p and δ-opioid receptor have been described, but MVB delivery of both is blocked by genetic ablation, siRNA-mediated down-regulation, or dominant negative interference with ESCRT components (Bilodeau et al., 2002; Hislop et al., 2004; Reggiori and Pelham, 2001). Similarly, Tf receptor in erythrocytes and viruses like HIV and EIAV bypass the need for Ubq and Hrs but bind directly to ESCRT components to effect MVB sorting or budding from the plasma membrane (Geminard et al., 2004; Morita and Sundquist, 2004). Like these proteins, efficient sorting of Pmel to ILVs does not require direct ubiquitylation, and sorting by association with an ubiquitylated partner is unlikely for several reasons. First, Pmel is extremely efficiently sorted to ILVs in HeLa cells, which do not normally express Pmel or potential melanocyte-specific X-Ubq partners such as MART-1 (Lévy et al., 2005). Second, partner-dependent sorting should be saturable, but Pmel is efficiently targeted to MVBs even when overexpressed. Moreover, a saturable partner cannot explain the increased numbers of MVBs and of ILVs per MVB in Pmel-overexpressing cells relative to controls. Third, overexpression of Hrs, Tsg101 or Vps4(K173A) had no effect on Pmel ILV localization in either melanocytic or HeLa cells in which the distribution of MART-1, EGFR, Ubq and endosomal SNAREs was dramatically altered. Given this phenotype in a large fraction of affected cells, the altered Pmel localization in cells with prolonged or extreme Hrs or Vps4(K173A) overexpression – matched by effects on LAMP-1, which is not an ILV cargo - is likely a consequence of secondary effects of these transgenes on endosomal membrane dynamics. Finally, Pmel distribution to ILVs and stage II melanosomes was not altered by depletion of Hrs using siRNA. Taken together, the data suggest that Pmel is sorted to ILVs by a novel mechanism that is likely independent of the ESCRT machinery. We predict that this pathway is shared by other long-lived ILV cargo.
ILV formation independent of ESCRT function
The defects in MVB formation observed in yeast ESCRT mutants and the failure to observe ILVs by conventional EM in mammalian cells expressing dominant negative ESCRT components have led to the thought that the ESCRT machinery drives membrane invagination in forming MVBs. However, here we show using cryosections that ILVs continue to form in cells with clearly disrupted ESCRT function. The ILVs observed in cells overexpressing Tsg101 or Hrs (also described in cells expressing dominant negative Vps4; Sachse et al., 2004; Yoshimori et al., 2000) lack the characteristic electron density of ILVs in untreated cells and so are likely depleted of protein cargo. Similar structures were observed in melanocytes depleted of Hrs by siRNA. While it is possible that these structures merely arise from an incomplete block in Hrs/ESCRT function, we consider this unlikely due to the dramatic effects on EGFR-EYFP, MART-1, X-Ubq, and syntaxin 7 localization. Furthermore, our observations are consistent with the existence in several cell types (Möbius et al., 2003; White et al., 2005), including melanocytes (Raposo et al., 2001; Theos et al., 2005a), of distinct populations of MVBs with different cargo on their ILVs. The MVBs with Pmel-containing ILVs are, however, likely distinct from those enriched in LBPA, since LBPA localizes to distinct organelles in melanocytes (unpublished data). Thus, multiple ILV populations may arise from separate ESCRT-dependent and –independent pathways. Whether the Pmel pathway requires annexin1, like that induced by EGF stimulation in other cell types (White et al., 2005), remains to be determined.
Lumenal information specifies Pmel sorting into ILVs
Cytoplasmic and transmembrane determinants are dispensable for sorting Pmel to ILVs in HeLa cells. To our knowledge, this is the first conclusive evidence for a role of a lumenal domain in directing cargo sorting to ILVs. Lumenal determinants that drive oligomerization or aggregation can inhibit endosomal recycling (Marsh et al., 1995) or misdirect cargo to lysosomes (Wolins et al., 1997) or exosomes (Vidal et al., 1997), but a similar mechanism is unlikely to account for Pmel sorting; sucrose gradient sedimentation analyses of Pmel and deletion mutants do not support a role for selective aggregation in ILV sorting, and we were unable to rescue ILV localization of ΔNTR or ΔPKD with inducible oligomerization domain fusions (data not shown). Thus, NTR and PKD are likely to mediate ILV sorting by a novel mechanism.
