Abstract
Although constantly exposed to the environment and “foreign bodies” such as contact lenses and unwashed fingertips, the ocular surface succumbs to infection relatively infrequently. This is, in large part, due to a very active and robust innate immune response mounted at the ocular surface. Studies over the past 20 years have revealed that small peptides with antimicrobial activity are a major component of the human innate immune response system. The ocular surface is no exception, with peptides of the defensin and cathelicidin families being detected in the tear film and secreted by corneal and conjunctival epithelial cells. There is also much evidence to suggest that the role of some antimicrobial peptides is not restricted to direct killing of pathogens, but, rather, that they function in various aspects of the immune response, including recruitment of immune cells, and through actions on dendritic cells provide a link to adaptive immunity. A role in wound healing is also supported. In this article, the properties, mechanisms of actions and functional roles of antimicrobial peptides are reviewed, with particular emphasis on the potential multifunctional roles of defensins and LL-37 (the only known human cathelicidin) at the ocular surface.
Keywords: antimicrobial peptide, cationic peptide, defensin, immune response, innate immunity, LL-37, microbicidal peptide, peptide, wound healing
I. INTRODUCTION
The body’s first line of defense against invading pathogens is the innate immune response. This response encompasses a variety of mechanisms, some of which (e.g., skin and mucous membranes) are designed to stop organisms from entering the body in the first place. Others, such as the presence of polymorphonuclear neutrophils in the tear film, are designed to stop an organism in its tracks if it does happen to breach the outer defenses, and, at the same time, to signal to the T lymphocytes of the adaptive immune system to prepare for action. The innate immune response utilizes a variety of different substances to kill invading organisms. These include enzymes of the respiratory burst, whose activity generates lethal reactive oxygen species within the phagocytic vacuole of neutrophils and secreted enzymes, such as lyzozyme and phospholipase A2, which are present in a variety of body fluids, including the tear film.1 The innate and adaptive arms of the immune system have been reviewed recently by Dempsey et al2 and Beutler.3
Studies over the past 20 years have revealed that the innate immune system also uses a variety of small peptides (defined as having less than 100 amino acids) to ward off unwanted invaders. The majority of these peptides have a positive charge due to an excess of positively charged amino acids, such as arginine and lysine; hence, they are frequently referred to as cationic antimicrobial peptides.
The first published report indicating the existence of such peptides in animal species appeared in 1963, but it was not until the early 1980s that the first peptides were identified.4 By that time, it was well established that plants use peptides as a major mechanism of defense.5 In 1981, Steiner et al6 described the sequence of two peptides, later called cecropins, that they had isolated from the moth Hyalophora cecropia the previous year.7 In 1983, Selsted et al8 described the first mammalian peptides, which were found in rabbit macrophages. In 1985, the first human antimicrobial peptides, which the authors referred to as “defensins” were isolated.9,10 Subsequent studies have shown that antimicrobial peptides are a common feature of the immune system of many species. A database maintained by the University of Trieste (http://www.bbcm.units.it/~tossi/pag1.htm) contains a list of over 800 antimicrobial peptides (and some proteins) from species as widely different as amoeba, plants, penguins, and humans. Various attempts have been made to classify the peptides. Most commonly, they are assigned to groups based upon secondary structural features, with three to four different groups being recognized: linear α-helical peptides (e.g., LL-37), peptides with β-strands linked by disulphide bridges (e.g., defensins), loop peptides (e.g., bactenecin), and those with a high proportion of specific amino acids (e.g., histatins).11–13 As discussed in more detail below, these peptides exhibit potent antimicrobial activity against bacteria, fungi, and some viruses, chiefly by disrupting the microbial membrane.
OUTLINE.
Introduction
-
Defensins at the ocular surface
Defensins: an overview
Defensins of the human ocular surface
Defensins at the ocular surface in non-human species
LL-37 and other antimicrobial peptides at the ocular surface
-
Antimicrobial role of defensins and other peptides at the ocular surface
Mechanism of antimicrobial activity
Antimicrobial activity at the ocular surface
Nonmicrobial roles of defensins and other antimicrobial peptides at the ocular surface
-
Potential of antimicrobial peptides as ocular therapeutics
Current status of antimicrobial peptide development
Possible uses of antimicrobial peptides in eye Care
Summary
Initial interest in these peptides focused on their antimicrobial activity; however, it soon became apparent that some of the peptides are also able to modulate various mammalian cell functions. This has led to the concept that, at least in mammalian systems, cationic antimicrobial peptides are multifunctional molecules.14–18 As discussed below, the peptides provide protection against invading organisms by direct microbicidal activity and by modulating the activity of a variety of other components of the immune system. Additionally, a growing number of studies document the participation of antimicrobial peptides in activities not directly related to the immune response.
In this review, the expression, mode of action, and functional roles of antimicrobial peptides are reviewed with emphasis on their microbicidal effect and potential other functions at the ocular surface. Because the defensins and the cathelicidin LL-37 are the most studied, they are the best understood of mammalian cationic antimicrobial peptides; therefore, their role forms the focal point of this review. For details on other antimicrobial peptide families, particularly those of lower species and plants, and anionic peptides with antimicrobial activity, the reader is referred to various review articles.5,12,19–23
II. DEFENSINS AT THE OCULAR SURFACE
A. Defensins: An Overview
Classic mammalian defensins are 29 to 45 amino acids in length and are characterized by the presence of six cysteine residues that interact to form three disulphide bonds and a β-sheet structure. They can be classified into two groups, referred to as α and β, depending upon the location and connectivity of the cysteines. For α-defensins, the pattern of connectivity is C1-C6, C2-C4 and C3-C5, whereas for β-defensins, the arrangement is C1-C5, C2-C4 and C3-C6.24 A third novel defensin class, referred to as θ (also minidefensins), has been identified in rhesus macaque leucocytes.25–27 The 18 amino acid θ-defensins are unique in that they are formed by splicing of two truncated α-defensins to create a circular structure. Six genes for human θ-defensins (termed retrocyclins) have been identified, but premature stop codons prevent their translation.28
In humans, six α and four β-defensins have been identified and characterized. The six α-defensins are human neutrophil peptide (HNP) 1 through 4 and human defensin (HD) 5 and 6. As their name suggests, HNP 1–4 are found in neutrophils, where they reach high concentrations within azurophil granules.9,10,29 They have also been found in monocytes and lymphocytes.30 HD5 and HD6 are present in the granules of Paneth cells, which are specialized host defense cells of the intestine.31,32 HD-5 has also been localized to the female reproductive tract.33 The α-defensins are expressed constitutively and are encoded as 90–100 amino acid prepropeptides.34,35 HNP 1–4 are synthesised, processed to the mature product, and packaged into azurophil granules as the neutrophils are developing in the bone marrow.36,37 HD-5 and -6 are stored as proforms, then are processed during or after release from the Paneth cell granules.24
The four β-defensins are named human β-defensin (hBD) 1–4 and are expressed chiefly by epithelial tissues, although expression by immune cells (monocytes, macrophages and dendritic cells) has been reported.38 Like α-defensins, β-defensins are encoded as larger precursor molecules; however, they are not stored in cytoplasmic granules.24 hBD-1, which was first isolated from human plasma,39 is constitutively expressed by a variety of epithelia, including airway epithelia,40 urogenital tissues,41 nasolacrimal duct,42 and mammary gland.43 hBD-2 and hBD-3 are inducible in many epithelia by bacterial products and cytokines.44–46 The expression of hBD-4 is more limited, with testes and epididymis having the highest levels.47,48 The expression of this peptide is inducible, although not by the cytokines reported to upregulate hBD-2 and hBD-3.47 In 2002, Schutte et al49 reported the discovery of 28 novel human β-defensin genes (DEFB) identified with use of a computational method. While some of these novel genes mapped to chromosome 8 in regions close to the known α-and β-defensin genes, others mapped to chromosomes 6 and 20. A number of these novel genes are now under investigation. Yamaguchi et al48 cloned two of the new genes, DEFB-5 and -6, and found that their products, named hBD-5 and -6, respectively, were specifically expressed in the epididymis. Recent cloning of five more of these novel genes (DEFB25–29, on chromosome 20) also shows that they are preferentially expressed by the male genital tract.50 Prematanachai et al51 studied the expression of DEFB-5 through 14 in gingival keratinocytes and found that DEFB-7, -9, -11, and -12 were constitutively expressed, whereas DEFB-8, and -14 were inducible by cytokines and infection. hBD-5 and the product of DEFB-9 have also been found to be expressed in the lung.52
B. Defensins of the Human Ocular Surface
The existence of defensins at the human ocular surface was first reported in 1998.53–55 Gottsch et al53 observed that α-defensin mRNA (the in situ hybridization probe they used was previously found to identify HNP-1 and -334) and protein (the antibody used detected HNP-1, -2 and -3) was detectable in human corneal stroma in cases of rejected transplants and post-infectious keratitis but not normal cornea. Haynes et al55,56 also observed positive immunoreactivity for HNP1–3 in inflamed conjunctiva and samples of normal tear film. Recently, Zhou et al57 confirmed the presence of HNP1–3 in tear film and, using liquid chromatography-mass spectrometry, they determined the levels of HNP-1 and -2 to be in the range 0.2–1 μg/ml. In each of these studies, the authors presumed that inflammatory cells, primarily neutrophils, were the source of α-defensins. Haynes et al55,56 also used RT-PCR to look for expression of the α-defensins HD-5 and HD-6; however, no evidence for the production of these defensins was found in any of the ocular samples they tested.
Both cornea and conjunctival epithelial cells express β-defensins. hBD-1 is constitutively expressed,54–56,58–61 whereas the expression of hBD-2 is variable.54,59–61 Indeed, we60 found this defensin to be expressed by the corneal epithelium in only two of eight cadaveric corneas tested (Figure 1). However, hBD-2 expression is known to be inducible.44 McNamara et al58 showed that hBD-2 expression by SV40-immortalized human corneal epithelial cells was upregulated by bacterial lipopolysaccharide by a pathway involving tyrosine kinase and p38 mitogen-activated protein (MAP) kinase activation.62 A later study also showed nuclear factor-κB (NFκB) to be involved.63 Studies of bacterial induction of hBD-2 expression in several cell types corroborate the involvement of MAP kinase and NFκB.64–68 Also, Birchler et al68 and Wang et al70 observed that upregulation of hBD-2 expression by the bacterial products lipoprotein and lipotechoic acid is mediated via Toll-like receptor 2.
Figure 1.
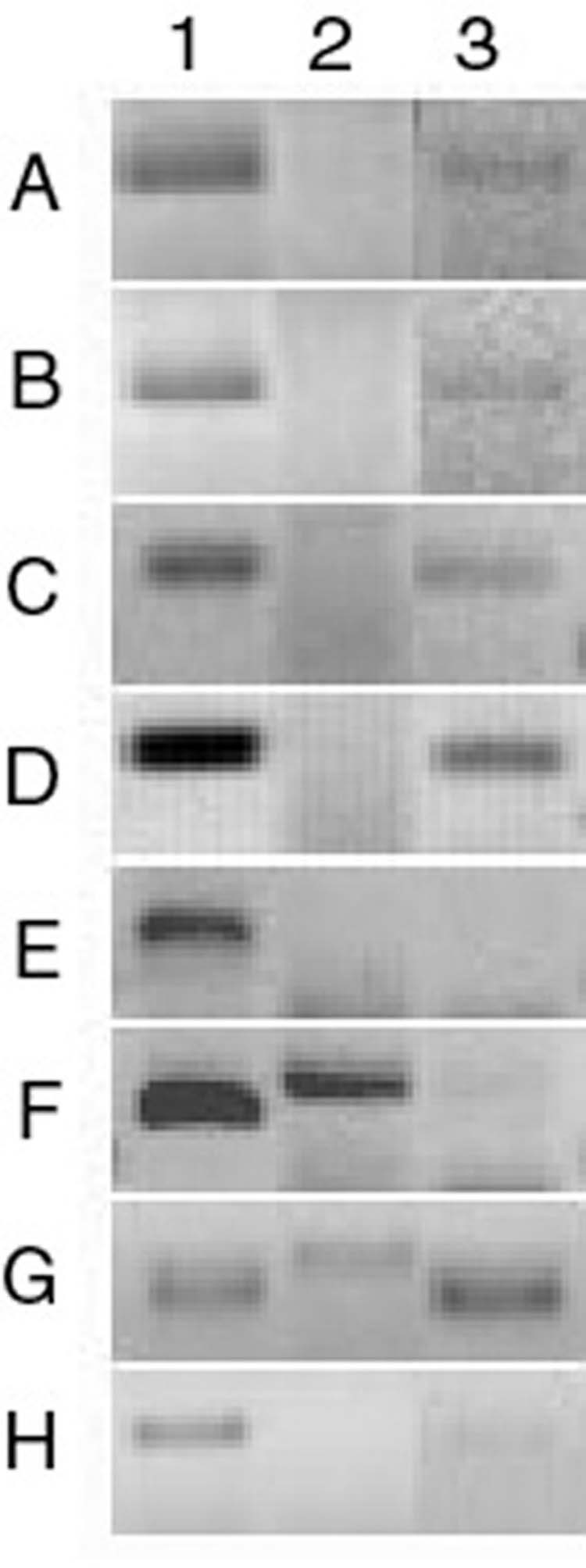
β-Defensin expression by human corneal epithelium. RT-PCR was performed using RNA isolated from epithelium scraped from eight cadaver corneas (A–H). All samples expressed hBD-1. Two samples (F and G) expressed hBD-2 and five (A–D, G) expressed hBD-3. 1 = hBD-1, 2 = hBD-2, 3 = hBD-3. (Reprinted from McDermott AM, Redfern RL, Zhang B, et al. Defensin expression by the cornea: multiple signaling pathways mediate IL-1β stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci, 2003; 44:1859-65, with permission of Association for Research in Vision and Ophthalmology.)
