Abstract
Background.
Cotrimoxazole prophylaxis is recommended for subgroups of human immunodeficiency virus (HIV)-infected adults and children to reduce all-cause morbidity and mortality. We investigated whether antenatal cotrimoxazole prophylaxis begun during pregnancy for HIV-infected pregnant women with low CD4 cell counts would affect birth outcomes.
Methods.
Cotrimoxazole prophylaxis was introduced as a routine component of antenatal care for HIV-infected women with CD4 cell counts <200 cells/μL during the course of a trial of mother-to-child HIV transmission in Lusaka, Zambia. Rates of preterm delivery, low birth weight, and neonatal mortality were compared for women with low CD4 cell counts before and after its introduction.
Results.
Among 255 women with CD4 cell counts <200 cells/μL, the percentage of preterm births (≤34 weeks of gestation) was lower (odds ratio [OR], 0.49 [95% confidence interval {CI}, 0.24-0.98]) after cotrimoxazole prophylaxis was introduced than before; there was a significant decrease in neonatal mortality (9% to 0%; P = .01) and a trend toward increased birth weight (β = 114 g [95% CI, -42 to 271 g]). In contrast, there were no significant changes in these parameters over the same time interval among women with CD4 cell counts ≥200 cells/μL.
Conclusion.
Antenatal provision of cotrimoxazole for HIV-infected pregnant women with low CD4 cell counts may have indirect benefits for neonatal health.
Cotrimoxazole (trimethoprim-sulfamethoxazole), originally recommended as prophylaxis against Pneumo-cystis pneumonia, was one of the first effective preventive interventions offered to HIV-infected adults and children in the United States and Europe [1]. Later clinical trials demonstrated significant reductions in allcause morbidity and mortality in African populations, suggesting wider benefits that possibly resulted from activity against various parasitic, fungal, and bacterial infections [2-5]. HIV/AIDS treatment guidelines recommend cotrimoxazole as a component of care for adults (including pregnant women) with low CD4 cell counts [6]. However, to avoid potential teratogenic effects of trimethroprim [7-9], treatment is only recommended after the first trimester of pregnancy [6, 10].
When enrollment into a clinical trial of mother-to-child HIV transmission in Lusaka, Zambia, first began, cotrimoxazole was not a routine component of care for HIV-infected pregnant women. During the course of the trial, guidelines for the local health services were refined to encourage its use for HIV-infected pregnant women. For the final quarter of the study, all women with CD4 cell counts <200 cells/μL were systematically provided cotrimoxazole from the second trimester as part of routine antenatal care. This change offered an unplanned “natural experiment” for the evaluation of the effects of antenatal cotrimoxazole prophylaxis on birth outcomes. We hypothesized that, in addition to the expected benefits for maternal health, there might be spillover benefits for neonatal health. In particular, because HIV-infected women are at high risk for preterm birth and for low birth weight infants [11]—and because antibiotic prophylaxis has been investigated as a means for the reduction of these adverse outcomes (with somewhat mixed results [12])—we investigated whether cotrimoxazole begun during pregnancy would affect birth outcomes among HIV-infected women.
SUBJECTS, MATERIALS, AND METHODS
Study design.
As part of a trial of short, exclusive breast-feeding to reduce mother-to-child HIV transmission and infant mortality [13], 1435 HIV-seropositive pregnant women were recruited at 2 clinics in Lusaka between April 2001 and September 2004. Cotrimoxazole prophylaxis was introduced as part of routine antenatal care beginning in November 2003. At enrollment, all women had CD4 cell counts measured (BD Immunocytometry Systems) and, from the beginning of November 2003, all those with CD4 cell counts <200 cells/μL who were at >14 weeks of gestation began receiving 2 single-strength tablets (400 mg of sulfamethoxazole and 80 mg of trimethoprim) daily. Treatment was delayed until 14 weeks of gestation for those who enrolled during the first trimester. Among all women in the study with CD4 cell counts <200 cells/μL, we compared rates of adverse pregnancy and birth outcomes before and after the introduction of cotrimoxazole prophylaxis.
Study procedures.
Routine antenatal care also included screening for syphilis by rapid plasma reagin tests and treatment of women and their partners with penicillin if found to be positive. All women received 800 mg of folate as part of daily prenatal multivitamin supplementation. Through October 2002, malaria prophylaxis was with monthly chloroquine treatment and thereafter consisted of intermittent presumptive treatment during each trimester with sulfadoxine-pyrimethamine. Antiretroviral treatment became available only in November 2004.
Blood samples were collected at enrollment to measure CD4 cell count, plasma viral load (Roche Amplicor version 1.5), and hemoglobin level (Hemocue system). Women were asked about HIV-related conditions, and World Health Organization clinical stage was assigned on the basis of self-reports. Height and weight were measured, and body mass index was calculated as kilograms per square meter.
