Abstract
Gene delivery is one of the biggest challenges in the field of gene therapy. It involves the efficient transfer of transgenes into somatic cells for therapeutic purposes. A few major drawbacks in gene delivery include inefficient gene transfer and lack of sustained transgene expression. However, the classical method of using viral vectors for gene transfer has circumvented some of these issues. Several kinds of viruses, including retrovirus, adenovirus, adeno-associated virus, and herpes simplex virus, have been manipulated for use in gene transfer and gene therapy applications. The transfer of genetic material into lacrimal epithelial cells and tissues, both in vitro and in vivo, has been critical for the study of tear secretory mechanisms and autoimmunity of the lacrimal gland. These studies will help in the development of therapeutic interventions for autoimmune disorders such as Sjögren’s syndrome and dry eye syndromes which are associated with lacrimal dysfunction. These studies are also critical for future endeavors which utilize the lacrimal gland as a reservoir for the production of therapeutic factors which can be released in tears, providing treatment for diseases of the cornea and posterior segment. This review will discuss the developments related to gene delivery and gene therapy in the lacrimal gland using several viral vector systems.
Keywords: Viral vectors, Lacrimal gland, Gene therapy, Gene delivery, Sjögren’s syndrome, Exocytosis
Abbreviations: Ad,adenovirus; IL,interleukin; IFN,interferon; TNF,tumor necrosis factor; β-gal,β-galactosidase
1. Introduction
Gene transfer and gene therapy in principle involve the development of efficient means for delivering gene(s) to the nuclei of somatic cells to replace a defective gene with a functionally normal one. This approach offers the hope of cures for various genetic and autoimmune diseases, including sickle cell disease [1], X-linked severe combined immunodeficiency disorder [2], Sjögren’s syndrome [3], rheumatoid arthritis [4], type I diabetes [5], multiple sclerosis [6], cystic fibrosis [7], and hemophilia [8]. Additionally, it also provides hope for long-term therapeutic benefits in contrast to the transient relief provided by conventional drug therapy.
One of the basic methods of gene transfer is to modify viruses into genetic shuttles which will deliver the gene of interest into the target cells. Much progress in gene delivery and therapy has been achieved with viral vectors due to their high transduction efficiency in cells in vivo. Viral vector systems, including retroviruses, lentiviruses, adenoviruses, and adeno-associated viruses, currently offer the best choice for efficient gene delivery [9, 10]. The most commonly used DNA viral vectors are adenoviruses (Ad) and adeno-associated viruses. These vectors have been extensively used in gene therapy of the eye [11]. They have been successfully used to mediate gene transfer for ocular neovascularization [12, 13], age-related macular degeneration [14], uveitis [15], diabetic retinopathy [16] corneal wound healing [17] and experimental autoimmune lacrimal gland disease [18, 19]. In this review, we discuss the viral gene delivery approaches performed in the lacrimal gland to understand and modulate lacrimal gland functions for therapeutic purposes.
2. Gene delivery by viral vectors in primary cultures of lacrimal gland tissue
Genes can also be introduced into cells to produce beneficial substances for therapeutic purposes. The lacrimal gland is responsible for the production and regulated release of tear proteins into ocular surface fluid. These proteins include nutrient factors which nurture the cornea, as well as factors which protect the ocular surface from pathogens. Delivery of genes for necessary products the lacrimal gland would chronically secrete could be a potential therapeutic approach for patients suffering from various diseases of the eye, including, glaucoma, dry eye, keratitis, and uveitis. Currently, these diseases require long-term administration of therapeutic preparations in the form of topical eye drops and eye ointments. Although this approach has the advantage in minimizing systemic side effects, the major drawback is the short residence time of the medication on the eye and hence the need for frequent applications which in some cases, could become a functional disability that would affect the patient’s quality of life. Many studies on gene therapy to the eye have been reported but the delivery and expression of foreign genes in the lacrimal gland for therapeutic purposes has not been extensively explored. As well, gene therapy to the lacrimal gland to specifically treat disorders of the lacrimal gland is of great interest, considering the large numbers of people who suffer from severe dry eye syndromes including the autoimmune disease, Sjögren’s syndrome.
Recently, Banin et al. demonstrated the first feasibility study of gene transfer ex vivo in rat lacrimal gland tissue fragments using viral vectors [20] such as vaccinia, Ad, and herpes simplex. The results showed that all the vectors were capable of delivering a reporter gene (β-galactosidase or β-gal) to the lacrimal gland but with different transduction efficiencies and tropisms. After 7 days of modified lacrimal gland fragment organ culture technique [21], β-gal expression was observed in 77% of tissue fragments exposed to vaccinia vector, 41% of fragments exposed to Ad and 13% of fragments exposed to herpes vectors. Upon histologic examination, vector-specific expression patterns of reporter genes were observed. The vaccinia vector preferentially delivered the β-gal gene to the lacrimal duct cells and acini while Ad vectors expressed β-gal mainly within the myoepithelial cells surrounding the lacrimal acini. It was also noted that β-gal expression in acinar cells transduced with Ad vectors was accompanied by degradation of these cells, possibly due to vector toxicity.
3. Ad-mediated gene therapy
3.1 Dry eye syndrome
Dry eye has been defined as “a disorder of the tear film due to tear deficiency or excessive evaporation that causes damage to the interpalpebral ocular surface and is associated with symptoms of discomfort” [22]. Systemic autoimmune diseases like Sjögren’s syndrome, rheumatoid arthritis, lupus erythematosus, and thyroiditis are considered as the major initiating factors in some kinds of dry eye disease. One of the most severe forms of dry eye is found in patients with Sjögren’s syndrome, an inflammatory autoimmune disorder characterized by lymphocytic infiltration and affecting approximately 4% of the population in the United States [23]. The immune-related lacrimal insufficiency reduces the quality and quantity of tear production below the level required to maintain a healthy and comfortable ocular surface. It is believed that a combination of immunologic, genetic, hormonal and environmental factors play a crucial role in the development of autoimmunity in the lacrimal gland [24,25]. The inflammatory infiltrates produce toxic factors that act as immune mediators, resulting in reduced secretory function caused by secretory tissue atrophy and dysfunction of the surviving tissue [26,27]. There is evidence that these lymphocytic infiltrates produce proinflammatory cytokines, including interleukin (IL)-1, -6, -12, and -18; interferon (IFN)-γ; and tumor necrosis factor (TNF)-α [28–32]. However, in addition to these proinflammatory cytokines, anti-inflammatory cytokines including IL-10, transforming growth factor (TGF)-β and IL-4 have also been detected [33,34]. Numerous studies indicate that regulation of anti-inflammatory cytokines (IL-10, IL- 4) or specific inhibitors of proinflammatory cytokines (sTNFR, IL-ra, anti-TNF-α) can play an important immunoregulatory role in inhibiting a disease process [35,36]. Among these cytokines, the local up-regulation of IL-10 and/or inhibition of TNF-α binding to target cells have received increased attention as promising therapeutic potentials against an immunopathological disease [37]
3.2 Autoimmune dacryoadenitis in animal models
The pathogenesis of dry eye syndrome has been difficult to fully elucidate due to the limited availability of lacrimal gland tissue samples from patients with Sjögren’s syndrome, thereby prompting the need for animal models of this disease. Murine models of autoimmune disease resembling secondary Sjögren’s syndrome have been established [38,39]. An induced lacrimal gland disease, autoimmune dacryoadenitis, has also been induced in mice and rats which to a certain extent resembles primary Sjögren’s syndrome in humans [40]. However, compared to studies of rodent models with induced lacrimal disease, fewer studies have been conducted on larger animals to effectively evaluate the efficiency of ocular therapies. Keratoconjunctivitis sicca has been studied in dogs [41] however, a spontaneous disease in dogs is challenging and they also require expensive maintenance care. Hence a rabbit model developed in this institute [42,43] was pursued for dry eye disease and experimental validation of therapies as an alternative option.