We have shown that hBD-2 is upregulated in regenerating corneal epithelium in an in vitro organ culture model of corneal epithelial wound healing.71 Additionally, we have shown that hBD-2 expression by corneal60 (Figure 2) and conjunctival epithelial cells61 is upregulated by the proinflammatory cytokines interleukin-1 (IL-1) and tumor necrosis factor (TNF)-α. In the case of corneal epithelial cells, the effects of IL-1 on hBD-2 expression were mediated via p38MAP kinase, JNK and NFκB.60 We have also observed that corneal (Figure 1) and conjunctival epithelial cells (Figure 3) express hBD-3. Some studies have indicated that expression of this defensin is inducible45,46,72 by TNFα and interferon-γ; however, we did not observe increased hBD-3 expression in either corneal (McDermott et al, unpublished observation) or conjunctival epithelial cells61 following cytokine treatment. We have also used RT-PCR to investigate the expression of three other β-defensins, hBD-4, -5 and -6, but did not find evidence of their production by cornea or conjunctival epithelial cells (McDermott, Proske & Huang, unpublished observation). β-Defensins have not been detected in the tear film.57
Figure 2.
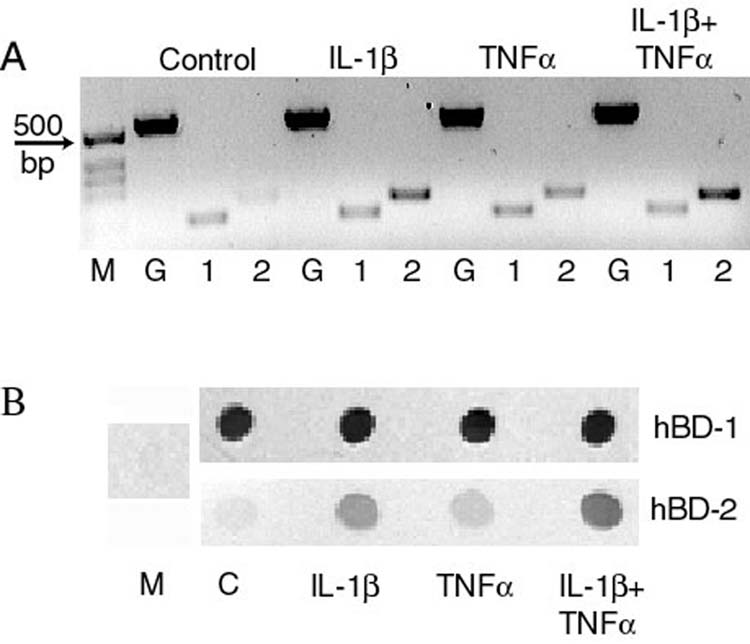
Effect of cytokines on β-defensin expression by primary cultured corneal epithelial cells. Cells were treated with 10ng/ml IL-1β, TNFα or both for 24 hours then RT-PCR (panel A) was used to study defensin mRNA expression and immunoblotting (panel B) was used to study peptide secretion in to the culture media. Panel A: M = size markers, G = housekeeping gene glyceraldehyde-3-phospahte dehydrogenase, 1 = hBD-1, 2 = hBD-2. Panel B: M = culture media that had not been in contact with cells, C = supernatant from control cells. Expression of hBD-1 is constitutive whereas hBD-2 is upregulated by IL-1β and TNFα. (Reprinted from McDermott AM, Redfern RL, Zhang B, et al. Defensin expression by the cornea: multiple signaling pathways mediate IL-1β stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci, 2003;44:1859-65, with permission of Association for Research in Vision and Ophthalmology.)
Figure 3.
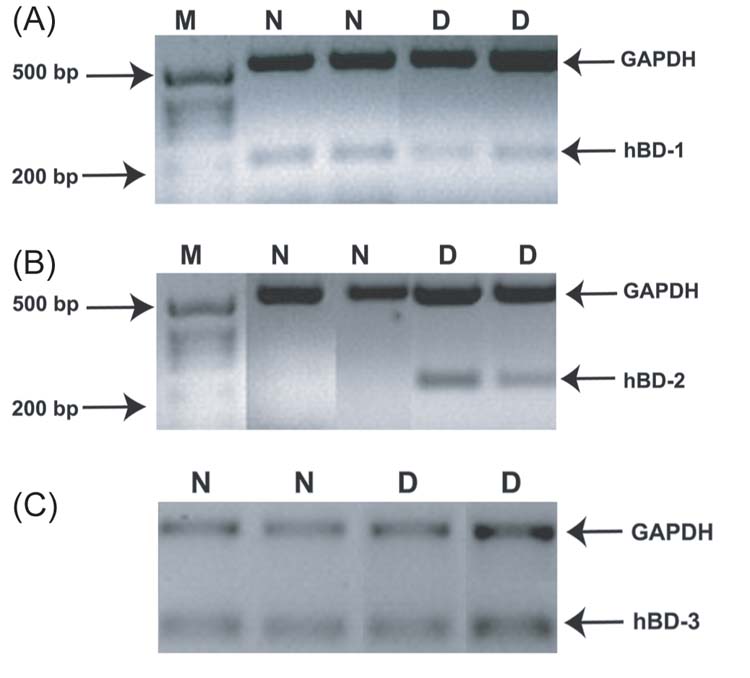
Expression of human β-defensin in conjunctival epithelium: relevance to dry eye disease. Cell samples were obtained by conjunctival impression cytology from normal subjects (N) and patients with moderately dry eyes (D). RT-PCR was used to investigate expression of hBD-1 (A), hBD-2 (B) and hBD-3 (C). M = size marker. hBD-1 and hBD-3 were constitutively expressed, whereas hBD-2 was only detected in samples from patients with dry eye. (Reprinted from Narayanan S, Miller WL, McDermott AM. Proinflammatory cytokines and Pseudomonas aeruginosa upregulate human β-defensin-2 in conjunctival epithelial cells : relevance to dry eye disease. Invest Ophthalmol Vis Sci 2003;44:3795-801, with permission of Association for Research in Vision and Ophthalmology.)
Because inflammation is a significant component of all forms of dry eye,73 we were interested to know what effect this condition had on the expression of β-defensins by conjunctival epithelial cells. We observed that conjunctival epithelium from patients with symptomatic mild to moderate dry eye expressed hBD-2, whereas samples from subjects without dry eye did not (Figure 3).61 There was no difference in the expression of hBD-1 and hBD-3 between the two groups. Kawasaki et al74 also noted increased expression of DEFB4 (formerly known as DEFB2), the gene for hBD-2, in conjunctival epithelial cells of patients with Sjogren syndrome, who have a very severe form of dry eye resulting from a systemic autoimmune condition. Based on the fact that hBD-2 expression can be upregulated by proinflammatory cytokines61 and that such cytokines are increased in dry eye,75,76 we hypothesized that increased hBD-2 expression is the result of enhanced inflammatory cytokine activity at the ocular surface in this condition. As discussed below, upregulated hBD-2 expression likely gives additional antimicrobial protection to the compromised ocular surface, and, through the nonmicrobicidal activities mediated by the peptide, may have other, although not necessarily beneficial, effects.
As summarized in Table 1, current data show that the epithelia of the human ocular surface normally express hBD-1 and hBD-3, whereas hBD-2 expression can be upregulated with appropriate stimuli, such as infection and inflammatory cytokines. Additionally, α-defensins HNP 1 through 3 are normally present in the tear film, and, during inflammation, they may be released in the cornea by infiltrating inflammatory cells.
Table 1.
Summary of Antimicrobial Peptides Present at the Human, Bovine and Rat Ocular Surface
| Species, location and peptide | Comment | |
|---|---|---|
| Human | ||
| Corneal epithelium: | hBD-1 | Constitutively expressed55, 56, 59, 60 |
| hBD-2 | Variably expressed, upregulated by proinflammatory cytokines, infection, injury55,56,58–60, 71 | |
| hBD-3 | Constitutively expressed60 | |
| CAP37 | 222 amino acid protein expressed in response to S. aureus and upregulated by proinflammatory cytokines97 | |
| LL-37 | Upregulated by injury and IL-1β94 | |
| Conjunctival epithelium: | hBD-1 | Constitutively expressed54–56, 59, 61 |
| hBD-2 | Variably expressed, upregulated by proinflammatory cytokines and in dry eye patients55, 56, 59, 61, 74 | |
| hBD-3 | Constitutively expressed61 | |
| Corneal stroma: | HNP-1, -3 | Present in cases of transplant rejection and post-infectious keratitis but not normal cornea, infiltrating neutrophils presumed to be the source53 |
| Tear film: | HNP-1, -2, -3 | Present in normal tear film,55–57 increased after ocular surface surgical procedure,57 neutrophils presumed to be the source |
| Other potential antimicrobial peptides at human ocular surface: | ||
| Thymosin β-4 | Expressed by corneal epithelium,107 recently shown to have antimicrobial activity102 | |
| Histatin 5 | Known antifungal activity, detected in tear film,121 corneal and conjunctival epithelia119* | |
| CCL28 | Antimicrobial chemokine116 detected in corneal and conjunctival epithelium119* | |
| CCL20 | Antimicrobial chemokine117–118 detected in IL-1α stimulated corneal fibroblasts122 | |
| LEAP-1, -2 | mRNA detected in corneal and conjunctival epithelia120* | |
| Dermcidin | mRNA detected in corneal and conjunctival epithelia120* | |
| Bovine | ||
| Corneal epithelium: | LAP | Present and upregulated after injury80* |
| TAP | Only significantly expressed after injury80* | |
| Conjunctival epithelium: | LAP | Constitutively expressed77* |
| TAP | Constitutively expressed80* | |
| Rat | ||
| Corneal epithelium: | rBD-1 | Constitutively expressed82* |
| rBD-2 | Only significantly expressed after injury82* | |
Indicates study in which only mRNA expression has been studied. hBD = human β-defensin; CAP37 = cationic antimicrobial protein 37; HNP = human neutrophil peptide; LEAP = liver expressed antimicrobial peptide; LAP = lingual antimicrobial peptide; TAP = tracheal antimicrobialpeptide; rBD = rat β-defensin.
C. Defensins at the Ocular Surface in Non-Human Species
Expression of β-defensins is not unique to human ocular surface epithelia. In 1997, Stolzenberg et al77 reported that bovine conjunctiva expressed lingual antimicrobial peptide (LAP), a peptide so named because it was first identified in tongue lesions.78 We have confirmed Stolzenberg’s observation and shown that tracheal antimicrobial peptide (TAP), the first β-defensin to be identified79 and the bovine homologue of hBD-2, is also expressed by bovine conjunctival epithelium.80 Additionally, we found that whereas bovine corneal epithelium constitutively expressed LAP, TAP was significantly expressed only after injury. Other studies in our laboratory have shown that rBD-1 and rBD-2, the rat homologues of hBD-1 and hBD-2, respectively,81 are expressed by rat cornea and conjunctiva and that rBD-2 expression is upregulated after corneal injury in vivo.82 Interestingly, Gottsch et al53 reported that IL-1α induced LAP expression in bovine keratocytes cultured in the presence of serum. We repeated their experiment using cultured human corneal fibroblasts and primers specific for human β-defensins, but we found no such response (McDermott and Redfern, unpublished observation).
III. LL-37 AND OTHER ANTIMICROBIAL PEPTIDES AT THE OCULAR SURFACE
To date, defensins have been the most studied of the human antimicrobial peptides; however, several others have been identified and are now the subject of intense investigation. In particular, the 37 amino acid linear peptide LL-37 has gained much attention. This peptide is derived by cleavage of a larger precursor referred to as human cationic antimicrobial protein 18 (hCAP18), which is the only known human member of a family of antimicrobial peptides called the cathelicidins (for recent reviews, see references 19 and 83). LL-37/hCAP18 was first cloned from bone marrow85,86 and is expressed by immune and inflammatory cells30,87,88 and epithelial tissue.89–91 LL-37 is released from its parent molecule by extracellular cleavage with proteinase 3.92 It has potent antibacterial activity and, in keeping with the observation that antimicrobial peptides are multifunctional molecules, exerts a variety of effects on mammalian cells.19,83,93 We used RT-PCR and immunoblotting to determine if ocular surface epithelial cells express LL-37. Low expression (mRNA and protein) of this antimicrobial peptide was detected in human corneal epithelial cells, and this was upregulated by injury and exposure to IL-1β.94 We also detected the peptide in cultured human conjunctival epithelial cells (Huang and McDermott, unpublished observation) and have shown that it stimulates corneal epithelial cell migration and kills ocular pathogens.95 These preliminary findings strongly suggest that LL-37 is an important ocular surface defense peptide.