What might this mechanism entail? PKD homology domains within cell-surface proteins in filamentous archaebacteria bind glycoproteins or glycolipids, and the PKD of PKD-1 has been proposed to mediate protein-protein or protein-carbohydrate interactions (Bycroft et al., 1999). An interaction of Pmel PKD with carbohydrate, like that of Alix (Matsuo et al., 2004) and Saposin C (Chu et al., 2005) with the lipid LBPA, may facilitate selective partitioning into lipid microdomains destined for the lumen, as proposed for Sna3p (Reggiori and Pelham, 2001). Rather than LBPA, the head groups of glycosphingolipids, enriched in ILVs in mammals, are excellent candidate ligands for PKD or NTR. Alternatively, physicochemical properties of Pmel, perhaps altered within endosomes by association with lipid microdomains and/or by acidification, may promote inward budding in much the same way as has been proposed for LBPA (Matsuo et al., 2004). Either of these mechanisms, but not association with a limiting binding protein, is consistent with the increase in MVB number and ILV content in HeLa cells overexpressing Pmel.
The MVB: A crucial intermediate in fibril formation
Our data show that Pmel must localize to ILVs, or to membrane domains destined for ILVs, for cleavage by a furin-like PC, crucial for downstream fibril formation. This is a novel example of ILV localization providing a specialized environment for protein-protein interactions, adding to those recently shown for HLA-DM: HLA-DR interactions in antigen presenting cells (Zwart et al., 2005) and the activation of the yeast integral membrane lipase Cvt17/Aut5p (Epple et al., 2003). ILV sorting may relieve a conformational restraint; the microenvironment of the limiting membrane may restrict access of the Pmel cleavage site to the PC, or the topology or lipid environment of the ILV may trigger a PC-cleavage-competent conformational change. Alternatively, the PC may itself localize to the ILV, such that delivery of Pmel to the ILV brings substrate and enzyme together. Consistent with this possibility, PC1 and PC2 are found in melanosome-enriched subcellular fractions of melanocytes (Peters et al., 2000) and localize to lipid microdomains (Arnaoutova et al., 2003; Blazquez et al., 2000). ILVs or ILV-associated lipids may also play additional roles in the formation of Pmel amyloid fibrils, as several amyloidogenic proteins, such as Aβ and prions, associate with lipids enriched in ILVs (Hooper, 2005). It is tempting to speculate that upon cleavage and release from the KLD, the RPT domain, shown here to be dispensable for sorting to ILVs but required for fibril formation, may interact with ILV-enriched lipids to facilitate the transition of Pmel to its amyloid form (Fowler et al., 2006). It will be important to determine whether similar processes facilitate pathological fibrillation in amyloidogenic diseases.
MATERIALS AND METHODS
Cell culture and transfection
MNT-1 and 1011-mel melanoma and HeLa cells were cultured as described (Berson et al., 2001; Raposo et al., 2001). HeLa and 1011-mel were transfected using FuGene-6 with 0.1–0.5 μg of DNA (Roche Applied Science, Indianapolis, IN) and analyzed 24–48h post-transfection. For siRNA, 106 MNT-1 cells were treated with 1 nmol siRNA duplexes and 50μl Oligofectamine (InVitroGen, Carlsbad, CA) in 5 ml serum-free OptiMEM for 4 h, then cultured in complete medium. 2 d later the transfection was repeated, and cells were cultured an additional day prior to analysis.
Chemicals and protease inhibitors
Reagents were obtained from Sigma (St. Louis, MO) unless otherwise noted. Protease inhibitors were from Roche Diagnostics (Indianapolis, IN).
Antibodies
αPep13h anti-peptide antibody to the C-terminus was affinity purified as described (Berson et al., 2001). MAbs HMB-50 (IgG2a), NKI-beteb (IgG2b), and HMB-45 (IgG1) to Pmel lumenal domain and A103 (for IFM and immunoblotting) or M2-7C10 (for IEM) to MART-1 were from LabVision (Fremont, CA), FK2 to X-Ubq from Biomol (Plymouth Meeting, PA), H4A3 to LAMP1 from Developmental Studies Hybridoma Bank (Iowa City, IA), mouse anti-HA from Covance (Denver, PA), rat anti-HA from Roche Diagnostics (Indianapolis, IN), and 7G7.B6 to Tac and 9E10 to myc from American Type Culture Collection (Manassas, VA). Rabbit antibodies to V5 were from ICL (Newburgh, OR), to myc from Santa Cruz Biotech (Santa Cruz, CA), to Hrs from S. Urbé (Univ. Liverpool, Liverpool, UK; Sachse et al., 2002), and to LAMP-1 from M. Fukuda (The Burnham Inst., La Jolla, CA; Fukuda et al., 1988). Alexa488- and Alexa594-conjugated species-specific secondary antibodies were from Molecular Probes, (Eugene, OR), and FITC- and Texas Red-conjugated isotype-specific secondary antibodies were from Southern Biotech (Birmingham, AL). Protein-A gold conjugates were from Cell Microscopy Center, Utrecht Medical School (Utrecht, The Netherlands).