Women were monitored via regularly scheduled visits during pregnancy. At delivery, obstetric and newborn details were recorded by study staff, who also collected delivery information from records for off-site deliveries. Women were encouraged to bring their infants to the clinic as soon as possible in the event of a home delivery. Gestational age at birth was calculated by adding the time (in days) between the date of enrollment and the date of delivery to the estimate of gestational age at enrollment, which was based on the reported last menstrual period (LMP). Estimates of gestational age based on clinical examination (fundal height) at enrollment, performed by midwives, were also available. Clinical chorioamnionitis was inferred if 1 or more of the following were noted: temperature during labor >38 °C, fetal tachycardia (>160 beats per minute [bpm]), maternal tachycardia (>110 bpm), foul-smelling fetus, or purulent/foul-smelling amniotic fluid. Home visits were scheduled at 4 days and at 2 and 3 weeks after delivery, and clinic visits were scheduled at 1 and 5 weeks postpartum. All maternal and infant deaths and hospital admissions were investigated by interview with the caregivers and/or health care personnel and by review of hospital records. The study was approved by the institutional review boards of the investigators’ institutions, and all participants provided written, informed consent.
Statistical methods.
A cutoff date of 14 November 2003 was selected as the date after which a pregnancy outcome had to have occurred among women with CD4 cell counts <200 cells/μL for it to be considered cotrimoxazole exposed. This allowed at least 2 weeks for the change in protocol to be implemented. Temporal changes in risk factors among women with low CD4 cell counts and in pregnancy outcomes among women with higher CD4 cell counts were also examined. For univariate analyses, the χ2 test was used to compare categorical variables unless expected cell sizes were <5, in which case Fisher’s exact test was used; the Cochrane-Armitage test for trend was used to compare distributions of ordered categorical data; the t test was used to compare normally distributed continuous variables; and the Wilcoxon rank sum test was used to compare nonnormal continuous variables. To adjust for possible confounding, multiple logistic regression with preterm birth as the outcome and multiple linear regression with birth weight as the outcome were performed. Variables that were significant in univariate analysis (P < .10) were included and were retained if significant (P < .05) or if their inclusion substantially changed estimates of the parameter describing the effect of cotrimoxazole. Missing values for hemoglobin level (n = 6) in the multiple regression models were imputed using the CD4 cell count stratum-specific mean. All statistical analyses were performed using SAS software (version 9.1; SAS).
RESULTS
Study cohort.
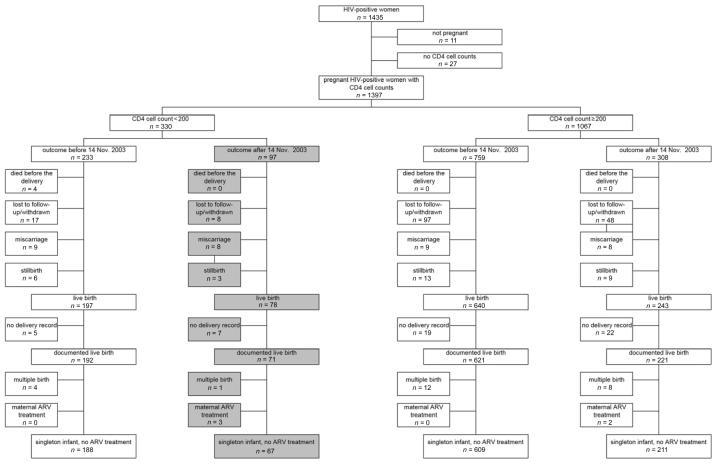
Twenty-four percent of the HIV-infected women enrolled in the cohort had CD4 cell counts <200 cells/μL. The present analysis focused on 255 women with low CD4 cell counts who had liveborn singleton infants—67 delivered after and 188 delivered before 14 November 2003. This excludes women who began receiving antiretroviral drugs while pregnant (figure 1). Enrollment of the 67 women who delivered after the cutoff date occurred between an estimated 11 and 39 weeks of gestation. Twelve of these women were enrolled before November 2003 but had >2 weeks of antenatal follow-up after this date. Six delivered within 1 week of enrollment, but 5 of these deliveries occurred >5 months after the protocol change was implemented. Therefore, all 6 women are included in the group presumed to have received cotrimoxazole.
Figure 1.
Enrollment and birth outcomes by CD4 cell count stratum and time of delivery. If the date of delivery was not known (lost to follow-up or maternal death), then women were grouped according to their expected date of delivery determined on the basis of the date of the last menstrual period and assuming a delivery at 38 weeks of gestation (the mean gestational age at delivery in this cohort). Shaded areas denote women with outcomes after 14 November 2003 who qualified for cotrimoxazole treatment during pregnancy. ARV, antiretroviral.
Birth outcomes after the introduction of cotrimoxazole prophylaxis.
Among singleton live births to women with CD4 cell counts <200 cells/μL, there were significant reductions in the percentage of preterm births (≤34 weeks of gestation), clinical chorioamnionitis, and neonatal mortality, as well as a trend toward a 115-g increase in mean birth weight, after the introduction of antenatal cotrimoxazole prophylaxis was observed (table 1). The reduction in the percentage of preterm births remained significant when the analysis was restricted to women enrolled before 32 weeks of gestation: 36% to 17% (P = .01). Neonatal hospitalization rates were not significantly reduced after 14 November 2003, and 1 infant was hospitalized with jaundice. Another infant in the postcotrimoxazole cohort was hospitalized with an absent urethral opening. The mother of this infant enrolled at 19 weeks gestational age.