Several other molecules may also fulfill this role. Cationic antimicrobial protein 37 (CAP37, azurocidin, heparin binding protein), a protein first isolated from human neutrophil granules,96 has been found to be expressed by rabbit corneal epithelium, stromal fibroblasts, and bulbar conjunctiva in response to infection with Staphylococcus aureus.97 The authors also reported that IL-1β and TNFα induced CAP37 expression in immortalized human corneal epithelial cells. Strictly speaking, being 222 amino acids in length, CAP37 cannot be called a peptide; nevertheless, it has features in common with its smaller relatives, including similarities in the mechanism of antimicrobial activity (such as sensitivity to ionic strength, see below). CAP37 has also been shown to modulate various mammalian cell functions,98 including stimulation of human corneal epithelial cell migration, proliferation, and regulation of gene expression.99,100
A further candidate for an ocular surface antimicrobial peptide is the 43 amino acid peptide Thymosin-β4 (Tβ-4). This peptide is a member of the thymosin family of G-actin sequestering peptides and has been shown to have additional biological effects, including chemotaxis, angiogenesis, and inhibition of inflammation,101 and, most recently, antimicrobial activity.102 Sosne et al have shown that exogenously applied Tβ-4 stimulated corneal epithelial healing and cytokine production after debridement with alcohol.103 Similarly, they found that Tβ-4 promoted wound healing after alkali injury, but, in this case, cytokine production was decreased.104 They have also shown that Tβ-4 stimulates migration of human corneal and conjunctival epithelial cells.103,105 Katz et al106 reported that Tβ-4 is a component of the normal human tear film, and recently Sosne et al107 reported that Tβ-4 levels increased in a mouse model of wound healing. Thus, Tβ-4 bears the hallmarks of a multifunctional peptide with antimicrobial activity, although, as an anionic peptide, its mechanism of action must be distinct from that of the cationic peptides, such as the defensins.
Other human peptides with known antimicrobial activity include histatins,108 human natriuretic peptides,109 dermcidin,110 hepcidin/liver-expressed antimicrobial peptide (LEAP)-1,111,112 LEAP-2,113 HE2α and HE2β1,114 and the following chemokines of the CXC and CC families: CXCL9, 10, 11115; CXCL4, CTAP-3, CCL5102; CCL28116; CCL20.117,118 Of these, corneal and/or conjunctival mRNA expression has been reported for histatins and CCL28119 and LEAP-1 and -2, and dermcidin.120 Histatin 5 has been detected in tear film121 and CCL20 mRNA was found to be markedly upregulated in human corneal fibroblasts in response to IL-1α.122 For most of these peptides, studies are required to show that translation and protein production does actually occur. Once complete, the results of such studies are likely to confirm that, in addition to defensins and LL-37, the ocular surface is equipped with a wide range of antimicrobial peptides.
IV. ANTIMICROBIAL ROLE OF DEFENSINS AND OTHER PEPTIDES AT THE OCULAR SURFACE
A. Mechanism of Antimicrobial Activity
As signified by their name, antimicrobial peptides have been identified based on their ability to kill or prevent the growth of microorganisms. While the individual peptides vary in their ability to kill different organisms, as a group they are active against both Gram positive and Gram negative bacteria, some fungi, and some viruses. As noted earlier, over 800 different antimicrobial peptides have been described; the vast majority are cationic and can be classified into several different groups based on their structure. Despite this significant diversity, it is generally accepted that the cationic peptides exert their antimicrobial activity in a similar fashion, although the details of this are poorly understood. By virtue of their positive charge, the peptides interact electrostatically with negatively charged components, particularly phospholipids, of microbial cell membranes, leading to increased permeability of the cell membrane and death. The relative selectivity of the peptides for microorganisms versus eukaryotic cells has been attributed to the low membrane potential, fewer negatively charged phospholipids, and high cholesterol levels of the latter.123,124 Several models have been proposed to describe the peptide-membrane interactions. For a more in-depth review than is given below, the reader is referred to articles by van’t Hof et al12 and Shai.125
In the barrel-stave model, peptide monomers associate with the membrane and then assemble together to form a stable transmembrane pore. The pore size increases as more peptide monomers are added, and leakage of intracellular contents through the pores eventually leads to cell death. The α-defensin HNP-2 has been reported to form stable pores with a maximum diameter of 2.5 nm, possibly representing a hexamer of defensin dimers.126
In the carpet model, the microbial membrane is first coated in a “carpet” of peptide; then, when the point of saturation is reached, the membrane collapses, creating “worm holes,” leading to lysis of the organism. The carpet model has been used to describe the mechanism of action of many antimicrobial peptides, including human β-defensins127 and LL-37.128
In the final model, the aggregate channel model, the peptides cluster into unstructured aggregates within the membrane, which causes the transient formation of channels through the membrane. These channels allow for the leakage of ions and, additionally, allow the peptides to cross the membrane and reach intracellular targets, where they exert their killing effect. The histatins are believed to reach their intracellular target, the mitochondria, in this way.129
By utilizing one of the approaches outlined above, antimicrobial peptides are able to kill Gram negative and positive bacteria and some fungi. Antiviral activity, particularly against some enveloped viruses, may be mediated by similar mechanisms130,131; however, other modes of action also appear to be involved. Indeed, HNP-1 and HD-5 have been observed to inhibit adenoviral infection in vitro,132,133 and, as adenovirus strains do not have an envelope, a mechanism other than membrane perturbation must be involved. Defensins have been shown to be active against herpes simplex virus (HSV) and human immunodeficiency virus (HIV).130,131,134 Recent studies suggest multiple modes of anti-HSV and -HIV activity, including inhibition of viral entry by masking of essential glycoproteins on the viral envelope or via a chemokine-like effect of the defensin, which results in downregulation of the coreceptors used by the viruses to gain entry to the host cell.135–138
In vitro studies show defensins and other peptides to have antimicrobial activity at concentrations in the micromolar range, with minimum inhibitory concentrations (MIC) of 1–100 μg/ml, depending upon peptide and organism.23,139,140 Within the confines of neutrophil granules, it is estimated that α-defensin concentrations can reach 1–10mg/ml, a level more than adequate for antimicrobial activity.139 The levels of α-defensins in body fluids is low, typically less than that required for antimicrobial activity, but they rise markedly in inflammation and infection to concentrations within the active range.141–143 Whether antimicrobial peptides secreted by epithelial surfaces can reach such high levels has been debated, but recent studies show that in some tissues it is possible. Liu et al reported that following stimulation with IL-1, levels of hBD-2 in organotypic epidermal cultures reached 3.5 to 16μM.144 Also, Oren et al observed that in human epidermis, hBD-2 is packed into lamellar bodies and localized to intercellular spaces, which would help increase local concentrations.145 Indeed, it had previously been suggested that high levels may actually be achieved in vivo by virtue of the peptides being concentrated at their sites of secretion.146 hCAP18, the parent molecule of LL-37, is present in plasma at a concentration of 1.2 μg/ml, but, interestingly, it is found in seminal plasma at seventy times this level.147
Although it has been extensively shown that defensins and other peptides have potent antimicrobial activity in vitro, conclusive proof of their activity in vivo is not as easily obtained. Nevertheless, a small number of studies do show that antimicrobial peptides are indeed important components of the innate immune system. Inactivation of the gene for matrilysin, an enzyme required for processing mouse intestinal α-defensins to their active form, increased the susceptibility of the animals to infection with Salmonella typhimurium.148 Mice lacking the gene Cnlp, which codes for CRAMP, the mouse homologue of LL-37, were found to be much more susceptible to skin infection caused by group A streptococcus.149 Knocking out the mouse β-defensin-1 gene led to delayed clearance of Haemophilus influenzae from the lung150 and an increase in Staphylococcus colonization in the bladder.151 Most recently, Salzmann et al reported that transgenic mice expressing the gene for human intestinal α-defensin HD-5 were much more resistant to an oral challenge with S. typhimurium than their wild-type counterparts.152
Other evidence supporting a role of antimicrobial peptides in vivo comes from a study of the severe recessive disorder morbus Kostmann.153 In this condition, an unknown mutation results in the failure of promyelocytes to develop into myelocytes, leaving patients with very few neutrophils. Although patients can now be treated with recombinant granulocyte-monocyte colony-stimulating factor to boost neutrophil numbers, they are still prone to recurrent infections, and, in particular, have severe oral infections. Putsep et al reported that such patients have normal neutrophils in terms of respiratory burst activity and lactoferrin production but that LL-37 is missing from both the neutrophils and saliva.153
B. Antimicrobial Activity at the Ocular Surface
Several studies show that antimicrobial peptides are effective against ocular surface pathogens in vitro. A variety of peptides have been tested, including defensins, magainins (antimicrobial peptides originally isolated from the African clawed frog154) and cecropins. Table 2 summarizes the peptides and the organisms against which they are effective.
Table 2.
Ocular Pathogens Susceptible to Antimicrobial Peptides In Vitro
| Peptide | Organism | Reference number |
|---|---|---|
| NP-1 (rabbit α-defensin) | S. aureus, P.aeruginosa, S. pneumoniae, α-hemolytic streptococcus, C. albicans, M. morangii | 155 |
| Magainins (MSI-103, MSI-94) | Acanthamoeba polyphaga | 156 |
| Shiva-11 (synthetic cecropin) | P. aeruginosa, S. aureus, S. pneumoniae, C. albicans | 157 |
| Protamine | P. aeruginosa, S. marcescens, S. aureus, S. intermedius | 158 |
| Melittin | P. aeruginosa, S. marcescens, S. aureus, S. intermedius | 158 |
| Cecropin A | P. aeruginosa, S. marcescens, S. aureus, S. intermedius | 158 |
| LL-37 | P. aeruginosa, HSV-1, Adenovirus (Ad3, 5, 8, 19) | 95, 159 |
| Defensins: HNP-1, hBD-1, hBD-2 | Adenovirus (Ad3, 5, 19) | 160 |
| hBD-2 | P. aeurginosa | McDermott & Huang unpublished |
These examples clearly demonstrate that antimicrobial peptides can kill a variety of known ocular pathogens in vitro. However, it remains to be determined if this is actually the case with the peptides that are present at the ocular surface in vivo. One question is whether or not the peptides reach high enough concentrations to have antimicrobial activity. Zhou et al reported tear fluid levels of HNP-1 and -2 to be in the range 0.2–1 μg/ml, and that these rose to 5–15 μg/ml in patients after ocular surgery, so reaching the necessary levels for microbicidal activity, at least against some organisms.57
Based on measurements of hBD-1 and hBD-2 secretion by cultured human corneal epithelial cells, we have estimated that human corneal epithelium is capable of producing microgram quantities of hBD-1 and hBD-2, but we do not know the actual concentrations.60 As is the case with skin,145 accumulation of the peptide in intercellular spaces may mean that very high concentrations are, indeed, achieved. Interestingly, several studies have shown that antimicrobial peptides can interact with each other and with other antimicrobial substances, such as lysozyme, in an additive or synergistic fashion.161–163 Therefore, the micromolar concentrations of peptide required for in vitro activity may not be required at the ocular surface, because there may be synergistic interactions between the several peptides present at the surface and also with components of the tear film, such as lysozyme.
An additional factor that must be considered when addressing the question of whether or not antimicrobial peptides are active at the ocular surface is the possibility that components of the tear film may compromise their activity. Indeed, Rich et al164 reported that the activity of the synthetic rabbit defensin NP-3a against P. aeruginosa was reduced in the presence of human tears in vitro, and we have observed similar effects on the activity of hBD-2.165 The tear film contains a significant amount of sodium chloride,166 and it is known that the antimicrobial activity of many peptides is sensitive to physiological salt concentrations, presumably because the salt interferes with the initial electrostatic interactions between peptide and microbial membrane.139 Thus, any peptide secreted onto the ocular surface by the epithelial cells, present in the tear film or exogenously applied (see Section VI.B), may be rendered ineffective by the salt in the surrounding environment. Interestingly, hBD-3, which we find to be constitutively expressed by the ocular surface epithelia, 60,61 is the defensin least sensitive to the effect of salt,45 a characteristic attributed to its having a higher net positive charge and an increased affinity to form dimers compared to hBD-1 and-2.167 It should also be noted that the effects of salt are dependent upon the concentration of peptide, with higher levels being minimally affected.168 Furthermore, it has been observed that synergistic interactions between peptides were able to overcome the detrimental effects of salt.168 Thus, while a high enough concentration of peptide at the ocular surface may circumvent the actions of salt, such a concentration may not be necessary, due to synergistic interactions between the different peptides present.
In fact, having synergistic interactions rather than high peptide concentrations may be the better option, as both defensins and LL-37 are cytotoxic to some mammalian cells at high concentrations. α-Defensins have been shown to be cytotoxic to various tumor cell lines,169 MRC-5 fibroblasts and primary cultured human umbilical vein endothelial cells,170 A549 lung epithelial cells,170,171 and alveolar macrophages.172 hBD-1 was found to be toxic to NIH-3T3 fibroblasts,173 and LL-37 was toxic to lymphocytes and peripheral blood leukocytes.174 Cytotoxicity was concentration-dependent, and, typically, it first became apparent at antimicrobial peptide concentrations of approximately 25 μg/ml, although this varied with the cell types being tested.
The mechanism of cytotoxicity has similarities to that of antimicrobial activity and involves an electrostatic (and initially reversible) interaction with the cell membrane, followed by pore formation and possible entry of the peptide into the target cell and DNA damage.175 However, while antimicrobial effects are apparent in minutes, cytotoxic effects take several hours to develop. Thus, it is possible that prolonged and excessive neutrophil infiltration and/or epithelial expression of hBD-2 and LL-37 may raise the concentration of antimicrobial peptides to a level that may actually cause damage to the ocular surface.
