Abstract
Background:
The effect of abrupt weaning, advocated as a safe transition from exclusive breastfeeding in HIV-exposed children, on the quantity of HIV viral load in breast milk (BMVL) is not known.
Objectives:
To determine the effect of abrupt cessation of breastfeeding on serum prolactin, pumped breast milk volume and BMVL obtained 2 weeks after rapid weaning in HIV-infected women.
Methods:
Women enrolled in a prospective study (ZEBS) were randomized to abruptly wean at 20 weeks postpartum or continue exclusive breastfeeding. Breast milk was obtained at 22 weeks by electric breast pump over 10 min from 222 women who had either weaned or continued to breastfeed. Pre- and post-pumping prolactin was measured. BMVL was measured at 20 and 22 weeks in 71 randomly selected women from both groups.
Results:
Baseline prolactin and breast milk volume was significantly lower among women who had weaned. Detectable (68 versus 42%; P 0.03) and median BMVL (448 versus < 50 copies/ml; P = 0.005) was significantly higher = among those who had weaned in comparison with those who were still breastfeeding and was significantly higher in the same women after weaning compared with 2 weeks earlier (P = 0.001). Conclusions: BMVL is substantially higher after rapid weaning and this may pose an increased risk of HIV transmission if children resume breastfeeding after a period of cessation. Increases in BMVL with differing degrees of mixed feeding needs to be assessed.
Keywords: HIV, breastfeeding, breast milk, viral load, weaning, prolactin mother-to-child transmission, postnatal HIV transmission
Introduction
The fact that HIV can be transmitted through breast milk poses a serious dilemma for women living in HIV-endemic and resource-poor areas. The benefits of exclusive breastfeeding (in the absence of HIV) are firmly established, and its protective effect against the major childhood killers, including diarrhea and pneumonia, make it one of the central interventions recommended for improving child survival [1]. However, in areas of high HIV prevalence where the protection afforded by exclusive breastfeeding might be the most critical, it could have a net harmful effect, producing more HIV transmission than fatal child infections averted.
A partial solution to this problem was suggested by the preliminary observation that exclusive breastfeeding conferred less risk of breast milk transmission than mixed feeding [2,3]. This has led to calls for early cessation of exclusive breastfeeding to provide the young infant some of the healthful immune and nutritive benefits of breast milk while minimizing the duration of exposure to HIV [3,4]. Rapid transition from exclusive breast milk to full replacement feedings might further decrease the risk of transmission by minimizing the concurrent exposure to non-breast milk substances and HIV-containing breast milk. It is speculated that pathogen- or allergen-containing foods taken with mixed feeding may increase gut permeability or mucosal inflammation [5], and/or incomplete breast emptying may lead to subclinical mastitis [6] and increased breast milk viral load [7]. Although it is difficult to conceive of other settings where the discomfort and potential harm produced by rapid weaning might have a net benefit, abrupt and complete cessation of exclusive breastfeeding may have an important role in reducing postnatal transmission of HIV.
Even if the overall benefit of abrupt weaning in the transition from exclusive breastfeeding to full replacement feeding is established, it will remain a difficult practice to achieve and sustain. In promoting this intervention, should it prove to be effective, it is important to consider the potential consequences of either incomplete abrupt weaning or resumption of breastfeeding after it has ceased.
The Zambia Exclusive Breastfeeding Study (ZEBS) aims to assess the risks associated with early and rapid weaning compared with the potential benefit of reduced late postnatal transmission [8]. We report here a comparison of viral load in breast milk obtained 2 weeks after rapid and complete weaning versus breast milk from women at the same postnatal age who continue to exclusively breastfeed. To assess the compliance of self-reported breastfeeding cessation we used plasma prolactin levels and breast milk volume as biologic confirmation of the decrease or cessation of lactation.