Table 1.
Birth outcomes among singleton liveborn infants born to 255 HIV-infected women with CD4 cell counts <200 cells/μL before and after the introduction of cotrimoxazole prophylaxis.
| Birth outcome | Before (n = 188) | After (n = 67) | P |
|---|---|---|---|
| Gestation | .02 | ||
| <32 weeks | 27 (14) | 2 (3) | |
| 32-33 weeks | 18 (10) | 4 (6) | |
| 34-36 weeks | 44 (23) | 22 (33) | |
| ≥37 weeks | 99 (53) | 39 (58) | |
| Mean ± SD, weeks | 37.0 ± 4.5 | 38.1 3.7 | .07 |
| Preterm birth (≤34 weeks) | 58 (31) | 12 (18) | .04 |
| Birth weighta | .24 | ||
| <1500 g | 6 (3) | 0 (0) | |
| 1500-1999 g | 6 (3) | 2 (3) | |
| 2000-2499 g | 23 (13) | 8 (12) | |
| ≥2500 g | 146 (81) | 55 (85) | |
| Mean ± SD,g | 2832 ± 566 | 2947 ± 504 | .15 |
| Chorioamnionitis | 12 (6) | 0 (0) | .04 |
| Neonatal (≤28 days) mortalityb | 17 (9) | 0 (0) | .01 |
| Neonatal (≤28 days) hospitalization | 26 (14) | 6 (9) | .30 |
| Sepsis-related hospitalization | 9 (5) | 1 (2) | .46 |
| Jaundice | 0 (0) | 1 (2) | .26 |
| Congenital malformations | 0 (0) | 1 (2) | .26 |
NOTE. Data are no. (%) of subjects, unless otherwise indicated.
Data on birth weight were available for 181 and 65 infants in the before and after groups, respectively.
The rates of losses to follow-up until day 28 were 6.9% before and 7.5% after 14 November 2003.
Women with low CD4 cell counts delivering before and after the introduction of cotrimoxazole prophylaxis were largely similar with respect to HIV-related characteristics and other medical and socioeconomic parameters related to adverse birth outcomes (table 2). To investigate whether temporal changes in the prevalence of other risk factors during the later period might account for the changes in birth outcomes observed after the introduction of cotrimoxazole prophylaxis, we investigated risk factors for preterm birth in this group. In a multiple logistic regression model, birth after the introduction of cotrimoxazole prophylaxis was associated with a significant reduction in the percentage of preterm births (odds ratio [OR], 0.46 [95% confidence interval {CI}, 0.22-0.96]) after adjustment for hemoglobin level (OR, 0.78 [95% CI, 0.63-0.97]), severe poverty (OR, 1.88 [95% CI, 1.03-3.34]), maternal education (OR, 1.64 [95% CI, 0.90-3.0]), and gestational age at enrollment (OR, 0.94 [95% CI, 0.90-0.99]). Enrollment earlier during pregnancy was most likely associated with increased risk, because enrollment late during pregnancy excludes the possibility for early preterm deliveries. Food insecurity was not a risk factor for preterm delivery, and, when included in the above model, the reduction in the percentage of preterm births during the postcotrimoxazole period remained unchanged.
Table 2.
Comparison of the cohorts of women with CD4 cell counts <200 cells/μL delivering liveborn singleton infants before and after the introduction of cotrimoxazole prophylaxis.
| Factor | Before (n = 188) | After (n = 67) | P |
|---|---|---|---|
| HIV-related factors at enrollment | |||
| CD4 cell count, median (IQR), cells/μL | 146 (102-169) | 128 (92-160) | .12 |
| HIV load, median (IQR), log10copies/mL | 5.1 (4.6-5.4) | 5.0 (4.6-5.5) | .51 |
| WHO stage 3 | 104 (55) | 33 (49) | .39 |
| Hemoglobin level <11 g/dL | 150 (80) | 47 (77) | .65 |
| Positive RPR test | 37 (20) | 9 (15) | .30 |
| Body mass index, mean ± SD, kg/m2 | 22.5 ± 3.1 | 23.0 ± 3.2 | .33 |
| Obstetric factors | |||
| Primipara | 19 (10) | 6 (9) | .79 |
| Gestational age at enrollment, mean ± SD, weeks | 26 ± 6.6 | 25 ± 7.2 | .65 |
| Characteristics at delivery | |||
| Place of birth | |||
| Home | 23 (12) | 6 (9) | .47 |
| Tertiary hospital | 38 (20) | 11 (16) | .50 |
| Primary care clinic | 126 (67) | 50 (75) | .25 |
| Male sex | 92 (49) | 35 (52) | .64 |
| Sociodemographic variables | |||
| Age, median (IQR), years | 27 (24-30) | 28 (24-31) | .49 |
| Married | 152 (81) | 55 (82) | .82 |
| ≥8 years of school | 93 (50) | 29 (43) | .38 |
| Severe povertya | 100 (53) | 36 (54) | .94 |
| Crowdingb | 46 (25) | 12 (18) | .29 |
| Food insecurityc | 55 (29) | 10 (15) | .02 |
NOTE. Data are no. (%) of subjects, unless otherwise indicated. IQR, interquartile range; RPR, rapid plasma reagin; WHO, World Health Organization.