Adenoviruses are the most common cause of acute viral infections of the cornea [44]. A rabbit model was used to determine the effects of an ocular Ad virus infection on lacrimal gland histopathology. Studies revealed that an Ad infection of the cornea resulted in increased numbers of RTLA+ and CD18+ cells and increased expression of MHC class II molecules in the lacrimal gland. However, Ad was not detected in the lacrimal tissue explanted 21 days after post-inoculation and it was unclear whether the changes were caused by the inoculated virus or through an aberrant expression of Class II histocompatibility antigens which involved T cell activation that led to autoimmunity in the lacrimal gland [44,45]. The possibilities suggested by these early studies for T-cell modulation of lacrimal gland function were instrumental in the establishment of a rabbit model for autoimmune dacryoadenitis which we have subsequently used for studying the mechanism of Sjögren’s syndrome and for identifying and evaluating therapeutic interventions. Autoimmune dacryoadenitis in rabbits was established through autologous mixed cell reactions that involved incubation of purified acinar cells prepared from one surgically excised inferior lacrimal gland and peripheral blood lymphocytes from the same animal [44]. Disease is then induced by injecting the donor rabbit’s remaining inferior lacrimal gland with the activated peripheral blood lymphocytes after 5 days of co-culture with the autologous acinar cells in the mixed cell reaction. After 2 weeks, abundant periductal foci of lymphocytes resembling the autoimmune lesions characteristic of Sjogren’s syndrome are seen [43]. The induced adenitis is accompanied by lacrimal gland dysfunction characterized by reduced tear production, reduced tear stability, abnormal corneal staining and an increased presence of CD4+ cells in the gland; features that mimic the clinical manifestations of Sjögren’s syndrome [46]. The severity of the induced disease shows no signs of abatement and increases with time over a period of 6 months after disease induction (unpublished data). Disease in the rabbit model has also been established through remote site injection of the activated lymphocytes (unpublished data).
3.3 Prophylactic effect of IL-10 in vivo
IL-10 is a pleiotropic cytokine produced by Th-2 type T cells, B cells, monocytes and macrophages and is considered an immunoregulatory cytokine because of its inhibitory effects on the expression of a large spectrum of proinflammatory cyokines (such as chemokines, MHC-II molecules, costimulatory molecules and other inflammatory mediators [47–49]. IL-10 was also found to suppress antigen– stimulated proliferation of murine Th-1 cells [50,51] suggesting that endogenous IL-10 down regulates cell-mediated immune responses in the development of autoimmune diseases. Its effector functions include induction of a shift of T-cell cytokine expression from a Th1 to a Th2 profile [51], and attenuation of the production of pro-inflammatory cytokines by macrophages [52–54] and polymorphonuclear neutrophils [55]. IL-10 has proven to be useful in several preclinical models of autoimmune diseases [56,57], but its administration is difficult as it needs multiple injections. IL-10 gene therapy using viral vectors stands out as an alternative method. Several reports have established that IL-10 gene therapy inhibits autoimmune diseases [58,59]. To determine whether the expression of the interleukin-10 gene suppressed lymphocytic proliferation in an in vitro autologous mixed cell reaction [37], lacrimal gland acinar epithelial cells were transduced with Ad vector encoding viral IL- 10 (vIL-10) (Fig.1). The transduction of lacrimal epithelial cells with IL-10 diminished lymphocytic proliferation in the mixed cell reaction. IL-10 product was transiently expressed with maximal production during the first week, after which detectable amounts declined with each successive week. Using the rabbit model of induced dacryoadenitis we reported that Ad-mediated gene transfer and expression of viral IL-10 resulted in prophylaxis, with diminution of lacrimal gland immunopathology and ocular surface disease [19]. The transduced vIL-10 encoded by the Epstein-Barr virus shares 84% sequence homology with human IL-10 and mimics several of its immunosuppressive activities. However, unlike human IL-10, it lacks the stimulatory effects on natural killer cells and cytotoxic T cells [60,61]. Ad-mediated IL-10 gene transfer to the lacrimal gland of rabbits with induced disease resulted in transient expression and secretion of vIL-10 in tears for less than 2 weeks. Short-lived Admediated vIL-10 expression has also been reported by De Kozak et al in treatment of experimental autoimmune uveoretinitis induced in mice and rats [62]. In that study, expression of the vIL-10 gene was associated with significantly decreased size and number of immune infiltrates, including CD4+, CD18+, RTLA+ cells and MHC class II molecule expressing cells. However, a significant increase in the number of CD8+ cells was observed [19]. These effects are in agreement with published data showing that vIL-10 exerts its immunosuppressive properties by down-regulating the MHC class II molecule and proinflammatory cytokine expression without stimulating cytotoxic T cells [37,46,63]. Prophylactic treatment of rabbits with vIL-10 before injection of activated autoreactive lymphocytes protected tear production and tear stability compared to animals with induced disease that did not receive Ad encoding vIL-10 [19].
Figure 1.
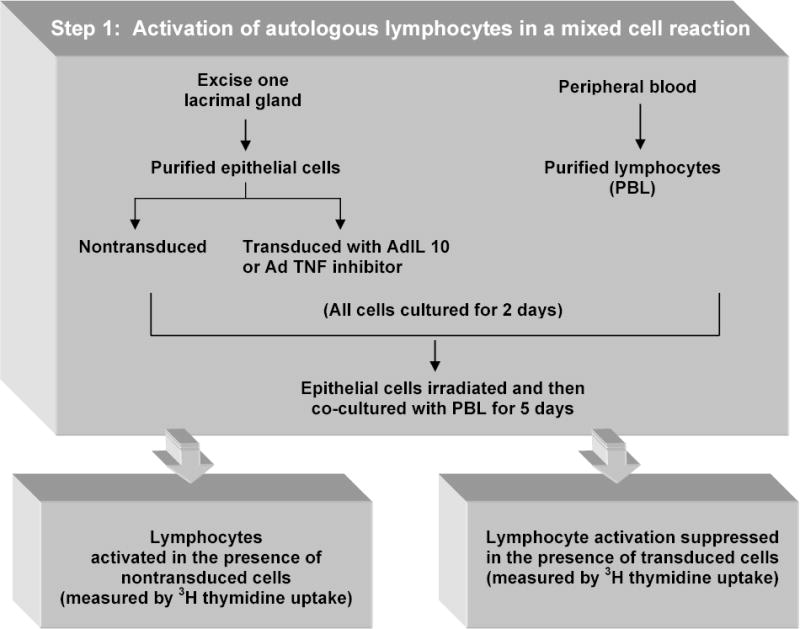
In vitro evaluation of anti-inflammatory gene therapy. This schematic describes an in vitro method for activating autologous lymphocytes in a mixed cell reaction and suppression of the lymphocyte activation by gene transfer. [Ref: 37].
3.4 Prophylactic effect of TNF-α in vivo
TNF-α, an interesting targets of immunotherapy, has been reported to play an important role in the pathogenesis of several immune mediated disorders, including rheumatoid arthritis [64,65] and Sjögren’s syndrome [66]. It is a pleiotropic inflammatory cytokine that promotes mononuclear cell infiltration in glands by inducing the secretion of several proinflammatory cytokines, expression of endothelial adhesion molecules, release matrix metalloproteinases from glandular epithelial cells, all of which promote the influx of mononuclear cells [67]. Also, TNF-α secretion by infiltrating T cells has been associated with apoptosis of glandular epithelial cells [68]. Since then many innovative strategies targeting TNF-α for the treatment of autoimmune diseases have been established. Inhibition of TNF-α has been used as an effective therapy in patients with rheumatoid arthritis and Crohn’s disease [69–71]. Neutralization of TNF-α was initially achieved using chimeric monoclonal antibodies in patients with rheumatoid arthritis. Kolls et al. reported the construction of an Ad vector encoding a chimeric TNF inhibitor, TNFRIp55-Ig [72]. The expression of TNFRIp55-Ig inhibitor gene suppressed lymphocytic proliferation in an in vitro autologous mixed cell reaction when the lacrimal epithelial cells were transduced with an Ad vector [37]. Expression of the AdTNFRIp55-Ig gene has been reported to successfully block the effect of TNF-α in several animal models [72–74]. The transgene product, a fusion protein formed by joining the human 55-kDa TNF receptor extracellular domain to a mouse IgG heavy chain, binds TNF by engaging two of its three receptor sites. Using this Ad vector we demonstrated a gene therapy treatment of rabbit lacrimal glands with established autoimmune dacryoadenitis [18,75]. The expression of TNFRIp55-Ig resulted in improvement of clinical features, which included increased basal tear production, increased tear stability and a reduction of corneal surface defects. These results suggest that the TNF inhibition altered the spectrum of cytokines in the local infiltrates to one that did not impair tear secretion. The therapeutic effect also reduced the intensity of immune cell infiltration in the gland (Fig.2).
Figure 2.