Methods
Study population
Data reported here are from a planned subset analysis of women enrolled in ZEBS, a detailed description of which is reported elsewhere [8]. In brief, ZEBS was designed to determine whether short exclusive breastfeeding with abrupt cessation at 4 months is feasible and can reduce postnatal HIV transmission and child mortality at 24 months postpartum. HIV-positive women attending prenatal care services at two primary health clinics in urban Lusaka, Zambia, were given nevirapine and counseled about the risks and benefits of infant feeding options. Women intending to breastfeed were recruited and a small number of HIV-negative women were also enrolled. Comprehensive lactation counseling promoted exclusive breastfeeding in all women up to 4 months. Half of the women were randomized to a counseling program encouraging abrupt breastfeeding cessation (within 24 h) to occur at the 4-month visit (group A), and half were randomized to the WHO recommendation to continue exclusive breastfeeding to 6 months with introduction of weaning foods thereafter (group B). The analysis subset was constructed from all study participants who delivered live children up to March 2003. Mixed breastfeeding is defined as the infant’s consumption of any substance other than breast milk and medications on one or more occasion in the prior 2-week period. Exclusive breastfeeding was defined as no episodes of non-breast-milk feeding in the prior 2 weeks.
After weaning, women in group A were counseled to manually express (and discard) milk for relief of discomfort. Both groups of women were seen again in the clinic 2 weeks later (4.5 months) when breast milk volume was measured by timed pumping (10 min per breast) using a Madela Lactina pulsatile electric breast pump (McHenry, Illinois, USA) set at a standard rate. Blood for prolactin was obtained immediately prior to application of the breast pump (baseline) and again 30 min after cessation of pumping. All mothers were asked to refrain from breastfeeding for 1 h prior to the baseline prolactin and breast milk pumping. If the mother claimed to have not completely weaned then sample collection was postponed until the next scheduled clinic visit at 5 months.
Breast milk collection
Breast milk was pumped into clean non-sterile bottles and transferred to a sterile collection cup and stored on-site at 4°C for up to 4 h. Samples were transferred the same day on ice to the laboratory where the volume was measured, the cell pellet was removed by low speed centrifugation and the remaining acellular aqueous and lipid phases were remixed, aliquoted into cryovials and frozen at 70°C until analyzed in the US.
Viral load measurements
Whole breast milk was centrifuged at 1600×g for 15 min to separate the aqueous supernatant from the lipid fraction. The aqueous portion was centrifuged again and the cell-free supernatant was removed. Breast milk HIV RNA viral load was measured in the supernatant using the Amplicor 1.5 kit with a lower limit of detection of 50 copies/ml, (Roche Molecular Systems, Branchburg, New Jersey, USA) [9] blinded to any clinical information.
Prolactin measurements
Whole blood samples were stored at room temperature for up to 4 h until transport to the processing laboratory where plasma was separated by low speed centrifugation and frozen for transport to the US. Prolactin was measured using the AxSYM Prolactin microparticle enzyme immunoassay technology (MEIA) (Abbott Laboratories, Abbott Park, Illinois, USA) according to the manufacturer’s instructions. The lower limit of detection of the AxSYM Prolactin assay is 0.6 ng/ml.
Statistical analysis
Categorical variables were compared between groups using chi-squared tests. Continuous variables were compared between groups using t-tests if normally-distributed or Kruskall-Wallis tests, if not. Paired Wilcoxon signed rank tests were used to compare changes in breast milk viral load between 4 and 4.5 months. Spearman rank order correlation coefficients (r) were used to describe associations between continuous variables.
Ethical approval
This study was approved by the Human Subjects Review Boards at each collaborating institution: Boston University, Columbia University, Children’s Hospital of Los Angeles, and University of Zambia.