Defined as severe if the household did not have a refrigerator, electricity, a water source inside the home or on own property, or a stove or hot plate for cooking and as mild if they had 1 or more of these.
Defined as 2 adults/room.
Defined as reporting that the household had no food at least 1 full day during the month preceding enrollment.
To test whether the change of malarial prophylaxis from chloroquine to sulfadoxine-pyrimethamine in early November 2002—a year before the introduction of cotrimoxazole prophylaxis—could explain part of the observed improvements in maternal and infant health, we conducted an analysis that was restricted to births after 14 November 2002. In this analysis, malarial prophylaxis was the same for all women (all received sulfadoxine-pyrimethamine). In the subgroup with CD4 cell counts <200 cells/μL (n = 166), the reduction in the percentage of preterm births was similar to that in the overall cohort (OR, 0.36 [95% CI, 0.17-0.79]) after adjustment for hemoglobin level, severe poverty, maternal education, and gestational age at enrollment. Neonatal mortality decreased from 8% to 0% (P = .02), and the frequency of chorioamnionitis decreased from 2% to 0% (P = .52).
In contrast to the improvement observed among severely immunosuppressed women, birth outcomes in the cohort of women with CD4 cell counts ≥200 cells/μL displayed no consistent patterns of improvement over the same time interval. Women with singleton live births before (n = 609) did not differ from women with singleton live births after (n = 211) 14 November 2003 with respect to the frequency of preterm births (130 [21%] vs. 45 [22%]; P = .97), clinical chorioamnionitis (15 [2.5%] vs. 2 [0.95%]; P = .26), and neonatal mortality (18 [3.0%] vs. 5 [2.4%]; P = .66).
Changes over time in associations between maternal CD4 cell count and birth weight.
Before the introduction of cotrimoxazole prophylaxis, there was a strong relationship between CD4 cell counts and birth weight (figure 2). This relationship was not linear but, rather, was logarithmic, with a mean increase of 276 g (95% CI, 248-504 g) per log10 increase in CD4 cell count; this resulted in an apparent threshold effect, with those with CD4 cell counts <100 cells/μL being the most at risk with respect to lower birth weight. During the postcotrimoxazole period, the decrease in birth weight was not apparent (β = 65 g [95% CI, -124 to 254 g] per log10 increase in CD4 cell count) (figure 2). Thus, a CD4 cell count during the precotrimoxazole period conferred a greater risk of low birth weight than during the postcotrimoxazole period. Birth weight improvement during the postcotrimoxazole period was most pronounced if CD4 cell counts were <100 cells/μL. The mean improvement in birth weight during the postcotrimoxazole period was 52 g (95% CI, -120 to 223 g; P = .55) for infants born to women with CD4 cell counts 100-200 cells/μL and was 319 g (95% CI, -21 to 659 g; P = .07) for infants born to women with CD4 cell counts <100 cells/μL.
Figure 2.
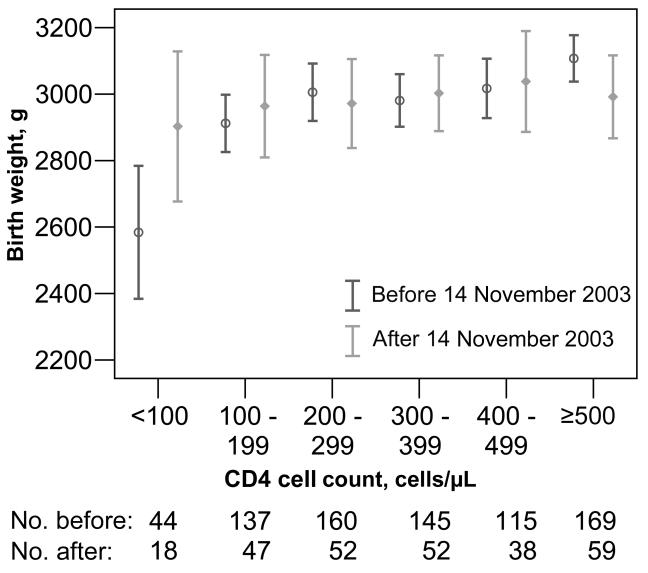
Mean birth weight in each CD4 cell count stratum, by birth before or after the introduction of cotrimoxazole prophylaxis. Only liveborn singleton infants without intrauterine exposure to maternal antiretroviral treatment were included (n = 1036); 39 infants with missing data were excluded. Bars show 95% confidence intervals. The nos. of infants included in each category are indicated.
Sensitivity analysis to investigate the effects of error in estimation of gestational age.