Plasmids and oligonucleotides
pCI-Pmel (long form) has been described (Berson et al., 2001). PmelΔNTR, ΔPKD, ΔRPT, and ΔKLD and the chimerae PTT and TPP were generated by site directed mutagenesis using two-step amplification (Higuchi et al., 1988) and primers listed in the Supplement. Sequences were verified by automated dideoxy sequencing. HA11-epitope tagged furin in pSX was from J. S. Bonifacino (National Institutes of Health, Bethesda, MD; Bosshart et al., 1994). Myc-tagged Hrs was from H. Stenmark (Dept. Biochemistry, The Norwegian Radium Hospital, Oslo, Norway; Raiborg et al., 2002). Vps4(K173A) with a C-terminal V5 epitope tag and human Tsg101 with an N-terminal HA epitope tag were from A. Piefer and P. Bates (Univ. of Pennsylvania, Philadelphia, PA). C-terminal EYFP fusion to human EGFR was from M. Lemmon (Univ. of Pennsylvania, Philadelphia, PA). Mouse rab27A with an N-terminal myc epitope tag was from J. Hammer (National Inst. of Health, Bethesda, MD). siRNA duplexes targeted to Hrs were as previously described (Bache et al., 2003b) and purchased from Qiagen S.A. (Courtaboeuf, France) along with the manufacturer’s standard negative control.
Metabolic labeling, immunoprecipitation and immunoblotting
Cells were metabolically pulse labeled with 35S-methionine/cysteine and chased, lysed in 1% (w/v) Triton X-100, and subject to immunoprecipitations, fractionation of eluted proteins by SDS-PAGE on 10% (except where indicated) polyacrylamide gels, and phosphorimaging analysis as described (Berson et al., 2000). Western blotting using whole cell lysates prepared with 1% SDS was as described (Berson et al., 2000) using 10% PAGE/5% methanol transfer buffer. Immobilon-P membranes (Millipore, Billerica, MA) with transferred proteins were probed with indicated antibodies, and bands were detected with alkaline-phosphatase-conjugated goat anti-rabbit or anti-mouse Ig, ECF (Amersham Biosciences) and phosphorimaging analysis using a Molecular Dynamics STORM 860 and Imagequest software (Amersham Biosciences, Piscataway, NJ).
IEM
Cells were fixed with 2% paraformaldehyde with or without 0.2% glutaraldehyde and single- or double-immunogold labeling of ultrathin cryosections was performed as described (Raposo et al., 1997; Raposo et al., 2001) using protein A conjugated to 10 nm or 15 nm gold particles (PAG-10 or -15).
IFM analyses
Transiently transfected HeLa, 1011-mel or MNT-1 cells were fixed with 2% formaldehyde in PBS and stained as described (Berson et al., 2000) with indicated primary antibodies and isotype- or species-specific secondary antibodies conjugated to FITC and Texas Red or Alexa488 and Alexa594 (and AMCA for triple labeling) respectively. Cells were analyzed on a Leica Microsystems (Bannockburn, IL) DM IRBE microscope, and images were captured using a Hamamatsu (Hamamatsu, Japan) Orca digital camera and Improvision (Lexington, MA) OpenLab software. Most images shown were processed from sequential z-series images, captured at 0.2 μm intervals, using OpenLab Volume Deconvolution.
FACS sorting
Cells were co-transfected with pEGFP-C3 (Clontech, Mountain View, CA) in a 1:10 ratio (EGFP:test plasmid). 24 h after transfection cells were harvested with 5 mM EDTA in PBS, centrifuged and resuspended in FACS buffer (PBS, 5% FBS, 1 mM EDTA) for sorting by EGFP fluorescence using a FACS Vantage SE cell sorter (Becton Dickinson). Samples for IEM were then washed in growth medium, and plated on tissue culture dishes at 37°C for 30min before fixation.