Results
Study participants
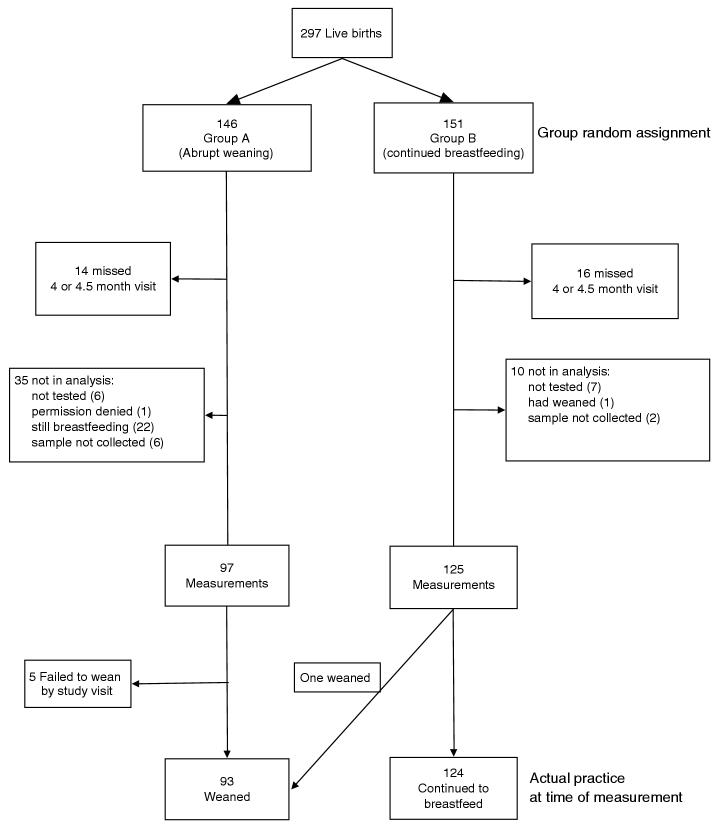
Fig. 1 outlines the derivation of the analysis sample for measurement of prolactin, breast milk volume and clinical parameters. This includes 222 HIV-positive women, 97 randomly assigned to group A and 125 assigned to group B, as well as 24 HIV-negative women (all in group B).
Fig. 1.
Flowchart of HIV-infected women who contributed blood prolactin and breast milk volume measurements by group randomization assignment and actual mode of feeding practice.
These were derived from the full cohort of 297 HIV-positive women (146 and 151 randomized to group A and B, respectively) with live-births occurring between the beginning of the study (May 2001) and March 2003. Among the 146 HIV-positive women assigned to group A, 97 (66.4%) had data available for this analysis, 14 (9.6%) did not attend the 4 or 4.5-month visit and 35 (24.0%) attended the visit but the samples were not collected either because the participant reported not having yet stopped breastfeeding (n = 22) or for other reasons. Among the 151 HIV-positive women assigned to group B, 125 (82.8%) had data available for this analysis, 16 (10.6%) did not complete the relevant postnatal study visit and 10 (6.6%) attended the visit but the samples were not collected. There were no systematic differences between the women for whom data were available in group A versus group B with regard to demographic, clinical or disease stage parameters.
Among the 97 HIV-positive women assigned to the early weaning group, 92 (95%) reported having stopped all breastfeeding before the post-weaning measurements were taken (one woman assigned to the continued breastfeeding group also stopped breastfeeding). Postweaning samples were obtained for 83 (85.6%) women at the scheduled 4.5-month visit, for 11 (11.3%) at the 5-month visit, and for three at a later time point. All except one of the 125 women in group B were still breastfeeding when the samples were obtained: 113 (90.4%) at the 4.5-month visit, 10 (8.0%) at the 5-month visit and two at a later visit. For clarity, the analysis was based on actual reported practice rather than group assignment. Results were essentially unchanged if an intent-to-treat analysis was performed (data not shown).
There were no significant differences in maternal age, parity, plasma viral load, CD4 cell count or body mass index (BMI) between the HIV-positive women who had weaned compared with those who were still breastfeeding at the time of measurement. Women who weaned were significantly more likely to have breast engorgement and to report fever since the last visit in comparison with women who continued to breastfeed (Table 1).
Table 1.