Gestational age was estimated on the basis of LMP reports at enrollment, which is an error-prone method. For 245 (96%) of the women in the low CD4 cell count stratum, gestational age at antenatal booking was also estimated by fundal height examination. Although these 2 measures were correlated (Pearson’s correlation coefficient, 0.62), for only 64% (n = 156) were these measurements within 2 weeks (plus or minus) of each other. In this subgroup (in which gestational age was, presumably, more accurately estimated), the effect of cotrimoxazole on preterm delivery (measured on the basis of LMP) was slightly stronger than that in the entire cohort (unadjusted OR, 0.43 [95% CI, 0.18-1.02]) and remained so after adjustment for hemoglobin level, severe poverty, maternal education, and gestational age at enrollment (adjusted OR, 0.33 [95% CI, 0.13-0.83]).
Use of fundal height-based estimates tended to yield older estimates of gestation at birth than did use of LMP-based estimates: the means for the low CD4 cell count stratum were 37.4 and 38.2 weeks for the LMP and fundal height estimates, respectively. If only the fundal height estimates were used, the temporal changes were most strongly related to a higher cutoff value of ≤36 weeks of gestation (OR, 0.49 [95% CI, 0.26-0.93]) after adjustment for hemoglobin level, severe poverty, maternal education, and gestational age at enrollment.
Do reductions in preterm birth explain reductions in neonatal mortality?
To investigate whether reduction in preterm birth might explain decreased mortality, we examined risk factors for neonatal death. Gestational age, birth weight, and chorioamnionitis were each significantly associated with neonatal mortality in the cohort overall (table 3) as well as in the low CD4 cell count stratum (data not shown). Before the introduction of cotrimoxazole prophylaxis, 10 (59%) of 17 neonatal deaths in the low CD4 cell count stratum were born at ≤34 weeks (OR, 3.66 [95% CI, 1.32-10.17]); 3 (18%) of 17 had indications of chorioamnionitis (OR, 3.86 [95% CI, 0.94-15.90]); and the mean birth weight was 668 g (95% CI, 372-963 g) lower among infants who died than among survivors.
Table 3.
Associations between neonatal mortality and gestational age, birth weight, and chorioamnionitis among 1075 HIV-infected women who gave birth to singleton liveborn infants and who did not receive antiretroviral treatment during pregnancy.
| Factor | Neonatal deaths/total no. (%) | OR (95% CI) |
|---|---|---|
| Gestational age | ||
| <30 weeks | 9/45 (20) | 12.1 (4.2-33) |
| 30-32 weeks | 4/71 (6) | 2.9 (0.7-9.7) |
| 33-34 weeks | 7/129 (5) | 2.8 (0.9-7.6) |
| 35-36 weeks | 7/186 (4) | 1.9 (0.6-5.2) |
| ≥37 weeks | 13/640 (2) | Reference |
| Birth weight | ||
| <1500 g | 4/9 (44) | 38.6 (6.6-200) |
| 1500-1999 g | 7/22 (32) | 22.5 (6.4-72) |
| 2000-2499 g | 8/96 (8) | 4.4 (1.5-12.1) |
| 2500-2999 g | 5/325 (2) | 0.8 (0.2-2.4) |
| ≥3000 g | 12/594 (2) | Reference |
| Chorioamnionitis | ||
| Yes | 7/29 (24) | 9.8 (3.3-25.7) |
| No | 33/1046 (3) | Reference |
NOTE. CI, confidence interval; OR, odds ratio.
Maternal outcomes after the introduction of cotrimoxazole prophylaxis.
Among all women with CD4 cell counts <200 cells/μL, 233 enrolled before and 94 enrolled after the introduction of cotrimoxazole prophylaxis (figure 1). There was a nonsignificant trend toward reduced maternal mortality (10 [4%] and 1 [1%]; P = .19) and hospital admissions (56 [24%] and 14 [15%]; P = .07) during the postversus precotrimoxazole period.
DISCUSSION
Our data suggest that, in addition to the expected benefits for maternal health, there may be neonatal benefits from providing cotrimoxazole prophylaxis to immunosuppressed HIV-infected women during pregnancy. We observed reductions in neonatal mortality, clinical chorioamnionitis, and preterm delivery among HIV-infected women with low CD4 cell counts coinciding in time with the addition of cotrimoxazole as a component of antenatal care.
Preterm birth can be caused by a variety of bacterial and parasitic infections that can be readily treated with cotrimoxazole. These include urinary tract infection [14], toxoplasmosis [15], malaria [16-18], and various other systemic infections, such as pneumonia [19]. Treatment of lower genital tract infections, including chlamydia and gonorrhea [20], may also have played some role, given that our protocol did not include systematic screening for these conditions. However, the frequency of asymptomatic lower genital tract infections may be insufficient to account for the size of the effect seen. Sustained use of cotrimoxazole throughout pregnancy may also have reduced the common condition of subclinical chorioamnionitis [21], which is consistent with our observation of a significant reduction in clinical chorioamnionitis.