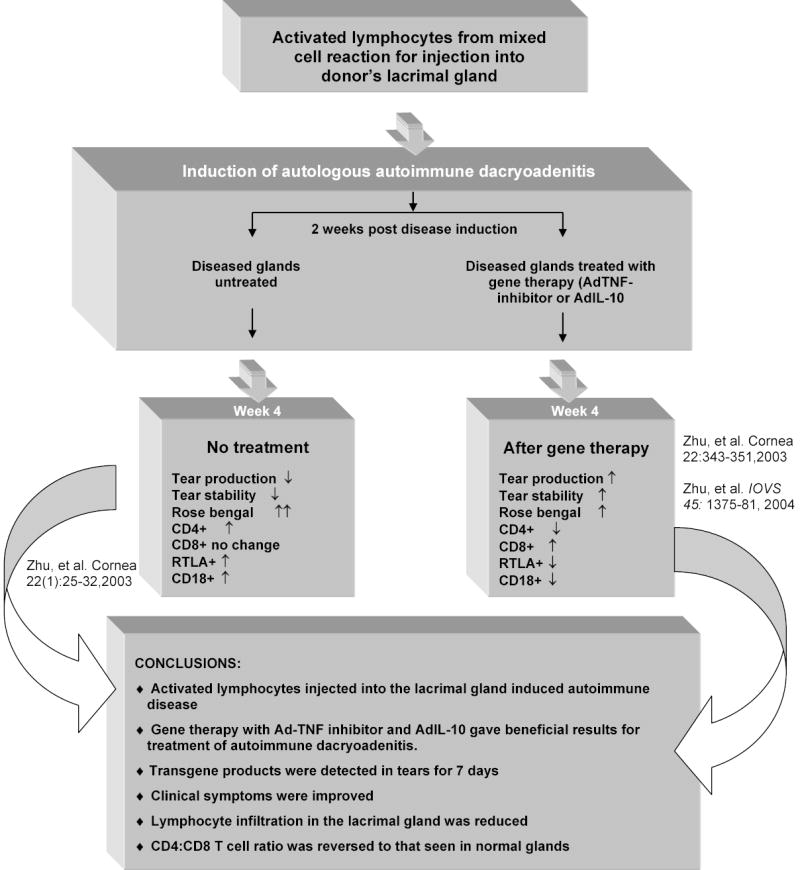
This schematic describes the method used to induce autoimmune dacryoadenitis and the effect of gene therapy on the clinical symptoms and gland histopathology [Ref: 46,75,19].
4. Ad-mediated gene transfer: In the study of lacrimal gland physiology
Since the initial reports of Ad-mediated expression of β-gal in lacrimal gland, we and others have subsequently optimized Ad-mediated gene transfer into lacrimal acini in primary culture, reaching a transduction efficiency of 80–90% in many cases with a relatively low multiplicity of infection (1–5). This achievement has been invaluable in the exploration of the function of different effectors in the lacrimal gland, suggesting that aspects of adenoviral uptake are unusually efficient in lacrimal acinar cells. Highlights from such studies are described below.
4.1 Androgen responses in primary cultures of lacrimal epithelial cells
Sjögren’s syndrome is most prevalent in women, with a gender ratio of 9:1. This huge gender difference may be partially attributable to the role that androgen-regulated transcription plays in lacrimal gland physiology [76–78]. Furthermore, androgens have been shown to suppress the inflammation in lacrimal glands of mouse models of Sjögren’s syndrome [79]. Studies also demonstrated the expression of various endogenous proteins including secretory component (SC), cystatin-related protein 1 and the C3 component of the prostatic binding protein in the primary lacrimal gland epithelial cell cultures were androgen responsive in male rats [80–82]. Vanaken et al. demonstrated androgen regulation in primary cultures of rat lacrimal gland through the use of recombinant adenoviral vectors [83]. The rat lacrimal gland primary cultures under androgen control were used as a homologous test system for tissue-specific transcription studies. By the use of two recombinant adenoviral vectors containing genomic fragments of the SC gene, they demonstrated the functionality of the sc promoter as well as its androgen regulation in this culture system.
4.2 Role of cytoplasmic dynein in apical secretory traffic in lacrimal acini
Conventional cytoplasmic dynein is a large multisubunit complex of ~1400 kDa consisting of two heavy chains and several intermediate and light chains [84]. The dynein heavy chains contains sites for MT binding and ATP hydrolysis, and is responsible for generation of mechanochemical force. Directed transport of vesicles by dynein requires a multiprotein complex called the dynactin complex [85]. Ad vectors with dynamitin constructs (Ad-Dynt) were used to study the participation of dynein-driven vesicle transport in stimulated secretory traffic to the apical membrane in lacrimal acini [86]. It was revealed that a cholinegeric agonist, carbachol, induced microtubule-dependent recruitment of cytoplasmic dyein and the dynactin complex into the subapical region and was inhibited by Admediated overexpression of dynamitin, suggesting that dynein activity drives this recruitment. Overexpression of dynamitin depletes subapical stores of rab3D, a member of the rab family of small GTP binding proteins that participate in membrane trafficking in eukaryotic cells, in resting acini, suggesting that dynein may also maintain this secretory vesicle population at the apical membrane. These data implicate cytoplasmic dynein in stimulated traffic to the apical plasma membrane in these secretory epithelial cells [86].
4.3 Role of PKC-α and PKC-ε on apical exocytosis of lacrimal acini
Protein kinase C (PKC) plays a major role in cholinergic and α1-adrenergic-stimulated lacrimal gland protein secretion [87]. However, as PKC is a family of at least 10 different isozymes, its role is somewhat complicated. The lacrimal gland contains at least four PKC isoforms, -α, -δ, -ε, and –λ [88]. Using adenoviral-mediated expression of PKC effectors and/or modified forms of these proteins, the major roles of PKC-α and PKC-ε on the apical exocytosis of lacrimal acini have been elucidated [89,90]. Hodges et al. demonstrated that PKC-α can be overexpressed using an adenoviral vector carrying the myristoylated PKC-α α constructs (myr-PKC α) in rat lacrimal gland acini [89]. Results showed that the overexpression of a constitutively active form of PKC-α increased basal protein secretion without altering Ca2+ handling in the lacrimal gland. The increase was dependent on the concentration of Ad used and therefore the amount of PKC-α expressed, implying that secretion can be stimulated by circumventing the release of neurotransmitters and instead through the activation of their receptors.
PKC-ε was first identified in association with actin as an effector of exocytosis in hippocampal neurons [91]. PKC-ε is known to influence cell adhesion and motility as it is associated with actin cytoskeleton reorganization [92–94]. However, little is known about the contribution of PKC-ε in regulation of actin filament remodeling in acinar exocytosis in epithelial cells. To investigate the involvement of apical actin remodeling in carbachol-stimulated exocytosis in reconstituted rabbit lacrimal acinar cells, Ad vectors with a dominant-negative (DN) PKC-ε constructs were used [90]. It was found that carbachol-stimulation increases PKC-ε association with apical actin filaments and actin-coated structures in lacrimal acini. To inhibit PKC-ε activity and probe its functional role in exocytosis, lacrimal acini were transduced at high efficiency with Ad vectors carrying the DN-PKC-ε constructs. Overexpression of PKC- ε resulted in profound changes in apical and basolateral actin filament organization in parallel with inhibition of the carbachol-stimulated secretion of protein and β- hexosaminidase. These data confirm the role of PKC-ε as an actin-binding protein recruited transiently to apical actin filaments and actin-coated structures, possibly, representing fusion intermediates, in carbachol-stimulated lacrimal acini. It was further established that its inhibition, through overexpression of DN-PKC-ε, stabilized actin-coated structures and correspondingly inhibited stimulated exocytosis of secretory products at the apical plasma membrane.
4.4 Role of actin and non-muscle myosin II in apical exocytosis of tear proteins
Green fluorescent protein (GFP)-actin has been used to measure the dynamics of actin in live cells [95–98]. Ad vectors encoding GFP-actin have been utilized to label the actin filament of lacrimal acini to obtain qualitative and quantitative measures of its dynamics [99]. The GFP-labeled apical actin filament array in lacrimal acini showed rapid carbachol-induced remodeling of the sub-apical actin network. Also, additional functional and morphological analyses of lacrimal acini exposed to the general myosin ATPase inhibitor, 2,3-butanedione monoxime and the more selective myosin light chain kinase inhibitor, demonstrated that the filamentous actin array beneath the apical plasma membrane of stimulated lacrimal acini participates actively in exocytosis, in conjunction with nonmuscle myosin II.