Supplementary Material
Acknowledgments
We thank J. Cornuel, S. Theos and K. Herman for technical assistance, A. Peden, S. Urbé, H. Stenmark, J. Lippincott-Schwartz, J. Bonifacino, M. Lemmon, M. Chou, M. Fukuda, J. Hammer, P. Bates, and A. Piefer for generous gifts of reagents, R. Rojas and J. Bonifacino for the siRNA protocol, K. Ferguson and J. Mendrola for helpful discussions and C. Burd and J. Bonifacino for critical review of the manuscript. This work was supported by National Institutes of Health grants R01 AR 041855 and R01 EY 015625, CNRS, Institut Curie, training grant T32-CA-009140 from the National Cancer Institute (ACT and STT), and American Cancer Society Fellowship PF-99-336-01-CIM (JFB).
References
- Arnaoutova I, Smith AM, Coates LC, Sharpe JC, Dhanvantari S, Snell CR, Birch NP, Loh YP. The prohormone processing enzyme PC3 is a lipid raft-associated transmembrane protein. Biochemistry. 2003;42:10445–10455. doi: 10.1021/bi034277y. [DOI] [PubMed] [Google Scholar]
- Babst M. A protein’s final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- Bache KG, Brech A, Mehlum A, Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J Cell Biol. 2003a;162:435–442. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache KG, Raiborg C, Mehlum A, Stenmark H. STAM and Hrs are subunits of a multivalent Ubiquitin-binding complex on early endosomes. J Biol Chem. 2003b;278:12513–12521. doi: 10.1074/jbc.M210843200. [DOI] [PubMed] [Google Scholar]
- Berson JF, Frank DW, Calvo PA, Bieler BM, Marks MS. A common temperature-sensitive allelic form of human tyrosinase is retained in the endoplasmic reticulum at the nonpermissive temperature. J Biol Chem. 2000;275:12281–12289. doi: 10.1074/jbc.275.16.12281. [DOI] [PubMed] [Google Scholar]
- Berson JF, Harper D, Tenza D, Raposo G, Marks MS. Pmel17 initiates premelanosome morphogenesis within multivesicular bodies. Mol Biol Cell. 2001;12:3451–3464. doi: 10.1091/mbc.12.11.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson JF, Theos AC, Harper DC, Tenza D, Raposo G, Marks MS. Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J Cell Biol. 2003;161:521–533. doi: 10.1083/jcb.200302072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau PS, Urbanowski JL, Winistorfer SC, Piper RC. The Vps27p-Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nature Cell Biol. 2002;4:534–539. doi: 10.1038/ncb815. [DOI] [PubMed] [Google Scholar]
- Bishop N, Woodman P. ATPase-defective mammalian VPS4 localizes to aberrant endosomes and impairs cholesterol trafficking. Mol Biol Cell. 2000;11:227–239. doi: 10.1091/mbc.11.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez M, Thiele C, Huttner WB, Docherty K, Shennan KI. Involvement of the membrane lipid bilayer in sorting prohormone convertase 2 into the regulated secretory pathway. Biochem J. 2000;349:843–852. doi: 10.1042/bj3490843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshart H, Humphrey J, Deignan E, Davidson J, Drazba J, Yuan L, Oorschot V, Peters PJ, Bonifacino JS. The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J Cell Biol. 1994;126:1157–1172. doi: 10.1083/jcb.126.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft M, Bateman A, Clarke J, Hamill SJ, Sandford R, Thomas RL, Chothia C. The structure of a PKD domain from polycystin-1: implications for polycystic kidney disease. EMBO J. 1999;18:297–305. doi: 10.1093/emboj/18.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309:127–130. doi: 10.1126/science.1110340. [DOI] [PubMed] [Google Scholar]
- Cerny J, Feng Y, Yu A, Miyake K, Borgonovo B, Klumperman J, Meldolesi J, McNeil PL, Kirchhausen T. The small chemical vacuolin-1 inhibits Ca(2+)-dependent lysosomal exocytosis but not cell resealing. EMBO Rep. 2004;5:883–888. doi: 10.1038/sj.embor.7400243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Witte DP, Qi X. Saposin C-LBPA interaction in late- endosomes/lysosomes. Exp Cell Res. 2005;303:300–307. doi: 10.1016/j.yexcr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- de Mazière AM, Muehlethaler K, van Donselaar E, Salvi S, Davoust J, Cerottini JC, Lévy F, Slot JW, Rimoldi D. The melanocytic protein melan-A/MART-1 has a subcellular localization distinct from typical melanosomal proteins. Traffic. 2002;3:678–693. doi: 10.1034/j.1600-0854.2002.30909.x. [DOI] [PubMed] [Google Scholar]