The tear film is a complex mixture of over 500 different proteins,176 some of which have been shown to be capable of inactivating some antimicrobial peptides. For example, α2-macroglobulin and serpins (serine protease inhibitors) both bind to and inactivate defensins.177,178 Although these proteins have been detected in tear film,179 their concentrations may not reach a level high enough to effectively impair peptide antimicrobial activity. But, if appropriate concentrations are achieved, these inhibitors may represent one mechanism for regulating the activity of the antimicrobial peptides and thus preventing unwanted cytotoxicity.
Given that antimicrobial peptides are active against ocular surface pathogens in vitro, that mechanisms exist to circumvent some of the potential interference from the tear film, and that the peptides are active in vivo (see section IV.A), the defensins, LL-37 and other peptides present at the ocular surface are likely to play a major role in defending the ocular surface from infection. Based on the previously described studies of peptide expression, it appears likely that hBD-1 and hBD-3 provide the main baseline defense against infection. Then, after injury, inflammation, or infection, hBD-2 and LL-37 are additionally expressed, and neutrophil infiltration causes an increase in α-defensins. As these antimicrobial peptides have differing spectra of activity44—for example, hBD-3 is particularly effective against Staphylococcus aureus,45 whereas hBD-2 has potent anti-pseudomonal activity44—and this enhances the ability of the ocular surface to ward off a wide range of pathogens.
V. NONMICROBICIDAL ROLES OF DEFENSINS AND OTHER ANTIMICROBIAL PEPTIDES AT THE OCULAR SURFACE
Defensins and other antimicrobial peptides were first identified through their ability to kill microorganisms; however, it is now recognized that many also have the capacity to modulate mammalian cell behavior. Indeed, the list of peptide nonmicrobicidal activities grows ever longer and more varied, with effects as disparate as defensin inhibition of adrenocorticotrophin activity (formerly, some defensins were also referred to as corticostatins),180 defensin regulation of smooth muscle cell contraction,181 and LL-37-induced angiogenesis.182 Because they occur via mechanisms completely independent of that responsible for antimicrobial activity (discussed below), these activities typically occur at peptide concentrations much lower than those required for effective antimicrobial activity and are not sensitive to the effects of salt.
Most attention has been paid to the effects of antimicrobial peptides, particularly those of defensins and LL-37, on immune cell functions. Defensins are chemotactic for T cells, monocytes, immature dendritic cells, neutrophils, and mast cells.183–188 They have also been shown to modulate monocyte and epithelial cell cytokine and chemokine production189–191 and stimulate histamine release from mast cells.192,193 Also, murine β-defensin-2 (mDF2β) stimulates dendritic cell activation and maturation.194 LL-37 has been found to exert very similar effects. This peptide is chemotactic for monocytes, T cells, neutrophils, and mast cells.195,196 It stimulates mast cell histamine release,193 modulates chemokine and chemokine receptor gene expression in macrophages, and stimulates airway epithelial cell release of the neutrophil chemoattractant IL-8.193,196,197 It has also been observed that LL-37 modifies dendritic cell differentiation—cells derived in the presence of LL-37 had altered characteristics, including increased endocytic capacity and secretion of T helper (Th)-1 cell-inducing cytokines and were able to generate an enhanced Th1 response.198
For both defensins and LL-37, strong evidence exists to suggest that their activities are mediated via specific cell surface receptors. The G-protein coupled receptor chemokine receptor 6 (CCR6) was found to mediate hBD-2-induced chemotaxis of immature dendritic cells and T cells.185 Toll-like receptor 4 mediates mDF2β effects on mouse dendritic cells,194 whereas hBD-2 effects on mast cells appeared to involve a G-protein linked receptor but not CCR6.187,193 The G-protein coupled receptor formyl peptide receptor-like 1 (FPRL-1) mediates LL-37-stimulated chemotaxis of neutrophils and monocytes195 but not mast cells.196 LL-37 has also been shown to transactivate the epidermal growth factor receptor (EGFR), possibly by first activating a metalloproteinase, which cleaves membrane-anchored EGFR ligands, which, in turn, bind to and activate the receptor.199 An interaction of LL-37 with the purinergic receptor P2X7, which leads to IL-1β release by monocytes, has also recently been observed.200
That defensins and LL-37 can directly and indirectly, via mast cell products and induction of chemokine secretion, attract various immune cells implies that in vivo these peptides can help mobilize the cellular components of the immune system in response to pathogen entry. Defensins may also participate in the immune response by causing neutrophil activation201 and modulating the activity of the classical complement pathway by binding to complement C1q.202,203
The aforementioned ability of defensins to inhibit adrenocorticotrophin activity,180 and, therefore, reduce production of anti-inflammatory glucocorticoids, may also be a mechanism by which these peptides can enhance the immune response. Additionally, LL-37 is able to bind and neutralize lipopolysaccharide and lipotechoic acid, and, thus, reduce the inflammatory response associated with these molecules.86,197,204
That defensins are chemoattractive for dendritic cells and that defensins and LL-37 can activate these cells has led to the suggestion that these peptides also provide a link between the innate and adaptive immune systems.185,186,198 Furthermore, some studies suggest that defensins can promote both humoral and cellular antigen-specific immune responses.205 Thus, in addition to their direct microbicidal role, antimicrobial peptides may participate in the immune response by recruiting and activating immune cells, regulating aspects of the inflammatory response, and promoting adaptive immunity. For more details on the evidence supporting the various roles of defensins and LL-37 in innate and adaptive immunity, the reader is referred to a recent review by Yang et al.18
The non-microbicidal roles of the peptides do not end with effects on the immune system. There are several lines of evidence to suggest that antimicrobial peptides may also be involved in wound healing. Defensins have been shown to stimulate proliferation of fibroblasts and epithelial cells.206,–211 Human α-defensins have been shown to induce wound closure in a culture model using NCI-H292 airway epithelial cells.212 Here the peptides stimulated both cell proliferation and migration via pathways involving MAP kinase to accelerate wound healing. HNP-1 has also been shown to enhance fibroblast expression of proα1(I) collagen but suppress that of matrix metalloproteinase-1.213 Whereas, increased hBD-1 expression is associated with keratinocyte differentiation,214 LL-37 has been shown to be angiogenic, acting via FPRL-1 to stimulate vascular endothelial cell proliferation and promote the development of vessel-like structures.182 LL-37 has also been implicated as an important factor in re-epithelialization of skin wounds, where it was suggested to stimulate cell proliferation.215 Although not conclusive, together these experimental findings do provide evidence for direct participation of antimicrobial peptides in wound healing responses.
Which, if any, of these nonmicrobicidal activities do antimicrobial peptides exert at the ocular surface, and are certain peptides more likely to mediate these effects? The second part of the question will be addressed first, as it is more easily answered. Antimicrobial peptides that first appear or whose expression increases under specific circumstances are the most likely to be involved in activities independent of antimicrobial activity. That is not to say that these peptides do not participate in killing of microorganisms, simply that in addition to this activity, they may have other actions. Thus, as hBD-2 and LL-37 are upregulated in regenerating corneal epithelium and in response to inflammatory cytokines,60,61,71,95 and given that α-defensins will be present only when neutrophil infiltration occurs, these are the most likely to show non-microbicidal activities. The non-microbicidal actions in which these antimicrobial peptides participate at the ocular surface can, for the most part, only be extrapolated from studies in nonocular tissues. For example, neutrophils are known to infiltrate the cornea after injury216–219; therefore, through their chemotactic properties, hBD-2 and LL-37188,195 may assist this recruitment. α-Defensins coming from the newly recruited neutrophils may, in turn, help mediate influx of additional neutrophils, perhaps by stimulating release of IL-8.191
Notably, we have found that defensins stimulate IL-8 secretion by cultured corneal fibroblasts.220 Recently, α-defensins have been shown to enhance the expression of the mucins MUC5B and MUC5AC by lung epithelial cells212; therefore, they may be able to stimulate the production of the MUC5AC by conjunctival goblet cells.221 Also, hBD-2 and LL-37 may stimulate the immature dendritic cells185,186,198 recently found to be resident in the corneal stroma222 and thus promote activation of the adaptive immune system.
hBD-2, LL-37 and α-defensins may also be involved in ocular surface wound healing. We71 have observed that hBD-2 is upregulated in regenerating corneal epithelium in an in vitro organ culture model of corneal epithelial wound healing (Figure 4). As IL-1 and TNFα are upregulated after injury,223–225 they are likely to be responsible for upregulated hBD-2 expression in our wounding model. In support of our in vitro observations, hBD-2 expression was found to be increased compared to normal controls in corneal epithelial cells from a patient with a severe corneal abrasion.58 Also, we have shown that corneal epithelial expression of rBD-2 (the rat homologue of hBD-2) is upregulated in an in vivo rat model of corneal scrape injury.82 We also observed that LL-37 expression was increased in regenerating epithelium in our in vitro culture model.95
Figure 4.

Expression of β-defensins in regenerating corneal epithelium. The corneal epithelium (“original”) was scraped from cadaver corneas, then the corneas were placed in to organ culture and regenerated epithelial cells (“regrown”) collected 24 or 48 hours later. RT-PCR (A) and immunoblotting (B) were used to study defensin expression in the samples. A: M = size marker; Lanes 1,4,7,10 are products for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase; Lanes 2,5,8,11. are products for hBD-1; Lanes 3,6,9,12 are products for hBD-2.B : Control are samples of synthetic defensin peptide (25ng/dot). hBD-1 is constitutively expressed but hBD-2 is only present in regrown samples. (Reprinted from McDermott AM, Redfern RL, Zhang B. Human β-defensin 2 is upregulated during re-epithelialization of the cornea. Curr Eye Res 2001;22:64-7, with permission of Current Eye Research.)
If defensins and/or LL-37 are important for corneal wound healing, then they would be expected to enhance one or more of the events that occur during the process. We have begun to address this, using in vitro assays to study corneal epithelial cell migration and proliferation, both important components of corneal wound healing. We find that while defensins alone cannot stimulate cell migration, both hBD-2 and HNP-1 can enhance fibronectin-stimulated migration.220 This is of particular significance, as it is fibronectin across which the epithelial cells must migrate in order for wound closure to occur.226,227 On the other hand, LL-37 alone can stimulate cell migration and also enhance fibronectin-induced migration.95 We have also observed that hBD-2 and HNP-1 can stimulate human corneal epithelial cell proliferation (McDermott and Proske, unpublished observation). These preliminary data do support a role for antimicrobial peptides in corneal epithelial wound healing; however, it remains to be determined if this is the case in vivo. Indeed, many factors that can modulate corneal epithelial cell migration and proliferation have been identified through in vitro experimentation,228–230 but none have yet been conclusively established as a regulator of wound healing in vivo.
Not all of the potential nonmicrobicidal effects of the antimicrobial peptides may be beneficial to the ocular surface. Excessive recruitment of immune cells may lead to uncontrolled inflammation and cause ocular surface damage. For example, defensin and LL-37 stimulation of mast cell chemotaxis and degranulation may be particularly harmful. Also, LL-37-mediated angiogenesis would certainly not be a good thing for the cornea, although it is not clear that this peptide can initiate growth of vessels in areas where they were previously nonexistent.
Figure 5 summarizes some of the major effects that defensins and LL-37 may have at the ocular surface, using the cornea as a model.
Figure 5.
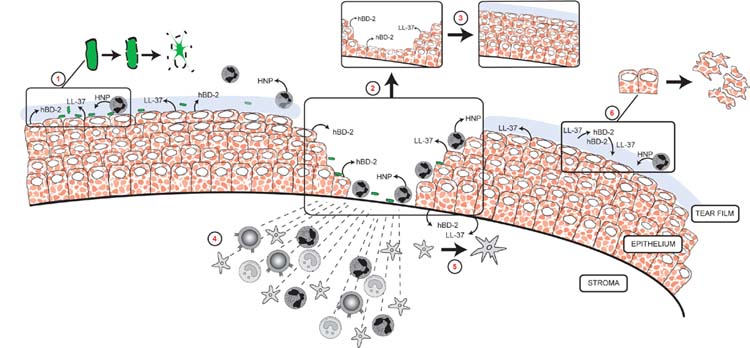
Potential roles for defensins and LL-37 in the cornea. Epithelial hBD-2 and LL-37 expression is increased in response to injury, inflammation and infection. HNP (α-defensins) are detected in normal tear film and would be in present in greater amounts after injury and during infection due to neutrophil infiltration. These peptides may then participate in the following: direct killing of pathogens (1); corneal epithelial wound healing by stimulating migration (2) and proliferation (3); recruitment of immune cells (4); promotion of adaptive immunity by activation of dendritic cells (5); cytotoxicity to ocular surface cells if in excess (6). hBD-1 and hBD-3 (not shown) are believed to provide a baseline defense against infection.