Characteristics of the 222 HIV-positive and 24 HIV-negative women included in the analysis by actual breastfeeding practices at the time of measurement of prolactin and breast milk volume.
| HIV-positive |
HIV-negative |
P-values |
|||
|---|---|---|---|---|---|
| 1. Stopped breastfeeding (n = 93) | 2. Still breastfeeding (n = 129) | 3. Still breastfeeding (n = 24) | 1 vs. 2 | 2 vs. 3 | |
| Child age (days) at measurementa | 143 (123-200) | 143 (137-190) | 142 (139-154) | NS | NS |
| Number of days ago breastfeeding stoppeda | 15 (4-84) | N/A | N/A | ||
| Maternal age (years)a | 26.4 (5.42) | 26.9 (5.50) | 26.6 (7.00) | NS | NS |
| Parityb | 3.3 (1.66) | 3.4 (1.69) | 3.2 (1.88) | NS | NS |
| CD4 cell count at enrollment during pregnancy (cells/μl)b | 357 (193) | 391 (204) | 817 (249) | NS | < 0.0001 |
| Plasma viral load at enrollment (median) | 44403 | 43006 | N/A | NS | |
| Body mass index when milk collectedb,e | 20.9 (3.80) | 20.5 (3.05) | 22.5 (3.67) | NS | 0.02 |
| Breast engorgementc,f | 7/87 (8.1) | 1/128 (0.8) | 1/24 (4.2) | 0.006 | NS |
| Report fever since last visitc,f | 19/87 (21.8) | 9/128 (7.03) | 2/24 (8.33) | 0.002 | NS |
| Mastitisc,f,g | 10/87 (11.5) | 1/128 (0.8) | 0/24 | 0.0005 | NS |
| Weaning periodc,f | |||||
| <2 days | 52/89 (58.4) | N/A | N/A | ||
| 2-7 days | 31/89 (34.8) | ||||
| >7 days | 6/89 (6.7) | ||||
| Amenorrheic 6 months post-partumc,f | 39/90 (43.3) | 107/129 (83.0) | 19/24 (79.2) | <0.0001 | NS |
| Blood prolactin leveld | 1. Stopped breastfeeding (n = 93) (ng/ml) | 2. Still breastfeeding (n = 129) (ng/ml) | 3. Still breastfeeding (n = 24) (ng/ml) | 1 vs. 2h | 2 vs. 3h |
|---|---|---|---|---|---|
| Pre-pumpingd | 25 (12-75) | 111 (68-155) | 120 (72-161) | <0.0001 | 0.89 |
| Post-pumpingd | 93 (43-157) | 169 (114-258) | 152 (121-198) | <0.0001 | 0.50 |
| Post- minus pre-d | 40 (7-104) | 45 (10-95) | 57 (10-94) | 0.81 | 0.95 |
| Breast milk volume | (ml) | (ml) | (ml) | ||
| Right plus leftd | 28 (7-75) | 55 (22-105) | 70 (30-115) | 0.0008 | 0.37 |
Values are:
mean (min - max).
mean (SD).
n (%).
median (interquartile range).
Valid weight and height data were available for 90 women in group 1, 127 in group 2 and 22 in group 3.
Clinical data were missing for some women; denominators are as shown.
Defined as breast engorgement, areas appearing red or shiny or any breast abscess detected on clinical examination or any maternal report of breast pain or other breast problems in the interval since the last visit if maternal fever was also reported since the last visit.
Two-sided P-value from Wilcoxon two-sample test (non-parametric test).
Prolactin and pumped breast milk volume as a measure of weaning compliance
Baseline plasma prolactin levels (just prior to pumping) were significantly lower in women who claimed to have weaned in comparison with women who reported continued breastfeeding providing confirmation that weaning had occurred. Prolactin levels measured after breast milk pumping increased to a similar extent in both groups (Table 1), but the percentage of women with very high prolactin levels was far greater among the breastfeeding women, presumably in response to the suckling stimulus of breast milk pumping (data not shown). There were no differences between HIV-positive and HIV-negative women who were still breast feeding.
The volume of breast milk pumped in 10 min was significantly less among women who had weaned in comparison with those still breastfeeding (Table 2). Among the 93 women who had weaned, 17.2% pumped no detectable milk, and 22.6% pumped less than 5 ml. By comparison only 4.7% of 129 women who were still breastfeeding pumped no milk and 9.3% pumped < 5ml (P < 0.008).