- Dell’Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J. 2000;14:1265–1278. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- Demirov DG, Ono A, Orenstein JM, Freed EO. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc Natl Acad Sci USA. 2002;99:955–960. doi: 10.1073/pnas.032511899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyotte A, Russell MRG, Hopkins CR, Woodman PG. Depletion of TSG101 forms a mammalian ‘Class E’ compartment: a multicisternal early endosome with multiple sorting defects. J Cell Sci. 2005;118:3003–3017. doi: 10.1242/jcs.02421. [DOI] [PubMed] [Google Scholar]
- Epple UD, Eskelinen EL, Thumm M. Intravacuolar membrane lysis in Saccharomyces cerevisiae. Does vacuolar targeting of Cvt17/Aut5p affect its function? J Biol Chem. 2003;278:7810–7821. doi: 10.1074/jbc.M209309200. [DOI] [PubMed] [Google Scholar]
- Février B, Raposo G. Exosomes: endosomal–derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Fowler DM, Koulov AV, Alory-Jost C, Marks MS, Balch WE, Kelly JW. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Viitala J, Matteson J, Carlsson SR. Cloning of cDNAs encoding human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Comparison of their deduced amino acid sequences. J Biol Chem. 1988;263:18920–18928. [PubMed] [Google Scholar]
- Geminard C, de Gassart A, Blanc L, Vidal M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic. 2004;5:181–193. doi: 10.1111/j.1600-0854.2004.0167.x. [DOI] [PubMed] [Google Scholar]
- Goila-Gaur R, Demirov DG, Orenstein JM, Ono A, Freed EO. Defects in human immunodeficiency virus budding and endosomal sorting induced by TSG101 overexpression. J Virol. 2003;77:6507–6519. doi: 10.1128/JVI.77.11.6507-6519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J. The endocytic pathway: a mosaic of domains. Nat Reviews, Mol Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nature Rev Mol Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop JN, Marley A, Von Zastrow M. Role of mammalian vacuolar protein-sorting proteins in endocytic trafficking of a non-ubiquitinated G protein-coupled receptor to lysosomes. J Biol Chem. 2004;279:22522–22531. doi: 10.1074/jbc.M311062200. [DOI] [PubMed] [Google Scholar]
- Hooper NM. Roles of proteolysis and lipid rafts in the processing of the amyloid precursor protein and prion protein. Biochem Soc Trans. 2005;33:335–338. doi: 10.1042/BST0330335. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nature Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Stefan CJ, Babst M, Emr SD. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J Cell Biol. 2003;162:413–423. doi: 10.1083/jcb.200302136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy F, Muehlethaler K, Salvi S, Peitrequin AL, Lindholm CK, Cerottini JC, Rimoldi D. Ubiquitylation of a melanosomal protein by HECT-E3 ligases serves as sorting signal for lysosomal degradation. Mol Biol Cell. 2005;16:1777–1787. doi: 10.1091/mbc.E04-09-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Seabra MC. The melanosome: membrane dynamics in black and white. Nature Rev Mol Cell Biol. 2001;2:738–748. doi: 10.1038/35096009. [DOI] [PubMed] [Google Scholar]
- Marsh EW, Leopold PL, Jones NL, Maxfield FR. Oligomerized transferrin receptors are selectively retained by a lumenal sorting signal in a long-lived endocytic recycling compartment. J Cell Biol. 1995;129:1509–1522. doi: 10.1083/jcb.129.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure J, Blanc NS, Matile S, Dubochet J, Sadoul R, Parton RG, Vilbois F, Gruenberg J. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nature Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Möbius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HFG, Slot JW, Geuze HJ. Recycling compartments and internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- Morita E, Sundquist WI. Retrovirus budding. Ann Rev Cell Dev Biol. 2004;20:395– 425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- Peters EMJ, Tobin DJ, Seidah NG, Schallreuter KU. Pro-opiomelanocortin-related peptides, prohormone convertases 1 and 2 and the regulatory peptide 7B2 are present in melanosomes of human melanocytes. J Invest Dermatol. 2000;114:430–437. doi: 10.1046/j.1523-1747.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nature Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol. 2003;15:446–455. doi: 10.1016/s0955-0674(03)00080-2. [DOI] [PubMed] [Google Scholar]