Because preterm delivery is strongly related to neonatal mortality [22] even in the context of HIV infection [23], its reduction may have been the main cause for the lower neonatal mortality rate. However, our sample size was inadequate to fully explore this issue statistically. It is also possible that cotrimoxazole may have had other beneficial effects that contributed to reductions in neonatal mortality, such as through effects on maternally acquired neonatal infections, such as Kleb-siella, Escherichia coli, Salmonella, Hemophilus influenza, and Streptococcus infections [24]. Trials have observed benefits of antibacterial interventions applied only during labor and delivery with respect to the morbidity and mortality of newborns of HIV-infected mothers [25-27]. Our results underscore the importance of combining antimicrobial interventions with antiviral interventions to more effectively reduce morbidity and mortality among infants born to HIV-infected mothers.
HIV-infected women are at high risk for adverse birth outcomes [11]. This risk may, in part, be attributable to high rates of coinfections and is increased with more-advanced HIV disease [28-31]. Our results are encouraging, because they suggest that high rates of adverse birth outcomes among HIV-infected women may be preventable. Although adverse pregnancy outcomes were concentrated among immunosuppressed women in our cohort, rates of preterm delivery, low birth weight, and neonatal mortality were also high among women with higher CD4 cell counts. It may be useful to investigate whether HIV-infected women with higher CD4 cell counts—or even HIV-uninfected women—would profit from antibiotic prophylaxis during pregnancy. A trial from Uganda examined the effects of a prophylactic regimen containing azithromycin, cefixime, and metronidazole in pregnant women. With an HIV infection prevalence of 14%, presumptive treatment resulted in significant reductions in neonatal mortality and a trend toward reduced preterm births [32], suggesting that antibiotic prophylaxis may benefit all pregnant women in areas with high neonatal morbidity and mortality rates.
A recent large trial among HIV-infected African women observed no beneficial effects on preterm birth and low birth weight of 2 courses of antibiotics—metronidazole and erythromycin given at 24 weeks, and metronidazole and ampicillin given during labor [33]. The authors suggest as explanation the failure of the regimen to reduce subclinical chorioamnionitis. Antibiotic prophylaxis has been extensively investigated as a strategy to reduce preterm delivery among HIV-uninfected women in the United States and elsewhere, but the benefits are not clear-cut [12]. Most reviews suggest that there is little benefit for general populations [12] but that there may be some benefit in certain high-risk groups, such as women with bacteriuria [34] or women with bacterial vaginosis and a history of preterm birth [35]. There are important differences between our study and many of these prior studies. First, none investigated cotrimoxazole, which has broad activity, particularly against systemic and parasitic infections. Furthermore, in our case, once cotrimoxazole was initiated, it was continued throughout pregnancy and breast-feeding. We also did not specifically screen for other common infections believed to be associated with preterm birth (with the exception of syphilis screening) and treated only symptomatic infections brought to the attention of health care providers. Our data suggest that the choice of antibiotic used may have a larger influence than previously supposed. The focus on lower genital tract infections and bacterial vaginosis may be misplaced. Broader consideration of all infections during pregnancy may be more productive.
Antenatal clinics are important sites, as they provide an infrastructure where HIV-infected women are identified and clinical interventions implemented. Despite concern about its potential untoward fetal effects, cotrimoxazole has been recommended for HIV-infected pregnant women on the basis of a favorable risk-benefit analysis [36]. One of the components of cotrimoxazole, trimethroprim, is a folate antagonist and has been associated with congenital malformations [7-9]. Our study was too small to be informative about birth defects, and women initiated prophylaxis only in the second trimester. Concern has also been raised about the use of sulfonamides during later pregnancy and close to delivery, because they may be associated with jaundice, hemolytic anemia, and—theoretically but not yet observed—kernicterus [37]. One child in our study was hospitalized with neonatal jaundice, and larger studies are needed to evaluate these outcomes more thoroughly.
The present study has several limitations. Estimates of gestational age relied on self-reported LMP dates, because more accurate methods (e.g., ultrasound) were not feasible. In light of comparison with birth weight and fundal height estimates in our data, it appears that the LMP estimates underestimate gestation by 2 weeks or more. However, the estimates were standard across the study population and across time and were based on interviews conducted at enrollment before any other outcomes were measured. Gestational age was strongly related to neonatal mortality in a dose-dependent manner, as expected. Although there undoubtedly was error, it is unlikely to have been differential during the 2 time periods. Nondifferential error tends to reduce the magnitude of associations [38]. Thus, although the occurrence of preterm birth may be overestimated in our data, it is unlikely that this affected comparison across the 2 time periods. Restriction of the analysis to gestation measurements confirmed by fundal height resulted in stronger associations, strengthening our confidence in the results despite the known difficulties in measuring gestational age in a low-resource setting such as this.