Table 2.
Characteristics of 71 HIV-positive women with post-pump viral loads.
| 1. Stopped breastfeeding (n = 31) | 2. Still breastfeeding (n = 40) | P-value | |
|---|---|---|---|
| Age of child (days) when measurements were takena | 143 (123-200) | 142 (139-161) | NS |
| No. of days ago breastfeeding stoppeda | 14 (4-41) | N/A | |
| Maternal age (years)b | 26.1 (5.74) | 25.8 (4.62) | NS |
| Parityb | 3.0 (1.49) | 3.3 (1.43) | NS |
| CD4 count at enrollment (cells/μl)b | 371 (211) | 410 (230) | NS |
| Plasma viral load at enrollment (median)b | 44403 | 32289 | NS |
| Body mass index when milk collectedb | 20.5 (4.21) | 20.6 (2.73) | NS |
| Report fever since last visitc | 9/29 (31.0) | 3/40 (7.50) | 0.01 |
| Mastitisc,d | 5/31 (16.1) | 1/40 (2.5) | 0.04 |
Values are:
mean (min - max).
mean (SD).
n (%).
Defined as breast engorgement, areas appearing red or shiny or any breast abscess detected on clinical examination or any maternal report of breast pain or other breast problems in the interval since the last visit if maternal fever was also reported since the last visit.
Clinical signs and duration of weaning
There were no significant differences in prolactin or breast milk volume by duration of time since cessation of breastfeeding. Although, there was a trend towards higher baseline prolactin with a short interval (< 7 days), and lower breast milk volume with a longer interval (> 14 days) since breastfeeding cessation, these differences did not reach statistical significance. There were also no significant differences by the duration of the weaning period (Table 3).
Table 3.
Factors associated with basal prolactin level (pre-pumping) and breast milk volume among 93 HIV-positive women who had stopped all breastfeeding by the time of measurement.
| n | Blood prolactin (pre-pumping) Median (IQR) | P-value | Breast milk volume (right plus left) Median (IQR) | P-value | |
|---|---|---|---|---|---|
| When breastfeeding stopped (days ago) | |||||
| 4-7 days | 6 | 85 (12-151) | 29 (14-30) | ||
| 8-14 days | 61 | 25 (12-66) | 45 (11-80) | ||
| 15-84 daysa | 26 | 24 (9-75) | NS | 17 (2-53) | NS |
| Duration of weaning | |||||
| <2 days | 52 | 26 (12-92) | 28 (5-73) | ||
| 2-7 days | 31 | 27 (14-59) | 28 (8-77) | ||
| >8 daysb | 6 | 12 (11-33) | NS | 15 (4-135) | NS |
| Breast engorgement on clinical examination | |||||
| Yes | 7 | 47 (12-153) | 80 (50-150) | ||
| No | 77 | 23 (12-75) | NS | 25 (6-65) | 0.01 |
| Maternal report of __ since stopping breastfeeding | |||||
| Engorgement | 67 | 25 (12-90) | 30 (9-80) | ||
| No engorgement | 22 | 26 (11-59) | NS | 17 (0-70) | NS |
| Breast pain | 69 | 27 (12-83) | 30 (7-77) | ||
| No breast pain | 20 | 18 (11-52) | NS | 15 (6-52) | NS |
| Feeling ill | 33 | 35 (12-4) | 52 (18-95) | ||
| Not feeling ill | 56 | 20 (11-62) | NS | 18 (3-48) | 0.009 |
| Fever | 19 | 61 (18-153) | 53 (18-140) | ||
| No fever | 65 | 21 (11-57) | 0.02 | 24 (5-60) | 0.02 |
Twenty-three of 26 were between 15 and 30 days with 1 at 25, one at 41 and 1 one at 84 days. This question was intended to elicit the time from when all breastfeeding stopped (not from when weaning started) until the day that the prolactin and breast milk measurements were taken.