VI. POTENTIAL OF ANTIMICROBIAL PEPTIDES AS OCULAR THERAPEUTICS
A. Current Status of Antimicrobial Peptide Development
To date, in the development of the cationic peptides for clinical use, the emphasis has been on their potential as antimicrobial agents. However, as more is learned and understood of the roles of these peptides in regulating acquired immunity and wound healing, investigation of these potential uses may become prominent. The emergence of organisms resistant to the drugs fashioned to eradicate them is the driving force behind efforts to discover new classes of antimicrobials. Achieving this goal is of utmost importance, as resistance to traditional pharmaceuticals develops and spreads rapidly, and some organisms now exist for which we have no adequate treatment. Thus, the discovery of antimicrobial peptides generated great excitement, as it brought forth a new class of molecule to be investigated. In particular, antimicrobial peptides are thought of as very attractive drug candidates, because they have a broad spectrum of activity and kill very rapidly, and because the likelihood that organisms will become resistant to them is believed to be low.22,123,231
As discussed by Zasloff,22 resistance may be unlikely to develop because the peptides target membranes; thus, resistance would necessitate a complete overhaul of the microbial membrane—“a ‘costly’ solution for most microbial species.” Also, development of specific proteases to degrade the peptides would be complex, and, as the strike against the microbe usually consists of multiple structurally different peptides, elimination of just one peptide may not be a good enough defense. However, while it may indeed be more difficult for an organism to counter an attack by a peptide, it is not impossible, and there are a growing number of literature reports documenting ways in which organisms circumvent peptide action.232 For example, the enteropathogen Shigella causes downregulation of hBD-1 and LL-37 expression.233 Some pathogens express phosphorylcholine on their cell surface; this mimics mammalian cell membrane lipids, therefore decreasing killing.234 Other organisms produce proteinases that can degrade and inactivate peptides,235 and, recently, it was reported that S. aureus produces staphylokinase, which is capable of forming a complex with and, therefore, inactivating α-defensins.236
As discussed by van’t Hof et al, difficulties associated with utilizing peptides as therapeutics include sensitivity to ionic strength, susceptibility to proteolysis, cytotoxicity, and cost of production.12 Despite this, a number of biotechnology/pharmaceutical companies are evaluating peptides from several sources for potential pharmaceutical use in a variety of clinical settings. Some peptides under development for pharmaceutical use are listed in Table 3, with notation of their stage of development and applications. To date, the peptide that advanced furthest in pharmaceutical development was pexiganan (Locilex™), a magainin. However, in a somewhat controversial decision, FDA denied approval, not for safety reasons or lack of efficacy, but because the peptide was no more effective than existing treatments.237 At the present time, there are no peptides in development with specific ocular applications; however, an internet search revealed that the biotechnology company Xoma had considered one of their bactericidal/permeability increasing protein238 derivatives (I-PREX™) as a possible treatment for infected corneal ulcers.
Table 3.
Summary of Antimicrobial Peptides in Clinical Development
| Company | Peptide–Name | Peptide–Origin | Application | Development Stage |
|---|---|---|---|---|
| Genaera (formerly Magainin) | Pexiganan (Locilex) | 22aa magainin | Infected diabetic foot ulcers | Denied FDA approval 1999 |
| Intrabiotics | Isegan | Protegrin | (a) Pneumonia in mechanically ventilated patients | Phase III—stopped June 2004 for safety reasons |
| (b) Lung infection in cystic fibrosis | Phase IIa | |||
| Micrologix | MBI-226 | No information | Catheter related blood stream infection | Phase III |
| MBI-594 | No information | Acne | Phase IIb | |
| Demgen (formerly Periodontix) | P113D | Histatin | Lung infection in cystic fibrosis November 2002 | Orphan drug designation |
| P113 | Histatin | Oral candadiasis in HIV | Phase I/II | |
| Xoma | XMP-629 | 9aa fragment of BPI* | Acne | Phase II |
| Entomed | ETD-151 | Heliothis virescens | Antifungal | Preclinical |
| ETD-P1263 | Insect defensin | MRSA | Preclinical | |
| AmPharma | No information | No information | (a) Systemic antibiotic/antifungal
(b) Incorporation in to surgical devices to prevent post-surgery infection |
Preclinical |
BPI is bactericidal/permeability increasing protein, a 50–55kDa protein of neutrophils238
B. Possible Uses of Antimicrobial Peptides in Eye Care
Exogenous antimicrobial peptides could be employed in several ways to improve/maintain the health of the ocular surface. As discussed in more detail below, one important modality would be as “stand-alone” topical antimicrobial drugs for the treatment and/or prophylaxis of ocular infections. Secondly, peptides may have a place as combined agents that are antimicrobial and can promote wound healing, which would be useful for treatment of injuries or of an ocular surface compromised by severe dry eye or diabetes, for example. Administration via a contact lens with the therapeutic antimicrobial peptide attached239 may be a useful alternative to the eye drop, as it may help overcome some of the dilution and flushing effects of the tear film.
The peptides might also be used as disinfectants in contact lens cleaning/storage solutions. Cecropin D5C, a synthetic insect antimicrobial peptide, has been shown to augment the antimicrobial activity of contact lens solutions against P. aeruginosa.240
Schwab et al tested defensin antimicrobial activity against S. aureus, S. pneumoniae, and P. aeruginosa at different temperatures in the corneal storage medium Optisol™.241 Because the peptides were effective at 4°C, the authors concluded that defensins exhibit promise as preservative agents for corneal storage. Interestingly, in a related study, the bovine antimicrobial peptide BNP-1 helped preserve endothelial cells in corneas stored in Optisol.242 Thus, the peptides, when added to corneal storage media, may have dual benefits.
Two papers report in vivo studies on the ability of topical antimicrobial peptides to prevent ocular surface infections. Nos-Barbera et al showed that rabbits infected by intrastromal injection with P. aeruginosa, then treated with synthetic peptides containing partial sequences from cecropin A (an insect peptide) and melittin (from bee venom) had reduced inflammatory signs and less bacterial damage than vehicle-treated controls.243 Mannis extensively tested the effect of a synthetic peptide named COL-1 (designed using computational drug design software) in a rabbit model of P. aeruginosa keratitis.244 Although the peptide showed potent antimicrobial activity in vitro, no clinical benefit was observed in the in vivo situation. In fact, the author reported that “rabbits treated with the peptide demonstrated more inflammation than controls.” He suggested that a possible reason for the lack of activity in vivo could be that the tear film components are a likely source of interference. As previously mentioned, tears have been shown to reduce peptide effectiveness in vitro;164,165 therefore, understanding how the peptides interact with tear film components is significant for successful development of antimicrobial peptides as topical ocular pharmaceuticals.
As previously noted, synergistic interactions exist between some of the peptides,161–163 so it may be that while one single peptide is not sufficient, two peptides applied together may be beneficial. Synergistic interactions with standard antibiotics have also been noted,245,246 so antimicrobial peptides may be utilized for augmentation of existing pharmaceuticals rather than as stand-alone pharmaceuticals.
Another approach would be to manipulate endogenous expression of the peptides, rather than adding them exogenously.247 This has been termed immunomodulation. For example, hBD-2 expression is known to be inducible, and this peptide is particularly effective against P. aeruginosa.44 Thus, it may be possible to upregulate hBD-2 expression in the corneal epithelium of patients at high risk of developing pseudomonas keratitis.
VII. SUMMARY
Current data show that the corneal and conjunctival epithelia express three β-defensins and LL-37, and the tear film contains α-defensins; thus, the ocular surface is well equipped with these important multifunctional effectors of innate immunity. hBD-1 and -3 are constitutively expressed, whereas hBD-2 and LL-37 are upregulated in response to stimuli such as injury and inflammation. In addition to affording protection by directly killing invading organisms, these peptides probably help regulate the ocular surface immune response by recruiting immune cells to a site of injury or infection and modulating the behavior and activity of these cells once they have arrived. Some of the antimicrobial peptides may also help restore the ocular surface after injury by promoting epithelial cell migration and proliferation. Finally, although very much still in the research and development stage, antimicrobial peptides may ultimately find a place as topical ocular pharmaceuticals and/or additives to eye care solutions and corneal preservation media.
Acknowledgments
The author wishes to thank Kimberly Thompson of the University of Houston, College of Optometry, Audio-Visual Department for drawing Figure 5.
Footnotes
The author has no financial interest in any concept or product discussed in this article.
Single copy reprint requests to: Alison M. McDermott, PhD (address below).
Abbreviations appear in boldface where they first appear with their definitions.
Supported by Texas Higher Education Coordinating Board Advanced Research Program grant and NIH grant EY13175.
References
- 1.Sack RA, Nunes I, Beaton A, Morris C. Host-defense mechanism of the ocular surfaces. Biosci Rep. 2001;21:463–80. doi: 10.1023/a:1017943826684. [DOI] [PubMed] [Google Scholar]
- 2.Dempsey PW, Vaidya SA, Cheng G. The art of war: Innate and adaptive immune responses. Cell Mol Life Sci. 2003;60:2604–21. doi: 10.1007/s00018-003-3180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–59. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Zeya HI, Spitznagel JK. Antibacterial and enzymic basic proteins from leukocyte lysosomes: separation and identification. Science. 1963;142:1085–7. doi: 10.1126/science.142.3595.1085. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Olmedo F, Molina A, Alamillo JM, et al. Plant defense peptides. Biopolymers. 1998;47:479–91. doi: 10.1002/(SICI)1097-0282(1998)47:6<479::AID-BIP6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Steiner H, Hultmark D, Engstrom A, et al. Sequence and specificity of two antibacterial peptides involved in insect immunity. Nature. 1981;292:246–8. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 7.Hultmark D, Steiner H, Rasmuson T, Boman HG. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980;106:7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- 8.Selsted ME, Brown DM, DeLange RJ, et al. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J Biol Chem. 1983;258:14485–9. [PubMed] [Google Scholar]
- 9.Ganz T, Selsted ME, Szklarek D, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–35. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selsted ME, Harwig SS, Ganz T, et al. Primary structures of three human neutrophil defensins. J Clin Invest. 1985;76:1436–9. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res. 2000;1:141–50. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van’t Hof W, Veerman ECI, Helmerhorst EJ, Amerongen AVN. Antimicrobial peptides: properties and applicability. Biol Chem. 2001;382:597–619. doi: 10.1515/BC.2001.072. [DOI] [PubMed] [Google Scholar]
- 13.Boman HG. Antimicrobial peptides: basic facts and emerging concepts. J Intern Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 14.Scott MG, Hancock REW. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit Revs Immunol. 2000;20:407–31. [PubMed] [Google Scholar]
- 15.Yang D, Chertov O, Oppenheim JJ. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell Mol Life Sci. 2001;58:978–89. doi: 10.1007/PL00000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just antimicrobicidal. Trends Immunol. 2002;23:291–6. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 17.Bals R, Wilson JM. Cathelicidins-a family of multifunctional antimicrobial peptides. Cell Mol Life Sci. 2003;60:711–20. doi: 10.1007/s00018-003-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang D, Biragyn A, Hoover DM, et al. Multiple roles of antimicrobial defensins, cathelicidins and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 19.Hancock REW, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotech. 1998;16:82–8. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 20.Otvos L. Antibacterial peptides isolated from insects. J Peptide Sci. 2000;6:497–511. doi: 10.1002/1099-1387(200010)6:10<497::AID-PSC277>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 21.Thomma BP, Cammue BP, Thevissen K. Plant defensins. Planta. 2002;216:193–202. doi: 10.1007/s00425-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 22.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 23.Brogden KA, Ackermann M, McCray PB, Tack BF. Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents. 2003;22:465–78. doi: 10.1016/s0924-8579(03)00180-8. [DOI] [PubMed] [Google Scholar]
- 24.Ganz T. Defensins: Antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 25.Tang YQ, Yuan J, Osapay G, et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 26.Leonova L, Kokryakov VN, Aleshina G, et al. Circular minidefensins and posttranslational generation of molecular diversity. J Leukoc Biol. 2001;70:461–4. [PubMed] [Google Scholar]
- 27.Tran D, Tran PA, Tang YQ, et al. Homomeric theta-defensins from Rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J Biol Chem. 2002;277:3079–84. doi: 10.1074/jbc.M109117200. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TX, Cole AM, Lehrer RI. Evolution of primate theta-defensins: a serpentine path to a sweet tooth. Peptides. 2003;24:1647–54. doi: 10.1016/j.peptides.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Wilde CG, Griffith JE, Marra MN, et al. Purification and characterization of human neutrophil peptide 4, a novel member of the defensin family. J Biol Chem. 1989;264:11200–3. [PubMed] [Google Scholar]
- 30.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–93. [PubMed] [Google Scholar]