5. Ad-mediated gene transfer: Modulation of secretory functions by Adv capsids in lacrimal epithelia
Although replication-deficient Ad vectors are leading candidates for gene therapy, there is a paucity of data on the cellular effects associated with Ad binding, internalization and trafficking to the nucleus, particularly in epithelial cells that represent normal targets for Ad infection. To study these effects, lacrimal gland acinar cells were exposed to replication-defective Ad serotype 5 (Ad5) containing a reporter gene (green fluorescent protein (GFP) or β-galactosidase (LacZ) or UV-inactivated Ad virus in vitro [100]. The organization and function of the lacrimal acinar secretory pathway in the reconstituted acinus-like structures were investigated. Exposure of lacrimal acini to replication-defective Ad constructs at high transduction efficiency (>80%) with a multiplicity of infection (MOI) of 5 for 16–18 h elicited a marked dispersal of rab3D, from its normally apical enrichment, a change independent of altered rab3D expression or membrane association. Rab3D is associated with the large pool of mature secretory vesicles beneath the apical plasma membrane in lacrimal gland as well as pancreas and parotid gland [86,101,102]. The dispersal of apical rab3D occurred independently of effects on the cytoskeleton or other membrane compartments or decreased protein synthesis. Stimulation of the Ad-transduced cells with carbachol resulted in a significant decrease in the release of protein and the secretory product, β-hexosaminidase. Furthermore, exposure of lacrimal acini to UV-inactivated Ad also depleted rab3D-enriched secretory vesicles in parallel with the inhibition of carbachol-stimulated release of protein and β-hexosaminidase, though the extent of the dispersal and inhibition, respectively, by UV-inactivated virus was slightly less than that caused by mock UV-inactivated Ad. However, the effects on acinar secretory functions were directly related to the duration of exposure to the Ad capsid, as lacrimal acini exposed to Ad constructs for a period of 4 h at MOI of 5 and ~80% transduction efficiency caused a 50% reduction in apical rab3D labeling.
Viral capsid proteins, exterior proteins associated with the protein-rich coat around the viral core, have been utilized for second- and third- generation gene delivery systems [103–106]. These systems have eliminated the presence of a complete viral vector particle but depend on the use of viral capsid proteins to interact with the host factors to facilitate entry of associated DNA. To determine the cellular effects of capsid proteins on lacrimal acinar cells, isolated Ad penton protein and knob protein, the region of the fiber protein known to interact with the cellular receptor, CAR, in the absence of the rest of the virus were investigated [107]. These proteins which have been used in non-viral gene transfer technique to enhance gene transfer to HeLa and 293 cells reflect the established infection pathway of Ad vectors [105]. It was found that treatment of lacrimal acini with recombinant Ad penton protein resulted in an almost complete loss of rab3D-enriched secretory vesicles [107]. The process occurs in parallel with an uncoupling of the stimulated secretory response, resulting in significantly increased basal protein release and significantly decreased carbachol-stimulated protein release. Knob protein treatment did not elicit significant change in the basal and carbachol-stimulated release of bulk protein. Additionally, penton protein caused additional cytoskeletal changes over and above the effects elicited by Ad alone including loss of the abundant apical actin network and bundling/disorganization of microtubules. However, knob protein was not found to elicit detectable microtubule organization. These results suggest that the penton protein, and not knob/fiber, is responsible for the deleterious changes in lacrimal gland function associated with chronic Ad exposure and that knob/fiber are better choices potentially for non-viral gene delivery into lacrimal acini.
6. Retroviral-mediated gene transfer: Immortalization of rabbit lacrimal gland epithelial cells
Immortalized cell lines have been extensively used in basic research as they offer the possibility of an inexhaustible supply of cells. Immortalized cell lines possessing morphological characteristics and physiological functions similar to that of primary cells can serve as models of animal and human tissues. Many different types of immortalized cell lines have been established over the past few years through various methods and some of them have even been successfully substituted for primary cells in many bioartificial organ systems [108–111]. An immortalized lacrimal epithelial cell line will be of great value to study the intracellular signaling pathways and lacrimal gland-associated gene expression studies. Also, a successful immortalized lacrimal epithelial cell line could serve as a cellular component for the proposed bioartificial lacrimal gland device which we are attempting to create in our laboratory [112].
Earlier attempts to culture and propagate primary lacrimal gland cells for extended periods of time have met with limited success [113–115]. Nguyen et al. were the first to establish an immortalized lacrimal epithelial cell line in a rabbit model using an immortalizing amphotropic retroviral vector containing the E6 and E7 genes of the human papillomavirus by injecting the retroviral vector into the orbital lacrimal glands of normal New Zealand White rabbits [116]. Two days after injection, cells were isolated from the lacrimal glands and were plated onto Matrigel®-coated culture plates. The cultured cells flattened out and grew in a monolayer typical of epithelial cells with a cobblestone appearance and this morphology was retained even at higher passages (p36-58). Ultrastructurally, the immortalized cells showed numerous interdigitating villi in the intercellular spaces and components of the cytoplasm such as intermediate filaments were observed. They retained many characteristics of primary lacrimal gland epithelial cells, including production and secretory granules and pharmacological responses to stimulation with carbachol. The cells were also characterized by immunoreactivity and positive staining was attained for the transferrin receptor and transferrin. Another immortalized rabbit lacrimal epithelial cell line using the simian virus 40 T antigen (SV40) was also established. However, the morphological and physiological characteristics of this cell line have not been reported directly [117].
Studies have shown that generation of cell lines immortalized by the introduction of viral oncoproteins alone tend to lose the normal phenotype of primary cells over extended periods of time. Hence, the reliability of cell lines generated through ‘viral transformation’ is questionable. However, different types of human cells have been efficiently transduced, expanded and characterized through the expression of human telomerase reverse transcriptase (hTERT) [118–122]. Cells immortalized through this technique, maintain a stable genotype and retain critical phenotypic markers. Although, the expression of TERT might be species specific, Thomas et al. reported the first use of hTERT expression in experimental xenotransplantation using bovine adrenal cells immortalized by transducing plasmids encoded for hTERT and SV40 T antigen [123]. The immortalized cells transplanted into severe combined immunodeficient (SCID) mice also formed functional tissue when replaced for the animals’ own adrenal glands. However, no immortalized lacrimal epithelial cell line using hTERT has been reported so far.
7. Conclusions and future directions
Recombinant adenoviral vectors have been extensively evaluated in the lacrimal gland and are found to be the most efficient gene transfer technique available to transduce lacrimal epithelial cells [20,46,83,86]. However, one major drawback in using adenoviral vectors is that they provide an unstable transgene expression because they rarely integrate into the transduced cell’s genome while vectors such as AAV provide prolonged transgene expression [124]. However, the limited capacity for gene delivery with respect to insert size severely limits the utility of AAV vectors. Also of concern is the demonstration of altered secretory functions associated with chronic exposure of the Ad penton protein. Retroviral vectors have been extensively used for their high efficacy; however, they do not have the ability to transduce non-dividing cells, a major limitation that has restricted their clinical use to gene therapy involving haematopoietic cells, rapidly dividing tumor cells, or ex vivo gene therapy of cells that can be propagated in cell culture [125]. Lentiviral vectors possess the ability to transduce non-dividing cells; however, safety concerns and the non-specific integration in the host cell’s chromosome mitigate the usefulness of these vectors for gene delivery.
Although high transduction efficiency and a sustained transgene expression present clear advantages of a viral gene delivery system, the strong immune response that these vectors elicit may pose a safety threat to the patient and to the immediate surrounding environment [126,127]. As immunogenicity arises from the viral capsid proteins that mediate gene delivery, engineering viral capsid proteins with altered ability to evoke immunity but with the same ability to manipulate cellular uptake would be necessary to overcome these issues. Also, the biological processes that underlie the cellular uptake and intracellular processing mechanisms need to be better understood in the lacrimal gland in particular to promote target-cell specificity of these vectors. Recent advances in improved vector design and vector purification have resulted in newer generations of viral vectors [128–130]. However, an ideal viral vector for tissue-specific transduction with regulated gene expression still remains elusive. Future progresses in vector engineering will create the means for effective gene delivery and will overcome the impediments to successful gene therapy.