Four reported between 8-14 days and two >14 days.
We found a non-significant increase in the volume of breast milk that was pumped among women with breast engorgement or breast pain and a significant increase in women who reported feeling ill or fever since weaning. Prolactin was significantly elevated among those who reported fever since breastfeeding ended (Table 3). There were no associations between prolactin and breast milk volume and either maternal age, parity, CD4 cell count or body mass index.
Exclusive breastfeeding and prolactin levels
All the women who had not stopped breastfeeding reported both day and night-time breast feeds over the previous 2 weeks and eight of 129 (6.2%) reported giving the child something other than breast milk over the same period. The median baseline prolactin levels were higher in those 121 women who reported only exclusive breastfeeding [median, 112; interquartile range (IQR), 79-161] compared with the eight women who reported mixed breastfeeding in the previous 2 weeks (median, 68; IQR, 47-110), but this did not reach statistical significance (P = 0.09). Post-pumping prolactin levels and pumped volumes were similar among exclusive and non-exclusive breastfeeding women.
Resumption of menses
Resumption of menses by 6 months post-partum was significantly more common among women who stopped breastfeeding than those who continued to breastfeed (Table 1). Among those who had stopped all breastfeeding, basal prolactin levels post-weaning were significantly lower (mean 36.9 ng/ml) among those who subsequently resumed menses by 6 months than those who did not resume menses by this time (mean 75.3 ng/ml) (P = 0.01). Post-pumping prolactin levels were also lower but the difference did not reach significance. Basal and post-pumping prolactin levels among those who were still breastfeeding were also significantly lower among those who subsequently resumed menses.
Breast milk viral load in a random subset of 71 women
To conserve resources, we randomly selected breast milk samples from 80 women (40 who had stopped breastfeeding and 40 who were still breastfeeding) among the 222 evaluable subjects to measure breast milk viral load. Thirty-one of the 40 who had weaned contained a sufficient quantity of breast milk (≥1 ml) for viral load testing; whereas all 40 women who continued to breastfeed had sufficient quantity for testing (Table 2). Detectable HIV RNA was measured in 21 of 31 (67.7%) women who had stopped breastfeeding compared with 17 of 40 (42.5%) women who were still breastfeeding (P = 0.03). The median RNA concentrations (if detectable) were 7930 copies/ml in those who had stopped and 904 copies/ml in those who were still breastfeeding (P = 0.005) (Fig. 2). HIV RNA concentrations > 1000 copies/ml were found among 45.2% of those who had stopped versus 12.5% of those who were still breastfeeding (P = 0.002).
Fig. 2.
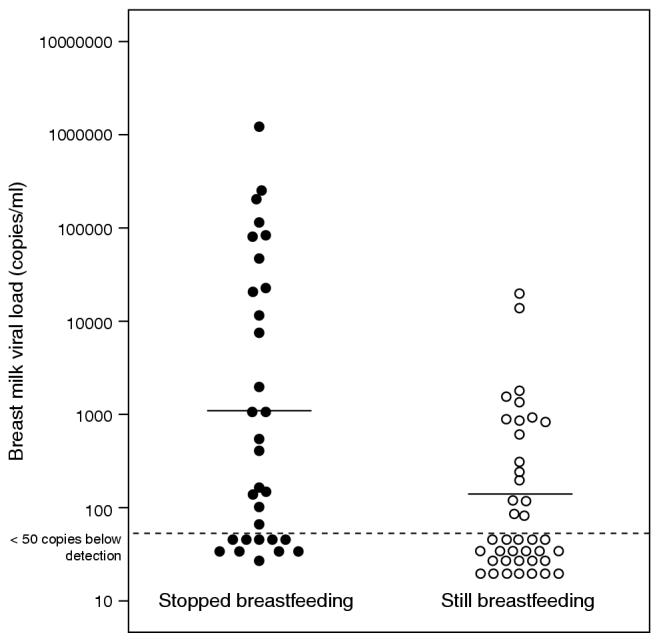
Breast milk HIV viral load among pumped breast milk from 31 women who recently weaned compared with breast milk from 40 lactating women of the same postnatal age.