- 31.Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial gene. J Biol Chem. 1992;267:23216–25. [PubMed] [Google Scholar]
- 32.Jones DE, Bevins CL. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of human bowel. FEBS Lett. 1993;315:187–92. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- 33.Quayle AJ, Porter EM, Nussbaum AA, et al. Gene expression, immunolocalization and secretion of human defensin-5 in female reproductive tract. Am J Pathol. 152:1247–58. [PMC free article] [PubMed] [Google Scholar]
- 34.Daher KA, Lehrer RI, Ganz T, Kronenberg M. Isolation and characterisation of human cDNA clones. Proc Natl Acad Sci USA. 1988;85:7327–31. doi: 10.1073/pnas.85.19.7327. Erratum in Proc Natl Acad Sci USA 1989;86:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valore EV, Ganz T. Posttranslational processing of defensins in immature human myeloid cells. Blood. 1992;79:1538–44. [PubMed] [Google Scholar]
- 36.Yount NY, Wang MS, Yuan J, et al. Rat neutrophil defensins. Precursor structures and expression during neutrophilic myelopoiesis. J Immunol. 1995;155:4476–84. [PubMed] [Google Scholar]
- 37.Arnljots K, Sorensen O, Lollike K, Borregaard N. Timing, targeting and sorting of azurophil granule proteins in human myeloid cells. Leukemia. 1998;12:1789–95. doi: 10.1038/sj.leu.2401202. [DOI] [PubMed] [Google Scholar]
- 38.Duits LA, Ravensbergen B, Rademaker M, et al. Expression of β-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunol. 2002;106:517–25. doi: 10.1046/j.1365-2567.2002.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bensch KW, Raida M, Magert HJ, et al. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–5. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 40.McCray PB, Bentley L. Human airway epithelia express a β-defensin. Am J Resp Cell Mol Biol. 1997;16:343–9. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- 41.Valore EV, Park CH, Quayle AJ, et al. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–42. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulsen FP, Pufe T, Schaudig U, et al. Detection of natural peptide antibiotics in human nasolacrimal ducts. Invest Ophthalmol Vis Sci. 2001;42:2157–63. [PubMed] [Google Scholar]
- 43.Jia HP, Starner T, Ackermann M, et al. Abundant human β-defensin-1 expression in milk and mammary gland epithelium. J Pediatr. 2001;138:109–12. doi: 10.1067/mpd.2001.109375. [DOI] [PubMed] [Google Scholar]
- 44.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 45.Harder J, Bartels J, Christophers E, et al. Isolation and characterization of human beta-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 46.Garcia JR, Jaumann F, Schulz S, et al. Identification of a novel, multifunctional beta-defensin (human beta-defensin 3) with specific antimicrobial activity. Its interaction with plasma membranes of Xenopus oocytes and the induction of macrophage chemoattraction. Cell Tissue Res. 2001;306:257–64. doi: 10.1007/s004410100433. [DOI] [PubMed] [Google Scholar]
- 47.Garcia JR, Krause A, Schulz S, et al. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819–21. [PubMed] [Google Scholar]
- 48.Yamaguchi Y, Nagase T, Makita R, et al. Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J Immunol. 2002;169:2516–23. doi: 10.4049/jimmunol.169.5.2516. [DOI] [PubMed] [Google Scholar]
- 49.Schutte BC, Mitros JP, Bartlett JA, et al. Discovery of five conserved beta-defensin gene clusters using a computational search strategy. Proc Natl Acad Sci USA. 2002;99:2129–33. doi: 10.1073/pnas.042692699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Jimenez FJ, Krause A, Schulz S, et al. Distribution of new human beta-defensin genes clustered on chromosome 20 in functionally different segments of epididymis. Genomics. 2003;81:175–83. doi: 10.1016/s0888-7543(02)00034-4. [DOI] [PubMed] [Google Scholar]
- 51.Premratanachai P, Joly S, Johnson GK, et al. Expression and regulation of novel human beta-defensins in gingival keratinocytes. Oral Microbiol Immunol. 2004;19:111–17. doi: 10.1111/j.0902-0055.2002.00127.x. [DOI] [PubMed] [Google Scholar]
- 52.Kao CY, Chen Y, Zhao YH, Wu R. ORFeome-based search of airway epithelial cell-specific novel human beta-defensin genes. Am J Respir Cell Mol Biol. 2003;29:71–80. doi: 10.1165/rcmb.2002-0205OC. [DOI] [PubMed] [Google Scholar]
- 53.Gottsch JD, Li Q, Ashraf MF, et al. Defensin gene expression in the cornea. Curr Eye Res. 1998;17:1082–6. doi: 10.1076/ceyr.17.11.1082.5235. [DOI] [PubMed] [Google Scholar]
- 54.Hattenbach LO, Gumbel H, Kippenberger S. Identification of beta-defensins in human conjunctiva. Antimicrob Agents Chemother. 1998;42:3332. doi: 10.1128/aac.42.12.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haynes RJ, Tighe PJ, Dua HS. Innate defence of the eye by antimicrobial defensin peptides. Lancet. 1998;352:451–2. doi: 10.1016/s0140-6736(05)79185-6. [DOI] [PubMed] [Google Scholar]
- 56.Haynes RJ, Tighe PJ, Dua HS. Antimicrobial defensin peptides of the human ocular surface. Br J Ophthalmol. 1999;83:737–41. doi: 10.1136/bjo.83.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou L, Huang LQ, Beuerman RW, et al. Proteomic analysis of human tears: defensin expression after ocular surface surgery. J Proteome Res. 2004;3:410–16. doi: 10.1021/pr034065n. [DOI] [PubMed] [Google Scholar]
- 58.McNamara NA, Van R, Tuchin OS, et al. Ocular surface epithelia express mRNA for human beta defensin-2. Exp Eye Res. 1999;69:483–90. doi: 10.1006/exer.1999.0722. [DOI] [PubMed] [Google Scholar]
- 59.Lehmann OJ, Hussain IR, Watt PJ. Investigation of beta-defensin gene expression in the ocular anterior segment by semiquantitative RT-PCR. Br J Ophthalmol. 2000;84:523–6. doi: 10.1136/bjo.84.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McDermott AM, Redfern RL, Zhang B, et al. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–65. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narayanan S, Miller WL, McDermott AM. Expression of human beta-defensins in conjunctival epithelium: relevance to dry eye disease. Invest Ophthalmol Vis Sci. 2003;44:3795–801. doi: 10.1167/iovs.02-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McNamara NA, Evans DJ, Van R, Fleiszig SM. Upregulation of human beta defensin-2 mRNA by Pseudomonas aeruginosa requires activation of p38MAPK (abstract) Invest Ophthalmol Vis Sci. 1999;40(suppl):2068. [Google Scholar]
- 63.Maltseva I, McNamara N, Fleiszig SMJ, Basbaum C. NFkappaB is involved in Pseudomonas aeruginosa-mediated transcriptional regulation of the human beta-defensin 2 gene in human corneal epithelial cells (abstract). ARVO e-abstract #3195, 2002 (www.arvo.org).
- 64.O’Neil DA, Porter EM, Elewaut D, et al. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–24. [PubMed] [Google Scholar]
- 65.Wada A, Ogushi K, Kimura T, et al. Helicobacter pylori-mediated transcriptional regulation of the human beta-defensin 2 gene requires NF-kappaB. Cell Microbiol. 2001;3:115–23. doi: 10.1046/j.1462-5822.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 66.Ogushi K, Wada A, Niidome T, et al. Salmonella enteritidis FliC (flagella filament protein) induces human beta-defensin-2 mRNA production by Caco-2 cells. J Biol Chem. 2001;276:30521–6. doi: 10.1074/jbc.M011618200. [DOI] [PubMed] [Google Scholar]
- 67.Krisanaprakornkit S, Kimball JR, Dale BA. Regulation of human beta-defensin-2 in gingival epithelial cells: The involvement of mitogen-activated protein kinase pathways, but not the NF-kappaB transcription factor family. J Immunol. 2002;168:316–24. doi: 10.4049/jimmunol.168.1.316. [DOI] [PubMed] [Google Scholar]
- 68.Tsutsumi-Ishii Y, Nagaoka I. NF-kappa B-mediated transcriptional regulation of human beta-defensin-2 gene following lipopolysaccharide stimulation. J Leukoc Biol. 2002;71:154–62. [PubMed] [Google Scholar]
- 69.Birchler T, Seibl R, Buchner K, et al. Human Toll-like receptor 2 mediates induction of the antimicrobial peptide human beta-defensin 2 in response to bacterial lipoprotein. Eur J Immunol. 2001;31:3131–37. doi: 10.1002/1521-4141(200111)31:11<3131::aid-immu3131>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Zhang Z, Louboutin JP, et al. Airway epithelia regulate expression of human beta-defensin-2 through Toll-like receptor 2. FASEB J. 2003;17:1727–9. doi: 10.1096/fj.02-0616fje. [DOI] [PubMed] [Google Scholar]
- 71.McDermott AM, Redfern RL, Zhang B. Human beta-defensin 2 is up-regulated during re-epithelialization of the cornea. Curr Eye Res. 2001;22:64–7. doi: 10.1076/ceyr.22.1.64.6978. [DOI] [PubMed] [Google Scholar]
- 72.Nomura I, Goleva E, Howell MD, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–9. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 73.Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocular Surface. 2004;2:124–30. doi: 10.1016/s1542-0124(12)70148-9. [DOI] [PubMed] [Google Scholar]
- 74.Kawasaki S, Kawamoto S, Yokoi N, et al. Up-regulated gene expression in the conjunctival epithelium of patients with Sjogren’s syndrome. Exp Eye Res. 2003;77:17–26. doi: 10.1016/s0014-4835(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 75.Pflugfelder SC, Jones D, Ji Z, et al. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–11. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 76.Solomon A, Dursun D, Liu Z, et al. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–92. [PubMed] [Google Scholar]
- 77.Stolzenberg ED, Anderson GM, Ackermann ME, et al. Epithelial antibiotic induced in states of disease. Proc Natl Acad Sci USA. 1997;94:8686–90. doi: 10.1073/pnas.94.16.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schonwetter BS, Stolzenberg ED, Zasloff MA. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–8. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 79.Diamond G, Zasloff M, Eck H, et al. Tracheal antimicrobial peptide, a novel cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–6. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tran NK, Huang L, Redfern RL, McDermott AM. Antimicrobial beta-defensins are expressed by bovine ocular surface cells and are upregulated during corneal epithelial regeneration (abstract) Invest Ophthalmol Vis Sci. 2001;42(suppl):4763. [Google Scholar]
- 81.Jia HP, Mills JN, Barahmand-Pour F, et al. Molecular cloning and characterization of rat genes encoding homologues of human beta-defensins. Infect Immun. 1999;67:4827–33. doi: 10.1128/iai.67.9.4827-4833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McDermott AM, Proske RJ, Woo HM, et al. Rat beta-defensin-2 is upregulated during corneal re-epithelialisation in vivo (abstract). ARVO e-abstract #4198, 2002 (www.arvo.org)
- 83.Nizet V, Gallo RL. Cathelicidins and innate defense against invasive bacterial infection. Scand J Infect Dis. 2003;35:670–6. doi: 10.1080/00365540310015629. [DOI] [PubMed] [Google Scholar]
- 84.Agerberth B, Gunne H, Odeberg J, et al. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–9. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cowland JB, Johnsen AH, Borregaard N. hCAP18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–6. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 86.Larrick JW, Hirata M, Balint RF, et al. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–7. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sorensen O, Arnljots K, Cowland JB, et al. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–803. [PubMed] [Google Scholar]
- 88.DiNardo A, Vitiello A, Gallo RL. Mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–8. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 89.Frohm MB, Agerberth B, Ahangari G, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 90.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP18 is expressed in the epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–6. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Frohm-Nilsson M, Sandstedt B, Sorensen O, et al. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–6. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sorensen OE, Follin P, Johnsen AH, et al. Human cathelicidin, hCAP18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–9. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 93.Niyonsaba F, Hirata M, Ogawa H, Nagaoka I. Epithelial cell-derived antibacterial peptides human beta-defensins and cathelicidin: multifunctional activities on mast cells. Curr Drug Targets Inflamm Allergy. 2003;2:224–31. doi: 10.2174/1568010033484115. [DOI] [PubMed] [Google Scholar]
- 94.Huang LH, Proske RJ, McDermott AM. Expression of the peptide antibiotic LL-37/hCAP 18 (cathelicidin) by human corneal epithelial cells (abstract). ARVO e-abstract #1335, 2003 (www.arvo.org)
- 95.Huang LC, Proske RJ, McDermott AM. Functional roles of the epithelial-derived antimicrobial peptide LL-37 at the ocular surface (abstract). ARVO e-abstract #4940, 2004 (www.arvo.org)
- 96.Shafer WM, Martin LE, Spitznagel JK. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphates. Infect Immun. 1984;45:29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruan X, Chodosh J, Callegan MC, et al. Corneal expression of the inflammatory mediator CAP37. Invest Ophthalmol Vis Sci. 2002;43:1414–21. [PubMed] [Google Scholar]
- 98.Watorek W. Azurocidin - inactive serine proteinase homolog acting as a multifunctional inflammatory mediator. Acta Biochim Pol. 2003;50:743–52. [PubMed] [Google Scholar]
- 99.Ruan X, Chodosh J, Lerner M, et al. CAP37, a multifunctional inflammatory mediator promotes corneal epithelial cell proliferation and migration (abstract) Invest Ophthalmol Vis Sci. 2001;42(suppl):4759. [Google Scholar]
- 100.Pereira H, Gonzalez ML, Ruan X, et al. Differential gene expression in human corneal epithelial cells (HCEC) in response to the inflammatory mediator, CAP37 (abstract). ARVO e-abstract #836, 2003 (www.arvo.org)
- 101.Huff T, Muller CSG, Otto AM, et al. beta-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–20. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 102.Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70:6524–33. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sosne G, Chan CC, Thai K, et al. Thymosin beta 4 promotes corneal wound healing and modulates inflammatory mediators in vivo. Exp Eye Res. 2001;72:605–8. doi: 10.1006/exer.2000.0985. [DOI] [PubMed] [Google Scholar]
- 104.Sosne G, Szliter EA, Barrett R, et al. Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp Eye Res. 2002;74:293–9. doi: 10.1006/exer.2001.1125. [DOI] [PubMed] [Google Scholar]
- 105.Sosne G, Hafeez S, Greenberry AL, 2nd, Kurpakus-Wheater M. Thymosin beta 4 promotes human conjunctival epithelial cell migration. Curr Eye Res. 2002;24:268–73. doi: 10.1076/ceyr.24.4.268.8414. [DOI] [PubMed] [Google Scholar]
- 106.Katz B, Wadhwa SD, Badamchian M, et al. Identification and quantification of thymosin β4 in human tears (abstract). ARVO e-abstract #2510, 2003 (www.arvo.org).