References
- 1.Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE, Acharya SA, Ellis J, London IM, Eaves CJ, Humphries RK, Beuzard Y, Nagel RL, Leboulch P. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- 2.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, Selz F, Hue C, Certain S, Casanova JL, Bousso P, Deist FL, Fischer A. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 3.Bolstad AI, Jonsson R. Gene therapeutics in Sjogren's syndrome. Expert Opin Biol Ther. 2005;5:763–772. doi: 10.1517/14712598.5.6.763. [DOI] [PubMed] [Google Scholar]
- 4.Adriaansen J, Vervoordeldonk MJ, Tak PP. Gene therapy as a therapeutic approach for the treatment of rheumatoid arthritis: innovative vectors and therapeutic genes. Rheumatology. 2006 Mar 29; doi: 10.1093/rheumatology/kel047. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 5.Giannoukakis N, Trucco M. Gene therapy for type 1 diabetes. Am J Ther. 2005;12:512– 528. doi: 10.1097/01.mjt.0000178774.39149.2d. [DOI] [PubMed] [Google Scholar]
- 6.Weiner LP, Louie KA, Atalla LR, Kochounian HH, Du J, Wei W, Hinton DR, Gordon EM, Anderson WF, McMillan M. Gene therapy in a murine model for clinical application to multiple sclerosis. Ann Neurol. 2004;55:390–399. doi: 10.1002/ana.10858. [DOI] [PubMed] [Google Scholar]
- 7.Zabner J, Couture LA, Gregory RJ, Graham SM, Smith AE, Welsh MJ. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]
- 8.Nathwani AC, McIntosh J, Davidoff AM. An update on gene therapy for hemophilia. Curr Hematol Rep. 2005;4:287–293. [PubMed] [Google Scholar]
- 9.Kootstra NA, Verma IM. Gene therapy with viral vectors. Annu Rev Pharmacol Toxicol. 2003;43:413–439. doi: 10.1146/annurev.pharmtox.43.100901.140257. [DOI] [PubMed] [Google Scholar]
- 10.Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80:35–47. [PubMed] [Google Scholar]
- 11.Bennet J. Commentary: an aye for eye gene therapy. Hum Gene Ther. 2006;17:177–179. doi: 10.1089/hum.2006.17.177. [DOI] [PubMed] [Google Scholar]
- 12.Lai YK, Sharma S, Lai CM, Brankov M, Constable IJ, Rakoczy PE. Virus-mediated secretion gene therapy--a potential treatment for ocular neovascularization. Adv Exp Med Biol. 2003;533:447–453. doi: 10.1007/978-1-4615-0067-4_57. [DOI] [PubMed] [Google Scholar]
- 13.Lai CM, Shen WY, Brankov M, Lai YK, Barnett NL, Lee SY, Yeo IY, Mathur R, Ho JE, Pineda P, Barathi A, Ang CL, Constable IJ, Rakoczy EP. Long-term evaluation of AAV-mediated sFlt-1 gene therapy for ocular neovascularization in mice and monkeys. Mol Ther. 2005;12:659–668. doi: 10.1016/j.ymthe.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Campochiaro PA, Nguyen QD, Shah SM, Klein ML, Holz E, Frank RN, Saperstein DA, Gupta A, Stout JT, Macko J, DiBartolomeo R, Wei LL. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006;17:167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 15.Fang IM, Lin CP, Yang CH, Chiang BL, Yang CM, Chau LY, Chen MS. Inhibition of experimental autoimmune anterior uveitis by adenovirus-mediated transfer of the interleukin-10 gene. J Ocul Pharmacol Ther. 2005;21:420–428. doi: 10.1089/jop.2005.21.420. [DOI] [PubMed] [Google Scholar]
- 16.Le Gat L, Gogat K, Bouquet C, Saint-Geniez M, Darland D, Van Den Berghe L, Marchant D, Provost A, Perricaudet M, Menasche M, Abitbol M. In vivo adenovirus-mediated delivery of a uPA/uPAR antagonist reduces retinal neovascularization in a mouse model of retinopathy. Gene Ther. 2003;10:2098–2103. doi: 10.1038/sj.gt.3302122. [DOI] [PubMed] [Google Scholar]
- 17.Mohan RR, Sharma A, Netto MV, Sinha S, Wilson SE. Gene therapy in the cornea. Prog Retin Eye Res. 2005;24:537–559. doi: 10.1016/j.preteyeres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Trousdale MD, Zhu Z, Stevenson D, Schechter JE, Ritter T, Mircheff AK. Expression of TNF inhibitor gene in the lacrimal gland promotes recovery of tear production and tear stability and reduced immunopathology in rabbits with induced autoimmune dacryoadenitis. J Autoimmune Dis. 2005;2:6. doi: 10.1186/1740-2557-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Z, Stevenson D, Schechter JE, Mircheff AK, Ritter T, Labree L, Trousdale MD. Prophylactic effect of IL-10 gene transfer on induced autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci. 2004;45:1375–1381. doi: 10.1167/iovs.03-0755. [DOI] [PubMed] [Google Scholar]
- 20.Banin E, Obolensky A, Piontek E, Falk H, Pikarsky E, Pe'er J, Panet A, Chowers I. Gene delivery by viral vectors in primary cultures of lacrimal gland tissue. Invest Ophthalmol Vis Sci. 2003;44:1529–1533. doi: 10.1167/iovs.02-0529. [DOI] [PubMed] [Google Scholar]
- 21.Hunt S, Spitznas M, Seifert P, Rauwolf M. Organ culture of human main and accessory lacrimal glands and their secretory behaviour. Exp Eye Res. 1996;62:541–554. doi: 10.1006/exer.1996.0064. [DOI] [PubMed] [Google Scholar]
- 22.Lemp M. Report of the National Eye Institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995;21:222–226. [PubMed] [Google Scholar]
- 23.Pillemer SR, Matteson EL, Jacobsson LT, Martens PB, Melton LJ, O'Fallon WM, Fox PC. Incidence of physician-diagnosed primary Sjogren syndrome in residents of Olmsted County, Minnesota. Mayo Clin Proc. 2001;76:593–599. doi: 10.4065/76.6.593. [DOI] [PubMed] [Google Scholar]
- 24.Fox RI. Sjogren's syndrome. Curr Opin Rheumatol. 1995;7:409–416. doi: 10.1097/00002281-199509000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan DA, Wickham DA, Krenzer KL, Rocha EM, Toda I. Aqueous tear deficiency in Sjögren’s syndrome: possible causes and potential treatment. In: Pleyer U, Hartmann C, Sterry W, editors. Oculodermal diseases-immunology of bullous oculo-muco-cutaneous disorders. Buren, The Netherlands: Aeolus Press; 1997. pp. 95–152. [Google Scholar]
- 26.Tsubota K, Toda I, Yagi Y, Ogawa Y, Ono M, Yoshino K. Three different types of dry eye syndrome. Cornea. 1994;13:202–209. doi: 10.1097/00003226-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Fox RI, Kang HI. Pathogenesis of Sjogren's syndrome. Rheum Dis Clin North Am. 1992;18:517–538. [PubMed] [Google Scholar]
- 28.Esch TR. Pathogenetic factors in Sjogren's syndrome: recent developments. Crit Rev Oral Biol Med. 2001;12:244–251. doi: 10.1177/10454411010120030301. [DOI] [PubMed] [Google Scholar]
- 29.Kolkowski EC, Reth P, Pelusa F, Bosch J, Pujol-Borrell R, Coll J, Jaraquemada D. Th1 predominance and perforin expression in minor salivary glands from patients with primary Sjogren's syndrome. J Autoimmun. 1999;13:155–162. doi: 10.1006/jaut.1999.0289. [DOI] [PubMed] [Google Scholar]
- 30.Sun D, Emmert-Buck MR, Fox PC. Differential cytokine mRNA expression in human labial minor salivary glands in primary Sjogren's syndrome. Autoimmunity. 1998;28:125–137. doi: 10.3109/08916939808996281. [DOI] [PubMed] [Google Scholar]
- 31.Magnusson V, Nakken B, Bolstad AI, Alarcon-Riquelme ME. Cytokine polymorphisms in systemic lupus erythematosus and Sjogren's syndrome. Scand J Immunol. 2001;54:55–61. doi: 10.1046/j.1365-3083.2001.00965.x. [DOI] [PubMed] [Google Scholar]
- 32.Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 33.Boumba D, Skopouli FN, Moutsopoulos HM. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjogren's syndrome. Br J Rheumatol. 1995;34:326–333. doi: 10.1093/rheumatology/34.4.326. [DOI] [PubMed] [Google Scholar]