- Raposo G, Kleijmeer MJ, Posthuma G, Slot JW, Geuze HJ. Immunogold labeling of ultrathin cryosections: application in immunology. In: Herzenberg LA, Weir D, Herzenberg LA, Blackwell C, editors. Handbook of Exp Immunol. Cambridge, MA: Blackwell Science, Inc; 1997. pp. 1–11. [Google Scholar]
- Raposo G, Marks MS. The dark side of lysosome-related organelles: specialization of the endocytic pathway for melanosome biogenesis. Traffic. 2002;3:237–248. doi: 10.1034/j.1600-0854.2002.030401.x. [DOI] [PubMed] [Google Scholar]
- Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J Cell Biol. 2001;152:809–823. doi: 10.1083/jcb.152.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Pelham HR. Sorting of proteins into multivesicular bodies: ubiquitin- dependent and -independent targeting. EMBO J. 2001;20:5176–5186. doi: 10.1093/emboj/20.18.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse M, Strous GJ, Klumperman J. ATPase-deficient hVPS4 impairs formation of internal endosomal vesicles and stabilizes bilayered clathrin coats on endosomal vacuoles. J Cell Sci. 2004;117:1699–1708. doi: 10.1242/jcs.00998. [DOI] [PubMed] [Google Scholar]
- Sachse M, Urbe S, Oorschot V, Strous GJ, Klumperman J. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol Biol Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe J, Bossi G, Griffiths GM. Linking albinism and immunity: the secrets of secretory lysosomes. Science. 2004;305:55–59. doi: 10.1126/science.1095291. [DOI] [PubMed] [Google Scholar]
- Strack B, Calistri A, Craig S, Popova E, Göttlinger HG. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114:689–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- Stuchell MD, Garrus JE, Muller B, Stray KM, Ghaffarian S, McKinnon R, Krausslich HG, Morham SG, Sundquist WI. The human endosomal sorting complex required for transport (ESCRT-I) and its role in HIV-1 budding. J Biol Chem. 2004;279:36059–36071. doi: 10.1074/jbc.M405226200. [DOI] [PubMed] [Google Scholar]
- Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, Bonifacino JS, Marks MS, Raposo G. Functions of AP-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell. 2005a;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos AC, Truschel ST, Raposo G, Marks MS. The Silver locus product Pmel17/gp100/Silv/ME20: Controversial in name and in function. Pigment Cell Res. 2005b;18:322–336. doi: 10.1111/j.1600-0749.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbé S, Sachse M, Row PE, Preisinger C, Barr FA, Strous GJ, Klumperman J, Clague MJ. The UIM domain of Hrs couples receptor sorting to vesicle formation. J Cell Sci. 2003;116:4169–4179. doi: 10.1242/jcs.00723. [DOI] [PubMed] [Google Scholar]
- Vidal M, Mangeat P, Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J Cell Sci. 1997;110:1867–1877. doi: 10.1242/jcs.110.16.1867. [DOI] [PubMed] [Google Scholar]
- von Schwedler UK, Stuchell M, Müller B, Ward DM, Chung HY, Morita E, Wang HE, Davis T, He GP, Cimbora DM, Scott A, Kräusslich HG, Kaplan J, Morham SG, Sundquist WI. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2005 doi: 10.1038/sj.emboj.7600759. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolins N, Bosshart H, Kuster H, Bonifacino JS. Aggregation as a determinant of protein fate in post-Golgi compartments: role of the luminal domain of furin in lysosomal targeting. J Cell Biol. 1997;139:1735–1745. doi: 10.1083/jcb.139.7.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T, Yamagata F, Yamamoto A, Mizushima N, Kabeya Y, Nara A, Miwako I, Ohashi M, Ohsumi M, Ohsumi Y. The mouse SKD1, a homologue of yeast Vps4p, is required for normal endosomal trafficking and morphology in mammalian cells. Mol Biol Cell. 2000;11:747–763. doi: 10.1091/mbc.11.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart W, Griekspoor A, Kuijl C, Marsman M, van Rheenen J, Janssen H, Calafat J, van Ham M, Janssen L, van Lith M, Jalink K, Neefjes J. Spatial separation of HLA-DM/HLA-DR interactions within MIIC and phagosome-induced immune escape. Immunity. 2005;22:221–233. doi: 10.1016/j.immuni.2005.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