Ethical constraints limit the possibility of conducting randomized trials to test the possible effects that interventions, such as cotrimoxazole, have on neonatal outcomes when their benefits for maternal health are already proven. One of the few ways to investigate the effects of cotrimoxazole is to use historical controls, as we and others have done [39-42]. Women in the pre- and postcotrimoxazole cohorts came from the same communities and were enrolled in the same trial, and outcomes were measured using the same protocol. We had the opportunity to examine and control for changes in other risk factors over time. However, the relevant sample size was small, and we cannot definitively exclude the possibility that other unmeasured factors changed with time and influenced the outcome. However, there were no systematic changes to the antenatal care protocols during this time period. The change in intermittent presumptive malarial treatment, which occurred earlier than did the introduction of cotrimoxazole prophylaxis, cannot explain the observed improvements in infant health. These observations should be examined in larger studies, particularly with concomitant use of antiretroviral drugs. As cotrimoxazole use becomes more widespread, drug resistance, including crossresistance to sulfadoxine-pyrimethamine, should be carefully monitored, because beneficial effects may be reduced. Our data support existing guidelines recommending cotrimoxazole prophylaxis for HIV-infected pregnant women [6, 10] and add the possibility that the implementation of these guidelines may have benefits for newborns in addition to the ones for mothers.
Acknowledgments
We are indebted to the study subjects for their dedicated participation in the study. We thank the staff of the Zambian Exclusive Breastfeeding Study, who provided high-quality implementation of the study protocol and dedicated care to the study participants. We also thank Drs. Chewe Luo, Jeff Stringer, and Sten Vermund for their support as well as Dr. Zena Stein for comments on early versions of the manuscript.
References
- 1.Fischl MA, Dickinson GM, La Voie L. Safety and efficacy of sulfamethoxazole and trimethoprim chemoprophylaxis for Pneumocystis carinii pneumonia in AIDS. JAMA. 1988;259:1185–9. doi: 10.1001/jama.259.8.1185. [DOI] [PubMed] [Google Scholar]
- 2.Chintu C, Bhat GJ, Walker AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–71. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 3.Anglaret X, Chene G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353:1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 4.Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–75. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 5.Maynart M, Lievre L, Sow PS, et al. Primary prevention with cotrimoxazole for HIV-1-infected adults: results of the pilot study in Dakar, Senegal. J Acquir Immune Defic Syndr. 2001;26:130–6. doi: 10.1097/00042560-200102010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Guidelines for preventing opportunistic infections among HIV-infected persons—2002 recommendations of the US Public Health Service and the Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51:1–52. [PubMed] [Google Scholar]
- 7.Hernandez-Diaz S, Werler MM, Walker AM, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343:1608–14. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- 8.Czeizel AE, Rockenbauer M, Sorensen HT, Olsen J. The teratogenic risk of trimethoprim-sulfonamides: a population based case-control study. Reprod Toxicol. 2001;15:637–46. doi: 10.1016/s0890-6238(01)00178-2. [DOI] [PubMed] [Google Scholar]
- 9.Lawson DH, Paice BJ. Adverse reactions to trimethoprim-sulfamethoxazole. Rev Infect Dis. 1982;4:429–33. doi: 10.1093/clinids/4.2.429. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Provisional WHO/UNAIDS recommendations on the use of contrimoxazole prophylaxis in adults and children living with HIV/AIDS in Africa. Afr Health Sci. 2001;1:30–1. [PMC free article] [PubMed] [Google Scholar]
- 11.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105:836–48. doi: 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- 12.Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P. Prophylactic antibiotic administration in pregnancy to prevent infectious morbidity and mortality. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD002250. CD002250. [DOI] [PubMed] [Google Scholar]
- 13.Thea DM, Vwalika C, Kasonde P, et al. Issues in the design of a clinical trial with a behavioral intervention—the Zambia exclusive breast-feeding study. Control Clin Trials. 2004;25:353–65. doi: 10.1016/j.cct.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Ovalle A, Levancini M. Urinary tract infections in pregnancy. Curr Opin Urol. 2001;11:55–9. doi: 10.1097/00042307-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Freeman K, Oakley L, Pollak A, et al. Association between congenital toxoplasmosis and preterm birth, low birthweight and small for gestational age birth. BJOG. 2005;112:31–7. doi: 10.1111/j.1471-0528.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 16.Ticconi C, Mapfumo M, Dorrucci M, et al. Effect of maternal HIV and malaria infection on pregnancy and perinatal outcome in Zimbabwe. J Acquir Immune Defic Syndr. 2003;34:289–94. doi: 10.1097/00126334-200311010-00005. [DOI] [PubMed] [Google Scholar]