- 34.Mitsias DI, Tzioufas AG, Veiopoulou C, Zintzaras E, Tassios IK, Kogopoulou O, Moutsopoulos HM, Thyphronitis G. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin Exp Immunol. 2002;128:562–568. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joosten LA, Helsen MM, van de Loo FA, van den Berg WB. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice: A comparative study using anti-TNF alpha, anti-IL-1 alpha/beta, and IL-1Ra. Arthritis Rheum. 1996;39:797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- 36.Joosten LA, Lubberts E, Durez P, Helsen MM, Jacobs MJ, Goldman M, van den Berg WB. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis: Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40:249–260. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z, Stevenson D, Ritter T, Schechter JE, Mircheff AK, Kaslow HR, Trousdale MD. Expression of IL-10 and TNF-inhibitor genes in lacrimal gland epithelial cells suppresses their ability to activate lymphocytes. Cornea. 2002;21:210–214. doi: 10.1097/00003226-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 38.Yeoman CM, Franklin CD. Assessment of sulphasalazine as a treatment modality in Sjogren's disease in NZB/NZW F1 hybrid mice. Clin Exp Rheumatol. 1996;14:53–57. [PubMed] [Google Scholar]
- 39.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 40.Liu SH, Prendergast RA, Silverstein AM. Experimental autoimmune dacryoadenitis. I. Lacrimal gland disease in the rat. Invest Ophthalmol Vis Sci. 1987;28:270–275. [PubMed] [Google Scholar]
- 41.Kaswan RL, Salisbury MA, Ward DA. Spontaneous canine keratoconjunctivitis sicca. A useful model for human keratoconjunctivitis sicca: treatment with cyclosporine eye drops. Arch Ophthalmol. 1989;107:1210–1216. doi: 10.1001/archopht.1989.01070020276038. [DOI] [PubMed] [Google Scholar]
- 42.Guo Z, Azzarolo AM, Schechter JE, Warren DW, Wood RL, Mircheff AK, Kaslow HR. Lacrimal gland epithelial cells stimulate proliferation in autologous lymphocyte preparations. Exp Eye Res. 2000;71:11–22. doi: 10.1006/exer.2000.0856. [DOI] [PubMed] [Google Scholar]
- 43.Guo Z, Song D, Azzarolo AM, Schechter JE, Warren DW, Wood RL, Mircheff AK, Kaslow HR. Autologous lacrimal-lymphoid mixed-cell reactions induce dacryoadenitis in rabbits. Exp Eye Res. 2000;71:23–31. doi: 10.1006/exer.2000.0855. [DOI] [PubMed] [Google Scholar]
- 44.Wood RL, Trousdale MD, Stevenson D, Azzarolo AM, Mircheff AK. Adenovirus infection of the cornea causes histopathologic changes in the lacrimal gland. Curr Eye Res. 1997;16:459–466. doi: 10.1076/ceyr.16.5.459.7046. [DOI] [PubMed] [Google Scholar]
- 45.Mircheff AK, Gierow JP, Lee LM, Lambert RW, Akashi RH, Hofman FM. Class II antigen expression by lacrimal epithelial cells: An updated working hypothesis for antigen presentation by epithelial cells. Invest Ophthalmol Vis Sci. 1991;32:2302–2310. [PubMed] [Google Scholar]
- 46.Zhu Z, Stevenson D, Schechter JE, Mircheff AK, Atkinson R, Trousdale MD. Lacrimal histopathology and ocular surface disease in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:25–32. doi: 10.1097/00003226-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pretolani M. Interleukin-10: an anti-inflammatory cytokine with therapeutic potential. Clin Exp Allergy. 1999;29:1164–1171. doi: 10.1046/j.1365-2222.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- 49.Mottonen M, Isomaki P, Saario R, Toivanen P, Punnonen J, Lassila O. Interleukin-10 inhibits the capacity of synovial macrophages to function as antigen-presenting cells. Br J Rheumatol. 1998;37:1207–1214. [PubMed] [Google Scholar]
- 50.Macatonia SE, Doherty TM, Knight SC, O'Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993;150:3755–3765. [PubMed] [Google Scholar]
- 51.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 55.Cassatella MA, Meda L, Bonora S, Ceska M, Constantin G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes: Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178:2207–2211. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wogensen L, Lee MS, Sarvetnick N. Production of interleukin 10 by islet cells accelerates immune-mediated destruction of beta cells in nonobese diabetic mice. J Exp Med. 1994;179:1379–1384. doi: 10.1084/jem.179.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bai XF, Zhu J, Zhang GX, Kaponides G, Hojeberg B, van der Meide PH, Link H. IL-10 suppresses experimental autoimmune neuritis and down-regulates TH1-type immune responses. Clin Immunol Immunopathol. 1997;83:117–126. doi: 10.1006/clin.1997.4331. [DOI] [PubMed] [Google Scholar]
- 58.Goudy K, Song S, Wasserfall C, Zhang YC, Kapturczak M, Muir A, Powers M, Scott-Jorgensen M, Campbell-Thompson M, Crawford JM, Ellis TM, Flotte TR, Atkinson MA. Adeno-associated virus vector-mediated IL-10 gene delivery prevents type 1 diabetes in NOD mice. Proc Natl Acad Sci U S A. 2001;98:13913–13918. doi: 10.1073/pnas.251532298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ko KS, Lee M, Koh JJ, Kim SW. Combined administration of plasmids encoding IL-4 and IL-10 prevents the development of autoimmune diabetes in nonobese diabetic mice. Mol Ther. 2001;4:313–316. doi: 10.1006/mthe.2001.0459. [DOI] [PubMed] [Google Scholar]
- 60.Go NF, Castle BE, Barrett R, Kastelein R, Dang W, Mosmann TR, Moore KW, Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990;172:1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vieira P, de Waal-Malefyt R, Dang MN, Johnson KE, Kastelein R, Fiorentino DF, deVries JE, Roncarolo MG, Mosmann TR, Moore KW. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991;88:1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Kozak Y, Thillaye-Goldenberg B, Naud MC, Da Costa AV, Auriault C, Verwaerde C. Inhibition of experimental autoimmune uveoretinitis by systemic and subconjunctival adenovirus-mediated transfer of the viral IL-10 gene. Clin Exp Immunol. 2002;130:212–223. doi: 10.1046/j.1365-2249.2002.01969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma Y, Thornton S, Duwel LE, Boivin GP, Giannini EH, Leiden JM, Bluestone JA, Hirsch R. Inhibition of collagen-induced arthritis in mice by viral IL-10 gene transfer. J Immunol. 1998;161:1516–1524. [PubMed] [Google Scholar]
- 64.Chu CQ, Field M, Feldmann M, Maini RN. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- 65.Firestein GS, Alvaro-Gracia JM, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347–3353. [PubMed] [Google Scholar]
- 66.Matsumura R, Umemiya K, Kagami M, Tomioka H, Tanabe E, Sugiyama T, Sueishi M, Kayagaki N, Yagita H, Okumura K. Expression of TNF-related apoptosis inducing ligand (TRAIL) on infiltrating cells and of TRAIL receptors on salivary glands in patients with Sjogren's syndrome. Clin Exp Rheumatol. 2002;20:791–798. [PubMed] [Google Scholar]
- 67.Moser R, Schleiffenbaum B, Groscurth P, Fehr J. Interleukin 1 and tumor necrosis factor stimulate human vascular endothelial cells to promote transendothelial neutrophil passage. J Clin Invest. 1989;83:444–455. doi: 10.1172/JCI113903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fox RI, Tornwall J, Michelson P. Current issues in the diagnosis and treatment of Sjogren's syndrome. Curr Opin Rheumatol. 1999;11:364–371. doi: 10.1097/00002281-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Finck BK. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 70.Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M, Harriman GR, Maini RN. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 71.Vermeire S, Noman M, Van Assche G, Baert F, Van Steen K, Esters N, Joossens S, Bossuyt X, Rutgeerts P. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn's disease: a prospective cohort study. Gastroenterology. 2003;125:32–39. doi: 10.1016/s0016-5085(03)00701-7. [DOI] [PubMed] [Google Scholar]