We were also able to compare breast milk viral load longitudinally (i.e. before and after weaning) among 29 of 31 women who had weaned. The pre-weaning sample was obtained at the visit when breastfeeding was due to stop, on average 2 weeks prior to the post-weaning measurement. Although a similar proportion of women, 18 of 29 (62.1%), had detectable virus before weaning as after weaning, the median pre-weaning breast milk viral load (if detectable) was only 353 copies/ml, and only 17.2% had > 1000 copies/ml. Breast milk viral load rose significantly from the pre-weaning sample to the postweaning sample (P = 0.001); a median increase of 15 822 copies/ml (38 to > 750 000 copies/ml). No increase in breast milk viral load occurred over an equivalent 2-week interval among women still breastfeeding at 5 months.
Among those who had stopped breastfeeding, there was a highly significant correlation between breast milk viral load and (1) baseline plasma prolactin (r 0.578; P = 0.0007); (2) post-pumping prolactin (r = 0.363; P = 0.04); and (3) a nearly significant correlation = with breast milk volume pumped (r 0.330; P 0.06). None of these three parameters was= associated with = breast milk viral load from the same women obtained prior to weaning 2 weeks earlier.
In the group with viral load measurements, 18 women reported that the duration of weaning was less than 2 days and 12 women reported that its duration was 2-7 days. None reported the weaning process lasting beyond 7 days. This information was missing for one person. There was no significant difference in post-weaning viral load among women who stopped within 2 days (median 313 copies/ml) versus those who stopped less rapidly (2-7 days; median 646 copies/ml). Although mastitis and reported fever were more common among women who had stopped breastfeeding (Table 2), we found no association between breast milk viral load and these two parameters but numbers were small.
Discussion
Breast milk viral load is an important determinant of postnatal HIV transmission [3,10-13] and is associated with high plasma viral load [14,15], advanced HIV disease [16,17], and mastitis [7,10]. Increases in mammary epithelial permeability which accompanies weaning in animals [18,19] and humans [20,21] may also increase breast milk viral load. This may lead to a substantially increased transmission risk among children who are partially weaned or in whom breastfeeding is resumed once stopped. In this study we show that breast milk obtained after complete cessation of breastfeeding had a substantially higher concentration of viral RNA than milk obtained from women who were still breastfeeding at the same post-natal age. It should be noted that only samples with 1 ml or more were able to be assayed for viral load and that approximately one-sixth of the women who had weaned produced less than this amount when pumped.
There were no significant differences between the two groups at baseline in maternal CD4 cell count or plasma viral load and any slight differences between these two groups seem unlikely to explain the large differences observed. Additionally, the presence of low breast milk viral load 2 weeks prior to weaning strongly supports the concept that weaning itself accounted for the increase in viral load rather than some confounding factor. The biological markers used to confirm self-report of weaning, pumped breast milk volume and plasma prolactin, provide objective evidence that the mode of feeding in the two groups during the weeks prior to viral load measurement were, in fact, markedly different.
Breast engorgement or fever reported since the last visit among the women who weaned is an indication of the discomfort and morbidity that may be associated with rapid or accelerated weaning. It may also be a marker of physiological processes similar to those that occur with clinical mastitis, which could account for the increase in viral load observed here. Milk fever, which has been described since the eighteenth century, is due to blocked milk flow occurring in the days after delivery in association with the onset of lactation [22]. Milk stasis, which may occur at any point during breastfeeding, may precede the development of mastitis. In both of these circumstances, mammary gland permeability is enhanced, potentially allowing easier flow of virus into the milk. Systemic infections may also play a role in increasing breast milk viral load.