- 107.Sosne G, Goldstein AL, Badamchian M, et al. Thymosin beta 4 expression in corneal wound healing (abstract). ARVO e-abstract #1425, 2004 (www.arvo.org)
- 108.Tsai H, Bobek LA. Human salivary histatins: promising anti-fungal therapeutic agents. Crit Rev Oral Biol Med. 1998;9:480–97. doi: 10.1177/10454411980090040601. [DOI] [PubMed] [Google Scholar]
- 109.Krause A, Liepke C, Meyer M, et al. Human natriuretic peptides exhibit antimicrobial activity. Eur J Med Res. 2001;6:215–8. [PubMed] [Google Scholar]
- 110.Schittek B, Hipfel R, Sauer B, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–7. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 111.Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulphide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–50. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 112.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–10. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 113.Krause A, Sillard R, Kleemeier B, et al. Isolation and biochemical characterization of LEAP-2, a novel blood peptide expressed in the liver. Protein Sci. 2003;12:143–52. doi: 10.1110/ps.0213603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henning von Horsten H, Derr P, Kirchhoff C. Novel antimicrobial peptide of human epididymal duct origin. Biol Reprod. 2002;67:804–13. doi: 10.1095/biolreprod.102.004564. [DOI] [PubMed] [Google Scholar]
- 115.Cole AM, Ganz T, Liese AM, et al. Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–7. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 116.Hieshima K, Ohtani H, Shibano M, et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol. 2003;170:1452–61. doi: 10.4049/jimmunol.170.3.1452. [DOI] [PubMed] [Google Scholar]
- 117.Yang D, Chen Q, Hoover DM, et al. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–55. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 118.Starner TD, Barker CK, Jia HP, et al. CCL20 is an inducible product of human airway epithelia with innate immune properties. Am J Respir Cell Mol Biol. 2003;29:627–33. doi: 10.1165/rcmb.2002-0272OC. [DOI] [PubMed] [Google Scholar]
- 119.Steele PS, Jumblatt MM. Defense proteins of the ocular surface (abstract). ARVO e-abstract #3792, 2004 (www.arvo.org)
- 120.Dua HS, Al-Abed M, Cade J, et al. The spectrum of expression of anti-microbial peptides at the ocular surface (abstract). ARVO e-abstract #4933, 2004 (www.arvo.org)
- 121.Steele PS, Jumblatt MM, Smith NB, Pierce WM. Detection of histatin 5 in normal human Schirmer strip samples by mass spectroscopy (abstract). ARVO e-abstract #98, 2002 (www.arvo.org)
- 122.Mahajan VB, Wei C, McDonnell PR., 3rd Microarray analysis of corneal fibroblast gene expression after interleukin-1 treatment. Invest Ophthalmol Vis Sci. 2002;43:2143–51. [PubMed] [Google Scholar]
- 123.Hancock RE. Peptide antibiotics. Lancet. 1997;349:418–22. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 124.Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochem Biophys Acta. 1999;1462:1–10. doi: 10.1016/s0005-2736(99)00197-2. [DOI] [PubMed] [Google Scholar]
- 125.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–48. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 126.Wimley WC, Selsted ME, White SH. Interactions between human defensins and lipid bilayers: evidence for formation of multimeric pores. Protein Sci. 1994;3:1362–73. doi: 10.1002/pro.5560030902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hoover DM, Chertov O, Lubkowski J. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J Biol Chem. 2000;275:32911–8. doi: 10.1074/jbc.M006098200. [DOI] [PubMed] [Google Scholar]
- 128.Oren Z, Lerman JC, Gudmundsson GH, et al. Structure and organization of the human antimicrobial peptide LL-37 in phospholipids membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J. 1999;341:501–13. [PMC free article] [PubMed] [Google Scholar]
- 129.Helmerhorst EJ, Breeuwer P, Van t’ Hof W, et al. The cellular target for histatin 5 on candida albicans is the energized mitochondrion. J Biol Chem. 1999;274:7286–91. doi: 10.1074/jbc.274.11.7286. [DOI] [PubMed] [Google Scholar]
- 130.Lehrer RI, Daher K, Ganz T, Selsted ME. Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J Virol. 1985;54:467–72. doi: 10.1128/jvi.54.2.467-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daher KA, Selsted ME, Lehrer RI. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986;60:1068–74. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gropp R, Frye M, Wagner TOF, Bargon J. Epithelial defensins impair adenoviral infection: implication for adenovirus-mediated gene transfer. Human Gene Ther. 1999;10:957–64. doi: 10.1089/10430349950018355. [DOI] [PubMed] [Google Scholar]
- 133.Bastian A, Schafer Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul Pept. 2001;101:157–61. doi: 10.1016/s0167-0115(01)00282-8. [DOI] [PubMed] [Google Scholar]
- 134.Nakashima H, Yamamoto N, Masuda M, Fujii N. Defensins inhibit HIV replication in vitro. AIDS. 1993;7:1129. doi: 10.1097/00002030-199308000-00019. [DOI] [PubMed] [Google Scholar]
- 135.Sinha S, Cheshenko N, Lehrer RI, Herold BC. NP-1, a rabbit alpha-defensin, prevents entry and intercellular spread of herpes simplex virus type 2. Antimicrob Agents Chemother. 2003;47:494–500. doi: 10.1128/AAC.47.2.494-500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Quinones-Mateu ME, Lederman MM, Feng Z, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 137.Wang W, Owen SM, Rudolph DL, et al. Activity of alpha-and theta-defensins against primary isolates of HIV-1. J Immunol. 2004;173:515–20. doi: 10.4049/jimmunol.173.1.515. [DOI] [PubMed] [Google Scholar]
- 138.Yasin B, Wang W, Pang M, et al. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J Virol. 2004;78:5147–56. doi: 10.1128/JVI.78.10.5147-5156.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ganz T, Lehrer RI. Defensins. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 140.Schroder JM. Epithelial peptide antibiotics. Biochem Pharmacol. 1999;57:121–34. doi: 10.1016/s0006-2952(98)00226-3. [DOI] [PubMed] [Google Scholar]
- 141.Shiomi K, Nakazato M, Ihi T, et al. Establishment of radioimmunoassay for human neutrophil peptides and their increases in plasma and neutrophil in infection. Biochem Biophys Res Commun. 1993;195:1336–44. doi: 10.1006/bbrc.1993.2190. [DOI] [PubMed] [Google Scholar]
- 142.Panyutich AV, Panyutich E, Krapivin VA, et al. Plasma defensin concentrations are elevated in patients with scepticemia or bacterial meningitis. J Lab Clin Med. 1993;122:202–7. [PubMed] [Google Scholar]
- 143.Mizukawa N, Sugiyama K, Ueno T, Mishima K, et al. Levels of human defensin-1, an antimicrobial peptide, in saliva of patients with oral inflammation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:539–43. doi: 10.1016/s1079-2104(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 144.Liu AY, Destoumieux D, Wong AV, et al. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the sate of differentiation. J Invest Dermatol. 2002;118:275–81. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 145.Oren A, Ganz T, Liu L, Meerloo T. In human epidermis, beta-defensin-2 is packaged in lamellar bodies. Exp Mol Pathol. 2003;74:180–2. doi: 10.1016/s0014-4800(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 146.Singh PK, Jia HP, Wiles K, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–6. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Malm J, Sorensen O, Persson T, et al. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immun. 2000;68:4297–302. doi: 10.1128/iai.68.7.4297-4302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wilson CL, Ouellette AJ, Satchell DP, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defence. Science. 1999;286:113–17. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 149.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 150.Moser C, Weiner DJ, Lysenko E, et al. beta-Defensin 1 contributes to pulmonary innate immunity in mice. Infect Immun. 2002;70:3068–72. doi: 10.1128/IAI.70.6.3068-3072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morrison G, Kilanowski F, Davidson D, Dorin J. Characterization of the mouse beta-defensin 1, Defb1, mutant mouse model. Infect Immun. 2002;70:3053–60. doi: 10.1128/IAI.70.6.3053-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Salzman NH, Ghosh D, Huttner KM, et al. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–6. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 153.Putsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observational study. Lancet. 2002;360:1144–9. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 154.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequency of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–53. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cullor JS, Mannis MJ, Murphy CJ, et al. In vitro antimicrobial activity of defensins against ocular pathogens. Arch Ophthalmol. 1990;108:861–4. doi: 10.1001/archopht.1990.01070080105044. [DOI] [PubMed] [Google Scholar]
- 156.Schuster FL, Jacob LS. Effects of magainins on ameba and cyst stages of Acanthamoeba polyphaga. Antimicrob Agents Chemother. 1992;36:1263–71. doi: 10.1128/aac.36.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gunshefski L, Mannis MJ, Cullor JS, et al. In vitro antimicrobial activity of Shiva-11 against ocular pathogens. Cornea. 1994;13:237–42. doi: 10.1097/00003226-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 158.Aliwarga Y, Hume EB, Lan J, Willcox MD, et al. Antimicrobial peptides: a potential role in ocular therapy. Clin Experiment Ophthalmol. 2001;29:157–60. doi: 10.1046/j.1442-9071.2001.00406.x. [DOI] [PubMed] [Google Scholar]
- 159.Gordon Y, Romanowski EG, Yates KA, McDermott AM. Human cathelicidin (LL-37/hCAP-18) demonstrates direct antiviral activity against adenovirus and herpes simplex virus in vitro (abstract). ARVO 3-abstract #2256, 2004 (www.arvo.org)
- 160.Romanowski EG, Yates KA, Gordon YJ. Human conjunctival defensins demonstrate direct antiviral activity against adenovirus in vitro (abstract). ARVO e-abstract #4648, 2003 (www.arvo.org)
- 161.Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 162.Yan H, Hancock RE. Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob Agents Chemother. 2001;45:1558–60. doi: 10.1128/AAC.45.5.1558-1560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 164.Rich D, Cullor J, Mannis MJ, et al. The in vitro activity of defensins against pseudomonas in the presence of human tears (abstract) Invest Ophthalmol Vis Sci. 1990;31(suppl):2203. [Google Scholar]
- 165.Huang L, Narayanan S, Redfern RL, McDermott AM. Human tears inhibit the antimicrobial activity of human beta-defensin-2 in vitro (abstract). ARVO e-abstract #82, 2002 (www.arvo.org).