- 17.Ayisi JG, van Eijk AM, ter Kuile FO, et al. The effect of dual infection with HIV and malaria on pregnancy outcome in western Kenya. AIDS. 2003;17:585–94. doi: 10.1097/00002030-200303070-00014. [DOI] [PubMed] [Google Scholar]
- 18.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 19.Madinger NE, Greenspoon JS, Ellrodt AG. Pneumonia during pregnancy: has modern technology improved maternal and fetal outcome? Am J Obstet Gynecol. 1989;161:657–62. doi: 10.1016/0002-9378(89)90373-6. [DOI] [PubMed] [Google Scholar]
- 20.Meis PJ, Goldenberg RL, Mercer B, et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1995;173:1231–5. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- 21.Chi BH, Mudenda V, Levy J, Sinkala M, Goldenberg RL, Stringer JS. Acute and chronic chorioamnionitis and the risk of perinatal human immunodeficiency virus-1 transmission. Am J Obstet Gynecol. 2006;194:174–81. doi: 10.1016/j.ajog.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 22.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 23.Wei R, Msamanga GI, Spiegelman D, et al. Association between low birth weight and infant mortality in children born to human immunodeficiency virus 1-infected mothers in Tanzania. Pediatr Infect Dis J. 2004;23:530–5. doi: 10.1097/01.inf.0000129691.42964.eb. [DOI] [PubMed] [Google Scholar]
- 24.Vergnano S, Sharland M, Kazembe P, Mwansambo C, Heath PT. Neonatal sepsis: an international perspective. Arch Dis Child Fetal Neonatal Ed. 2005;90:F220–4. doi: 10.1136/adc.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taha TE, Biggar RJ, Broadhead RL, et al. Effect of cleansing the birth canal with antiseptic solution on maternal and newborn morbidity and mortality in Malawi: clinical trial. BMJ. 1997;315:216–20. doi: 10.1136/bmj.315.7102.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaillard P, Mwanyumba F, Verhofstede C, et al. Vaginal lavage with chlorhexidine during labour to reduce mother-to-child HIV transmission: clinical trial in Mombasa, Kenya. AIDS. 2001;15:389–96. doi: 10.1097/00002030-200102160-00012. [DOI] [PubMed] [Google Scholar]
- 27.Mandelbrot L, Msellati P, Meda N, et al. 15 month follow up of African children following vaginal cleansing with benzalkonium chloride of their HIV infected mothers during late pregnancy and delivery. Sex Transm Infect. 2002;78:267–70. doi: 10.1136/sti.78.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stratton P, Tuomala RE, Abboud R, et al. Obstetric and newborn outcomes in a cohort of HIV-infected pregnant women: a report of the women and infants transmission study. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:179–86. doi: 10.1097/00042560-199902010-00011. [DOI] [PubMed] [Google Scholar]
- 29.Dreyfuss ML, Msamanga GI, Spiegelman D, et al. Determinants of low birth weight among HIV-infected pregnant women in Tanzania. Am J Clin Nutr. 2001;74:814–26. doi: 10.1093/ajcn/74.6.814. [DOI] [PubMed] [Google Scholar]
- 30.Temmerman M, Chomba EN, Ndinya-Achola J, Plummer FA, Coppens M, Piot P. Maternal human immunodeficiency virus-1 infection and pregnancy outcome. Obstet Gynecol. 1994;83:495–501. doi: 10.1097/00006250-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Kumar RM, Uduman SA, Khurranna AK. Impact of maternal HIV-1 infection on perinatal outcome. Int J Gynaecol Obstet. 1995;49:137–43. doi: 10.1016/0020-7292(95)02356-h. [DOI] [PubMed] [Google Scholar]
- 32.Gray RH, Wabwire-Mangen F, Kigozi G, et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. Am J Obstet Gynecol. 2001;185:1209–17. doi: 10.1067/mob.2001.118158. [DOI] [PubMed] [Google Scholar]
- 33.Goldenberg RL, Mwatha A, Read JS, et al. The HPTN 024 Study: the efficacy of antibiotics to prevent chorioamnionitis and preterm birth. Am J Obstet Gynecol. 2006;194:650–61. doi: 10.1016/j.ajog.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD000490. CD000490. [DOI] [PubMed] [Google Scholar]
- 35.McDonald H, Brocklehurst P, Parsons J. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD000262.pub2. CD000262. [DOI] [PubMed] [Google Scholar]
- 36.Forna F, McConnell M, Kitabire FN, et al. Systematic review of the safety of trimethoprim-sulfamethoxazole for prophylaxis in HIV-infected pregnant women: implications for resource-limited settings. AIDS Rev. 2006;8:24–36. [PubMed] [Google Scholar]
- 37.Briggs GG, Freeman RK, Yaffe SJ. 6th ed. Lippincott Williams & Wilkins; Philadelphia: 2002. Drugs in pregnancy and lactation. 1393/t-97/t. [Google Scholar]
- 38.Rothman JK, Greenland S. Modern epidemiology. 2nd ed. Lippincott Williams & Wilkins; Philadelphia: 1998. p. 127. [Google Scholar]
- 39.Zachariah R, Spielmann MP, Harries AD, Salaniponi FL. Voluntary counselling, HIV testing and sexual behaviour among patients with tuberculosis in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7:65–71. [PubMed] [Google Scholar]
- 40.Grimwade K, Sturm AW, Nunn AJ, Mbatha D, Zungu D, Gilks CF. Effectiveness of cotrimoxazole prophylaxis on mortality in adults with tuberculosis in rural South Africa. AIDS. 2005;19:163–8. doi: 10.1097/00002030-200501280-00008. [DOI] [PubMed] [Google Scholar]
- 41.Mwaungulu FB, Floyd S, Crampin AC, et al. Cotrimoxazole prophylaxis reduces mortality in human immunodeficiency virus-positive tuberculosis patients in Karonga District, Malawi. Bull World Health Organ. 2004;82:354–63. [PMC free article] [PubMed] [Google Scholar]
- 42.Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–34. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]