- 72.Kolls J, Peppel K, Silva M, Beutler B. Prolonged and effective blockade of tumor necrosis factor activity through adenovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1994;91:215–219. doi: 10.1073/pnas.91.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ritter T, Schroder G, Risch K, Vergopoulos A, Shean MK, Kolls J, Brock J, Lehmann M, Volk HD. Ischemia/reperfusion injury-mediated down-regulation of adenovirus-mediated gene expression in a rat heart transplantation model is inhibited by co-application of a TNFRp55-Ig chimeric construct. Gene Ther. 2000;7:1238–1243. doi: 10.1038/sj.gt.3301222. [DOI] [PubMed] [Google Scholar]
- 74.Brennan FM, Feldmann M. Cytokines in autoimmunity. Curr Opin Immunol. 1996;8:872–877. doi: 10.1016/s0952-7915(96)80018-5. [DOI] [PubMed] [Google Scholar]
- 75.Zhu Z, Stevenson D, Schechter JE, Mircheff AK, Crow RW, Atkinson R, Ritter T, Bose S, Trousdale MD. Tumor necrosis factor inhibitor gene expression suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:343–351. doi: 10.1097/00003226-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 76.Sullivan DA, Sato EH. Potential therapeutic approach for the hormonal treatment of lacrimal gland dysfunction in Sjogren's syndrome. Clin Immunol Immunopathol. 1992;64:9–16. doi: 10.1016/0090-1229(92)90052-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sullivan DA, Wickham LA, Rocha EM, Krenzer KL, Sullivan BD, Steagall R, Cermak JM, Dana MR, Ullman MD, Sato EH, Gao J, Rocha FJ, Ono M, Silveira LA, Lambert RW, Kelleher RS, Tolls DB, Toda I. Androgens and dry eye in Sjogren's syndrome. Ann N Y Acad Sci. 1999;876:312–324. doi: 10.1111/j.1749-6632.1999.tb07656.x. [DOI] [PubMed] [Google Scholar]
- 78.Sullivan DA, Krenzer KL, Sullivan BD, Tolls DB, Toda I, Dana MR. Does androgen insufficiency cause lacrimal gland inflammation and aqueous tear deficiency? Invest Ophthalmol Vis Sci. 1999;40:1261–1265. [PubMed] [Google Scholar]
- 79.Sullivan DA, Edwards JA. Androgen stimulation of lacrimal gland function in mouse models of Sjogren's syndrome. J Steroid Biochem Mol Biol. 1997;60:237–245. doi: 10.1016/s0960-0760(96)00190-2. [DOI] [PubMed] [Google Scholar]
- 80.Sullivan DA, Kelleher RS, Vaerman JP, Hann IE. Androgen regulation of secretory component synthesis by lacrimal gland acinar cells in vitro. J Immunol. 1990;145:4238–4244. [PubMed] [Google Scholar]
- 81.Rocha FJ, Wickham LA, Pena JD, Gao J, Ono M, Lambert RW, Kelleher RS, Sullivan DA. Influence of gender and the endocrine environment on the distribution of androgen receptors in the lacrimal gland. J Steroid Biochem Mol Biol. 1993;46:737–749. doi: 10.1016/0960-0760(93)90314-m. [DOI] [PubMed] [Google Scholar]
- 82.Vercaeren I, Vanaken H, Devos A, Peeters B, Verhoeven G, Heyns W. Androgens transcriptionally regulate the expression of cystatin-related protein and the C3 component of prostatic binding protein in rat ventral prostate and lacrimal gland. Endocrinology. 1996;137:4713–4720. doi: 10.1210/endo.137.11.8895338. [DOI] [PubMed] [Google Scholar]
- 83.Vanaken H, Gerard RD, Verrijdt G, Haelens A, Rombauts W, Claessens F. Tissue-specific androgen responses in primary cultures of lacrimal epithelial cells studied by adenoviral gene transfer. J Steroid Biochem Mol Biol. 2001;78:319–328. doi: 10.1016/s0960-0760(01)00113-3. [DOI] [PubMed] [Google Scholar]
- 84.Holzbaur EL, Vallee RB. DYNEINS: molecular structure and cellular function. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- 85.Gill SR, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Jerdeva G, Yarber FA, da Costa SR, Xie J, Qian L, Rose CM, Mazurek C, Kasahara N, Mircheff AK, Hamm-Alvarez SF. Cytoplasmic dynein participates in apically targeted stimulated secretory traffic in primary rabbit lacrimal acinar epithelial cells. J Cell Sci. 2003;116:2051–2065. doi: 10.1242/jcs.00398. [DOI] [PubMed] [Google Scholar]
- 87.Zoukhri D, Hodges RR, Sergheraert C, Dartt DA. Cholinergic-induced Ca2+ elevation in rat lacrimal gland acini is negatively modulated by PKCdelta and PKCepsilon. Invest Ophthalmol Vis Sci. 2000;41:386–392. [PubMed] [Google Scholar]
- 88.Zoukhri D, Hodges RR, Sergheraert C, Toker A, Dartt DA. Lacrimal gland PKC isoforms are differentially involved in agonist-induced protein secretion. Am J Physiol. 1997;272:263–269. doi: 10.1152/ajpcell.1997.272.1.C263. [DOI] [PubMed] [Google Scholar]
- 89.Hodges RR, Raddassi I, Zoukhri D, Toker A, Kazlauskas A, Dartt DA. Effect of overexpression of constitutively active PKCalpha on rat lacrimal gland protein secretion. Invest Ophthalmol Vis Sci. 2004;45:3974–3981. doi: 10.1167/iovs.04-0508. [DOI] [PubMed] [Google Scholar]
- 90.Jerdeva GV, Yarber FA, Trousdale MD, Rhodes CJ, Okamoto CT, Dartt DA, Hamm-Alvarez SF. Dominant-negative PKC-epsilon impairs apical actin remodeling in parallel with inhibition of carbachol-stimulated secretion in rabbit lacrimal acini. Am J Physiol Cell Physiol. 2005;289:1052–1068. doi: 10.1152/ajpcell.00546.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prekeris R, Mayhew MW, Cooper JB, Terrian DM. Identification and localization of an actin-binding motif that is unique to the epsilon isoform of protein kinase C and participates in the regulation of synaptic function. J Cell Biol. 1996;132:77–90. doi: 10.1083/jcb.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Akita Y. Protein kinase C-epsilon (PKC-epsilon): its unique structure and function. J Biochem. 2002;132:847–852. doi: 10.1093/oxfordjournals.jbchem.a003296. [DOI] [PubMed] [Google Scholar]
- 93.Besson A, Wilson TL, Yong VW. The anchoring protein RACK1 links protein kinase Cepsilon to integrin beta chains. Requirements for adhesion and motility. J Biol Chem. 2002;277:22073–22084. doi: 10.1074/jbc.M111644200. [DOI] [PubMed] [Google Scholar]
- 94.Song JC, Rangachari PK, Matthews JB. Opposing effects of PKCalpha and PKCepsilon on basolateral membrane dynamics in intestinal epithelia. Am J Physiol Cell Physiol. 2002;283:1548–1556. doi: 10.1152/ajpcell.00105.2002. [DOI] [PubMed] [Google Scholar]
- 95.Choidas A, Jungbluth A, Sechi A, Murphy J, Ullrich A, Marriott G. The suitability and application of a GFP-actin fusion protein for long-term imaging of the organization and dynamics of the cytoskeleton in mammalian cells. Eur J Cell Biol. 1998;77:81–90. doi: 10.1016/S0171-9335(98)80075-7. [DOI] [PubMed] [Google Scholar]
- 96.Tyska MJ, Mooseker MS. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys J. 2002;82:1869–1883. doi: 10.1016/S0006-3495(02)75537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loomis PA, Zheng L, Sekerkova G, Changyaleket B, Mugnaini E, Bartles JR. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J Cell Biol. 2003;163:1045–1055. doi: 10.1083/jcb.200309093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol. 2004;164:887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jerdeva GV, Wu K, Yarber FA, Rhodes CJ, Kalman D, Schechter JE, Hamm-Alvarez SF. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J Cell Sci. 2005;118:4797–4812. doi: 10.1242/jcs.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y, Xie J, Yarber FA, Mazurek C, Trousdale MD, Medina-Kauwe LK, Kasahara N, Hamm-Alvarez SF. Adenoviral capsid modulates secretory compartment organization and function in acinar epithelial cells from rabbit lacrimal gland. Gene Ther. 2004;11:970–981. doi: 10.1038/sj.gt.3302247. [DOI] [PubMed] [Google Scholar]