- 166.Van Haeringen NJ. Clinical biochemistry of tears. Surv Ophthalmol. 1981;26:84–96. doi: 10.1016/0039-6257(81)90145-4. [DOI] [PubMed] [Google Scholar]
- 167.Schibli DJ, Hunter HN, Aseyev V, et al. The solution structures of human beta-defensins lead to a better understanding of the potent antibacterial activity of hBD-3 against Staphylococcus aureus. J Biol Chem. 2002;277:8279–89. doi: 10.1074/jbc.M108830200. [DOI] [PubMed] [Google Scholar]
- 168.Nagaoka I, Hirota S, Yomogida S, et al. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res. 2000;49:73–9. doi: 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- 169.Lichtenstein A, Ganz T, Selsted ME, Lehrer RI. In vitro tumour cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood. 1986;68:1407–10. [PubMed] [Google Scholar]
- 170.Okrent DG, Lichtenstein AK, Ganz T. Direct cytotoxicity of polymorphonuclear leukocyte granule proteins to human lung-derived cells and endothelial cells. Am Rev Respir Dis. 1990;141:179–85. doi: 10.1164/ajrccm/141.1.179. [DOI] [PubMed] [Google Scholar]
- 171.Van Wetering S, Mannesse-Lazermos SP, Dijkman JH, Hiemstra PS. Effect of neutrophil serine proteinases and defensins on lung epithelial cells: modulation of cytotoxicity and IL-8 production. J Leukoc Biol. 1997;62:217–26. doi: 10.1002/jlb.62.2.217. [DOI] [PubMed] [Google Scholar]
- 172.Spencer LT, Paone G, Krein PM, et al. Role of human neutrophil peptides in lung inflammation associated with alpha1-antitrypsin deficiency. Am J Physiol Lung Cell Mol Physiol. 2004;286:L514–20. doi: 10.1152/ajplung.00099.2003. [DOI] [PubMed] [Google Scholar]
- 173.Zucht HD, Grabowsky J, Schrader M, et al. Human beta-defensin-1: A urinary peptide present in variant molecular forms and its putative functional implication. Eur J Med Res. 1998;3:315–23. [PubMed] [Google Scholar]
- 174.Johansson J, Gudmundsson GH, Rottenberg ME, et al. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718–24. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 175.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Ann Rev Immunol. 1993;11:105–28. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 176.Fung K, Morris C, Duncan M. Mass spectrometric techniques applied to the analysis of human tears: A focus on the peptide and protein constituents. Adv Exp Med Biol. 2002;506(Part A):601–5. doi: 10.1007/978-1-4615-0717-8_84. [DOI] [PubMed] [Google Scholar]
- 177.Panyutich A, Ganz T. Activated alpha-2 macroglobulin is a principal defensin-binding protein. Am J Respir Cell Mol Biol. 1991;5:101–6. doi: 10.1165/ajrcmb/5.2.101. [DOI] [PubMed] [Google Scholar]
- 178.Panyutich AV, Hiemstra PS, van Wetering S, Ganz T. Human neutrophil defensins and serpins form complexes and inactivate each other. Am J Respir Cell Mol Biol. 1995;12:351–7. doi: 10.1165/ajrcmb.12.3.7873202. [DOI] [PubMed] [Google Scholar]
- 179.Sathe S, Sakata M, Beaton AR, Sack RA. Identification, origins and the diurnal role of the principal serine protease inhibitors in human tear fluid. Curr Eye Res. 1998;17:348–62. doi: 10.1080/02713689808951215. [DOI] [PubMed] [Google Scholar]
- 180.Cervini LA, Gray WR, Kaiser R, et al. Rat corticostatin R4: synthesis, disulphide bridge assignment, and in vivo activity. Peptides. 1995;16:837–42. doi: 10.1016/0196-9781(95)00040-q. [DOI] [PubMed] [Google Scholar]
- 181.Nassar T, Akkawi S, Bar-Shavit R, et al. Human alpha-defensin regulates smooth muscle cell contraction: a role for low-density lipoprotein receptor-related protein/alpha2-macroglobulin receptor. Blood. 2002;100:4026–32. doi: 10.1182/blood-2002-04-1080. [DOI] [PubMed] [Google Scholar]
- 182.Koczulla R, von Dengenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–20. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chertov O, Michiel DF, Xu L, et al. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935–40. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 185.Yang D, Chertov O, Bykovskaia SN, et al. beta-Defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 186.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000;68:9–14. [PubMed] [Google Scholar]
- 187.Niyonsaba F, Iwabuchi K, Matsuda H, et al. Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol. 2002;14:421–6. doi: 10.1093/intimm/14.4.421. [DOI] [PubMed] [Google Scholar]
- 188.Niyonsaba F, Ogawa H, Nagaoka I. Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology. 2004;111:273–81. doi: 10.1111/j.0019-2805.2004.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Misuno NI, Kolesnikova TS, Lerer RI, et al. [Effects of human defensin HNP-1 on the production of tumour necrosis factor-alpha by human blood monocytes in vitro] Biull Eksp Biol Med. 1992;113:524–7. [PubMed] [Google Scholar]
- 190.Chaly YV, Paleolog EM, Kolesnikova TS, et al. Neutrophil alpha-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–66. [PubMed] [Google Scholar]
- 191.van Wetering S, Mannesse-Lazeroms SP, Van Sterkenburg MA, et al. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol. 1997;272:L888–96. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- 192.Befus AD, Mowat C, Gilchrist M, et al. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J Immunol. 1999;163:947–53. [PubMed] [Google Scholar]
- 193.Niyonsaba F, Someya A, Hirata M, et al. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur J Immunol. 2001;31:1066–75. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 194.Biragyn A, Ruffini PA, Leifer CA, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 195.De Yang, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule-and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Niyonsaba F, Iwabuchi K, Someya A, et al. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–6. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Scott MG, Davidson DJ, Gold MR, et al. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–91. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 198.Davidson DJ, Currie AJ, Reid GS, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172:1146–56. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 199.Tjabringa GS, Aarbiou J, Ninaber DK, et al. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 200.Elssner A, Duncan M, Gavrillin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1β processing and release. J Immunol. 2004;172:4987–94. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 201.Yomogida S, Nagaoka K, Saito K, Yamashita T. Evaluation of the effects of defensins on neutrophil functions. Inflamm Res. 1996;45:62–7. doi: 10.1007/BF02265117. [DOI] [PubMed] [Google Scholar]
- 202.Prohaszka Z, Nemet K, Csermely P, et al. Defensins purified from human granulocytes bind C1q and activate the classical complement pathway like the transmembrane glycoprotein gp41 of HIV. Mol Immunol. 1997;34:809–16. doi: 10.1016/s0161-5890(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 203.Van den Berg RH, Faber-Krol MC, van Wetering S, et al. Inhibition of activation of classical pathway of complement by human neutrophil defensins. Blood. 92:3898–903. [PubMed] [Google Scholar]
- 204.Larrick JW, Hirata M, Zheng H, et al. A novel granulocyte-derived peptide with lipopolysaccharide-neutralizing activity. J Immunol. 1994;152:231–40. [PubMed] [Google Scholar]
- 205.Lillard JW, Jr, Boyaka PN, Chertov O, et al. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA. 1999;96:651–6. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhu QZ, Singh AV, Bateman A, et al. The corticostatic (anti-ACTH) and cytotoxic activity of peptides isolated from fetal, adult and tumour-bearing lung. J Steroid Biochem. 1987;27:1017–22. doi: 10.1016/0022-4731(87)90184-1. [DOI] [PubMed] [Google Scholar]
- 207.Kudryashov BA, Kondashevskaya MV, Lyapina LA, et al. [Action of defensin on healing of aseptic skin wounds and on vascular permeability] Biull Eksp Biol Med. 1990;109:391–3. [PubMed] [Google Scholar]
- 208.Bateman A, Singh A, Congote LF, Solomon S. The effect of HP-1 and related neutrophil granule peptides on DNA synthesis in HL60 cells. Regul Pept. 1991;35:135–43. doi: 10.1016/0167-0115(91)90476-w. [DOI] [PubMed] [Google Scholar]
- 209.Murphy CJ, Foster BA, Mannis MJ, et al. Defensins are mitogenic for epithelial cells and fibroblasts. J Cell Physiol. 1993;155:408–13. doi: 10.1002/jcp.1041550223. [DOI] [PubMed] [Google Scholar]
- 210.Aarbiou J, Ertmann M, van Wetering S, et al. Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J Leukoc Biol. 2002;72:167–74. [PubMed] [Google Scholar]
- 211.Muller CA, Markovic-Lipkovski J, Klatt T, et al. Human alpha-defensins HNPs-1, -2,and -3 in renal cell carcinoma: influences on tumor cell proliferation. Am J Pathol. 2002;160:1311–24. doi: 10.1016/s0002-9440(10)62558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Aarbiou J, Verhoosel RM, van Wetering S, et al. Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am J Respir Cell Mol Biol. 2004;30:193–201. doi: 10.1165/rcmb.2002-0267OC. [DOI] [PubMed] [Google Scholar]
- 213.Oono T, Shirafuji Y, Huh WK, et al. Effects of human neutrophil peptide-1 on the expression of interstitial collagenase and type I collagen in human dermal fibroblasts. Arch Dermatol Res. 2002;294:185–9. doi: 10.1007/s00403-002-0310-6. [DOI] [PubMed] [Google Scholar]
- 214.Frye M, Bargon J, Gropp R. Expression of human β-defensin-1 promotes differentiation of keratinocytes. J Mol Med. 2001;79:275–82. doi: 10.1007/s001090100200. [DOI] [PubMed] [Google Scholar]
- 215.Heilborn JD, Nilsson MF, Kratz G, et al. The cathelicidin antimicrobial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–89. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 216.Weimar V. Polymorphonuclear invasion of wounded corneas. J Exp Med. 1957;105:141–6. doi: 10.1084/jem.105.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kao WW, Zhu G, Benza R, et al. Appearance of immune cells and expression of MHC II DQ molecules by fibroblasts in alkali-burned corneas. Cornea. 1996;15:397–408. doi: 10.1097/00003226-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 218.O’Brien TP, Li Q, Ashraf MF, et al. Inflammatory response in the early stages of wound healing after excimer laser keratectomy. Arch Ophthalmol. 1998;116:1470–4. doi: 10.1001/archopht.116.11.1470. [DOI] [PubMed] [Google Scholar]
- 219.Gan L, Hamberg-Nystrom H, Fagerholm P, et al. Cellular proliferation and leukocyte infiltration in to the rabbit cornea after photorefractive keratectomy. Acta Ophthalmol Scand. 2001;79:488–92. doi: 10.1034/j.1600-0420.2001.790512.x. [DOI] [PubMed] [Google Scholar]
- 220.Redfern RL, Proske RJ, McDermott AM. Effect of defensins on corneal cell migration and cytokine production (abstract). ARVO e-abstract #4866, 2004 (www.arvo.org)
- 221.Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004;78:173–85. doi: 10.1016/j.exer.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 222.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:581–9. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 223.Ayliffe W, Espaillat A, Foster CS, Lee SJ. Polymerase chain reaction analysis of TNFα gene expression in rat corneal wound healing (abstract) Invest Ophthalmol Vis Sci. 1993;34(suppl):3325. [Google Scholar]
- 224.Planck SR, Rich LF, Ansel JC, et al. Trauma and alkali burns induce distinct patterns of cytokine gene expression in the rat cornea. Ocul Immunol Inflamm. 1997;5:95–100. doi: 10.3109/09273949709085057. [DOI] [PubMed] [Google Scholar]
- 225.Sotozono C, Kinoshita S. Growth factors and cytokines in corneal wound healing, in Nishida T (ed). Corneal healing response to injuries and refractive surgeries. The Hague, Kugler Publications, 1998, pp 29–40
- 226.Gipson IK, Watanabe H, Zieske JD. Corneal wound healing and fibronectin. Int Ophthalmol Clin. 1993;33:149–163. doi: 10.1097/00004397-199303340-00013. [DOI] [PubMed] [Google Scholar]
- 227.Schultz GS. Modulation of corneal wound healing, in Krachmer JH, Mannis MJ, Holland EJ (eds). Cornea, Volume 1. St Louis, Mosby, 1997, pp183–98
- 228.Reid TW. Growth control of the cornea and lens epithelial cells. Prog Retin Eye Res. 1994;13:508–54. [Google Scholar]
- 229.Imanishi J, Kamiyama K, Iguchi I, et al. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19:113–29. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 230.Kurpakus-Wheater M, Kernacki KA, Hazlett LD. Maintaining corneal intergrity. How the window stays clear. Prog Histochem Cytochem. 2001;36:185–259. [PubMed] [Google Scholar]
- 231.Hancock REW, Scott MG. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci USA. 97:8856–61. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ganz T. Fatal attraction evaded: How pathogenic bacteria resist cationic polypeptides. J Exp Med. 2001;193:F31–3. doi: 10.1084/jem.193.9.f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Islam D, Bandholtz L, Nilsson J, et al. Down regulation of bactericidal peptides in enteric infections: A novel immune escape mechanism with bacterial DNA as a potential regulator. Nature Med. 2001;7:180–5. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 234.Lysenko ES, Gould J, Bals R, et al. Bacterial phosphorylcholine decreases susceptibility to the antimicrobial peptide LL-37/hCAP18 expressed in the upper respiratory tract. Infect Immun. 2000;68:1664–71. doi: 10.1128/iai.68.3.1664-1671.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Schmidtchen A, Frick IM, Andersson E, et al. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Molec Microbiol. 2002;46:157–68. doi: 10.1046/j.1365-2958.2002.03146.x. [DOI] [PubMed] [Google Scholar]
- 236.Jin T, Bokarewa M, Foster T, et al. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J Immunol. 2004;172:1169–76. doi: 10.4049/jimmunol.172.2.1169. [DOI] [PubMed] [Google Scholar]
- 237.Moore A. The big and small of drug discovery. EMBO Reports. 2003;2:114–17. doi: 10.1038/sj.embor.embor748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–54. [PubMed] [Google Scholar]
- 239.Willcox M, Cole N, Lan J, et al. Contact lenses coated with protamine reduce bacterial adhesion and the production of contact lens induced acute red eye (CLARE) (abstract). ARVO e-abstract #1568, 2004 (www.arvo.org)
- 240.Sousa LB, Mannis MJ, Schwab IR, et al. The use of synthetic Cecropin (D5C) in disinfecting contact lens solutions. CLAO J. 1996;22:114–17. [PubMed] [Google Scholar]
- 241.Schwab IR, Dries D, Cullor J, et al. Corneal storage medium preservation with defensins. Cornea. 1992;11:370–5. doi: 10.1097/00003226-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 242.Reid TW, Steward K, Johnason M, et al. Studies on the effect of the bovine neutrophil antibiotic dodeca-peptide (BNP-1) on the viability of human corneal endothelial cells stored in optisol (abstract) Invest Ophthalmol Vis Sci. 1998;39(suppl):358. [Google Scholar]
- 243.Nos-Barbera S, Portoles M, Morilla A, et al. Effect of hybrid peptides of cecropin A and melittin in an experimental model of bacterial keratitis. Cornea. 1997;16:101–6. [PubMed] [Google Scholar]
- 244.Mannis M. The use of antimicrobial peptides in ophthalmology: an experimental study in corneal preservation and the management of bacterial keratitis. Trans Am Ophthalmol Soc. 2002;100:243–71. [PMC free article] [PubMed] [Google Scholar]
- 245.Midorikawa K, Ouhara K, Komatsuzawa H, et al. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun. 2003;71:3730–9. doi: 10.1128/IAI.71.7.3730-3739.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Maisetta G, Batoni G, Esin S, et al. Activity of human beta-defensin 3 alone or combined with other antimicrobial agents against oral bacteria. Antimicro Agents Chemother. 2003;47:3349–51. doi: 10.1128/AAC.47.10.3349-3351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Finlay BB, Hancock REW. Can innate immunity be enhanced to treat microbial infections? Nat Rev Microbiol. 2004;2:497–504. doi: 10.1038/nrmicro908. [DOI] [PubMed] [Google Scholar]