- 101.Ohnishi H, Ernst SA, Wys N, McNiven M, Williams JA. Rab3D localizes to zymogen granules in rat pancreatic acini and other exocrine glands. Am J Physiol. 1996;271:531–538. doi: 10.1152/ajpgi.1996.271.3.G531. [DOI] [PubMed] [Google Scholar]
- 102.Valentijn JA, Sengupta D, Gumkowski FD, Tang LH, Konieczko EM, Jamieson JD. Rab3D localizes to secretory granules in rat pancreatic acinar cells. Eur J Cell Biol. 1996;70:33–41. [PubMed] [Google Scholar]
- 103.Fender P, Ruigrok RW, Gout E, Buffet S, Chroboczek J. Adenovirus dodecahedron, a new vector for human gene transfer. Nat Biotechnol. 1997;15:52–56. doi: 10.1038/nbt0197-52. [DOI] [PubMed] [Google Scholar]
- 104.Medina-Kauwe LK, Kasahara N, Kedes L. 3PO, a novel nonviral gene delivery system using engineered Ad5 penton proteins. Gene Ther. 2001;8:795–803. doi: 10.1038/sj.gt.3301448. [DOI] [PubMed] [Google Scholar]
- 105.Medina-Kauwe LK, Maguire M, Kasahara N, Kedes L. Nonviral gene delivery to human breast cancer cells by targeted Ad5 penton proteins. Gene Ther. 2001;8:1753–1761. doi: 10.1038/sj.gt.3301583. [DOI] [PubMed] [Google Scholar]
- 106.Mastrobattista E, van der Aa MA, Hennink WE, Crommelin DJ. Artificial viruses: a nanotechnological approach to gene delivery. Nat Rev Drug Discov. 2006;5:115–21. doi: 10.1038/nrd1960. [DOI] [PubMed] [Google Scholar]
- 107.Hamm-Alvarez SF, Xie J, Wang Y, Medina-Kauwe LK. Modulation of secretory functions in epithelia by adenovirus capsid proteins. J Control Release. 2003;93:129–140. doi: 10.1016/j.jconrel.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 108.Sussman NL, Gislason GT, Kelly JH. Extracorporeal liver support: Application to fulminant hepatic failure. J Clin Gastroenterol. 1994;18:320–324. [PubMed] [Google Scholar]
- 109.Kobayashi N, Okitsu T, Nakaji S, Tanaka N. Hybrid bioartificial liver: establishing a reversibly immortalized human hepatocyte line and developing a bioartificial liver for practical use. J Artif Organs. 2003;6:236–244. doi: 10.1007/s10047-003-0235-7. [DOI] [PubMed] [Google Scholar]
- 110.Ozgen N, Terashima M, Aung T, Sato Y, Isoe C, Kakuta T, Saito A. Evaluation of long-term transport ability of a bioartificial renal tubule device using LLC-PK1 cells. Nephrol Dial Transplant. 2004;19:2198–2207. doi: 10.1093/ndt/gfh399. [DOI] [PubMed] [Google Scholar]
- 111.Saito A. Research into the development of a wearable bioartificial kidney with a continuous hemofilter and a bioartificial tubule device using tubular epithelial cells. Artif Organs. 2004;28:58–63. doi: 10.1111/j.1525-1594.2004.07323.x. [DOI] [PubMed] [Google Scholar]
- 112.Selvam S, Thomas PB, Trousdale MD, Stevenson D, Schechter JE, Mircheff AK, Jacob JT, Smith RE, Yiu SC. Journal of Biomedical Materials Research. Part B: Applied Biomaterials. Towards Tissue-Engineered Tear Secretory System: Functional Lacrimal Gland Acinar Cells Cultivated on Matrix Protein-Coated Substrata. in press. [DOI] [PubMed] [Google Scholar]
- 113.Hann LE, Kelleher RS, Sullivan DA. Influence of culture conditions on the androgen control of secretory component production by acinar cells from the rat lacrimal gland. Invest Ophthalmol Vis Sci. 1991;32:2610–2621. [PubMed] [Google Scholar]
- 114.Meneray MA, Fields TY, Bromberg BB, Moses RL. Morphology and physiologic responsiveness of cultured rabbit lacrimal acini. Invest Ophthalmol Vis Sci. 1994;35:4144–4158. [PubMed] [Google Scholar]
- 115.Rismondo V, Gierow JP, Lambert RW, Golchini K, Feldon SE, Mircheff AK. Rabbit lacrimal acinar cells in primary culture: morphology and acute responses to cholinergic stimulation. Invest Ophthalmol Vis Sci. 1994;35:1176–1183. [PubMed] [Google Scholar]
- 116.Nguyen DH, Beuerman RW, Halbert CL, Ma Q, Sun G. Characterization of immortalized rabbit lacrimal gland epithelial cells. In Vitro Cell Dev Biol Anim. 1999;35:198–204. doi: 10.1007/s11626-999-0027-3. [DOI] [PubMed] [Google Scholar]
- 117.Iserovich P, Yiming M, Wang Z, Bildin VN, Reinach PS, Fischbarg J. Epidermal growth factor stimulates fluid transport in SV40 transformed rabbit lacrimal gland cells. Adv Exp Med Biol. 2002;506:243–247. doi: 10.1007/978-1-4615-0717-8_33. [DOI] [PubMed] [Google Scholar]
- 118.Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 119.Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, Dimaio JM, Milchgrub S, Smith AL, Souza RF, Gilbey L, Zhang X, Gandia K, Vaughan MB, Wright WE, Gazdar AF, Shay JW, Minna JD. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 120.Shao R, Guo X. Human microvascular endothelial cells immortalized with human telomerase catalytic protein: a model for the study of in vitro angiogenesis. Biochem Biophys Res Commun. 2004;321:788–794. doi: 10.1016/j.bbrc.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 121.Kamata N, Fujimoto R, Tomonari M, Taki M, Nagayama M, Yasumoto S. Immortalization of human dental papilla, dental pulp, periodontal ligament cells and gingival fibroblasts by telomerase reverse transcriptase. J Oral Pathol Med. 2004;33:417–423. doi: 10.1111/j.1600-0714.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- 122.Xiaoxue Y, Zhongqiang C, Zhaoqing G, Gengting D, Qingjun M, Shenwu W. Immortalization of human osteoblasts by transferring human telomerase reverse transcriptase gene. Biochem Biophys Res Commun. 2004;315:643–651. doi: 10.1016/j.bbrc.2004.01.102. [DOI] [PubMed] [Google Scholar]
- 123.Thomas M, Yang L, Hornsby PJ. Formation of functional tissue from transplanted adrenocortical cells expressing telomerase reverse transcriptase. Nat Biotechnol. 2000;18:39–42. doi: 10.1038/71894. [DOI] [PubMed] [Google Scholar]
- 124.Harui A, Suzuki S, Kochanek S, Mitani K. Frequency and stability of chromosomal integration of adenovirus vectors. J Virol. 1999;73:6141–6146. doi: 10.1128/jvi.73.7.6141-6146.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1:91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 126.Raper SE, Yudkoff M, Chirmule N, Gao GP, Nunes F, Haskal ZJ, Furth EE, Propert KJ, Robinson MB, Magosin S, Simoes H, Speicher L, Hughes J, Tazelaar J, Wivel NA, Wilson JM, Batshaw ML. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther. 2002;13:163–175. doi: 10.1089/10430340152712719. [DOI] [PubMed] [Google Scholar]
- 127.Check E. A tragic setback. Nature. 2002;420:116–118. doi: 10.1038/420116a. [DOI] [PubMed] [Google Scholar]
- 128.Auricchio A, Kobinger G, Anand V, Hildinger M, O'Connor E, Maguire AM, Wilson JM, Bennett J. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet. 2001;10:3075–3081. doi: 10.1093/hmg/10.26.3075. [DOI] [PubMed] [Google Scholar]
- 129.Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, Samulski RJ. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Von Seggern DJ, Aguilar E, Kinder K, Fleck SK, Gonzalez Armas JC, Stevenson SC, Ghazal P, Nemerow GR, Friedlander M. In vivo transduction of photoreceptors or ciliary body by intravitreal injection of pseudotyped adenoviral vectors. Mol Ther. 2003;7:27–34. doi: 10.1016/s1525-0016(02)00030-8. [DOI] [PubMed] [Google Scholar]