Weaning normally begins with the introduction of complementary foods which necessarily reduces the amount of milk consumed by the child and thereby produced by the mother. This involves a gradual process of involution of the breast which has been associated with steadily increasing milk sodium concentrations during the final phases of the weaning period [21]. Increased breast milk sodium levels, defined as sub-clinical mastitis (not necessarily in the setting of weaning), are associated with increased breast milk viral load [10,23]. This would suggest that breast milk viral load is increased during the final stages of mixed feeding, which may account for a significant portion of the increased postnatal transmission associated with mixed feeding. If true, it will be important to establish the role of high breast milk viral load in postnatal transmission during mixed feeding.
Prolactin
Prolactin has a well-established role in human milk production and lactational amenorrhea [24,25]. It has been shown to increase significantly in response to the stimulus of breastfeeding [26-29], diminish with the onset of non-exclusive breastfeeding [30] and fall precipitously after suckling has ended [31]. Hyperprolactinemia is also known to occur in up to one-fifth of HIV-infected non-lactating individuals [32,33] but has not been assessed in lactating HIV-infected women.
To increase the reliability of self-reported weaning information, we used both breast milk volume obtained during a structured pumping session and prolactin level as biologic confirmation that weaning had occurred. We show here that cessation of breastfeeding at 4 months postpartum is highly associated with diminished plasma prolactin and extracted breast milk volume 2 weeks later in HIV-infected women. This indicates that these parameters may be useful in determining the degree of compliance among groups of HIV-infected women who have chosen to rapidly wean their children off full breastfeeding. However, we did not find a specific pre- or post-pumping prolactin level that could be used as a reliable threshold. Although this provided reassurance that compliance with the primary intervention for the breastfeeding study (rapid weaning) was high, the clinical utility of these findings is limited. We did find a nearly significant association between baseline level of prolactin and mixed feeding over the prior 2 weeks. However, the number of mixed-feeding women was small (8/129), limiting our ability to find such an association. None-theless, our result is consistent with findings from women who are not HIV infected [30,34,35], with the proposed mechanism being an alteration in the frequency, intensity or duration of the breastfeeding stimulus. Further research is needed to determine whether baseline prolactin could be used as a surrogate measure of the degree of non-exclusive breastfeeding.
We found a strong association between cessation of breastfeeding and subsequent resumption of menses. We also show that low prolactin levels predict early (6 months) return of menses, regardless of the woman’s weaning status. The level of prolactin in HIV-infected lactating women has not previously been measured. The role of prolactin in lactational amenorrhea in the absence of HIV-infection is well established and, from our results, appears to be intact among HIV-infected, lactating or recently weaned women, despite the presence of hyperprolactinemia found among HIV-infected men and women [32,33]. However, the significance of increased prolactin in these circumstances remains controversial [36]. We have also found that basal prolactin levels in actively breastfeeding women are not different according to HIV infection status, and do not correlate with low BMI or CD4 cell count at enrollment.
In conclusion, we have shown that prolactin and pumped breast milk volume were highly associated with reported weaning behavior. We have also shown that large increases in breast milk viral load occurred after rapid cessation of breastfeeding. These findings indicate that the HIV exposure risk to the child through breastmilk that would be consumed during re-lactation is greater than it would be prior to cessation. Further research is needed to determine whether partial breastfeeding cessation (i.e. mixed feeding) is also associated with increases in breast milk viral load. If so, this may have important implications for how HIV-infected women are counseled and choose to make the transition from exclusive breastfeeding.
Acknowledgements
We are indebted to the study subjects for their dedicated participation in the study. We also thank the staff of the ZEBS project who provided high quality implementation of the study protocol and dedicated care to the study participants. We thank the Madela Corporation for donating the Lactina breast pumps and supplies. We also thank Ellen Piwoz for her help in study design and development of the counseling intervention; Chewe Luo, Jeff Stringer, and Sten Vermund for their support; and Deirdre Pierotti and Jonathon Simon for their administrative and moral support.
Appendix A.1.
Contributions of authors Concept, study design and protocol development (alphabetical order): Chipepo Kankasa, MD; Louise Kuhn, PhD; Moses Sinkala, MD; Donald M. Thea, MD.
Monitoring of the study (alphabetical order): Chipepo Kankasa, MD; Prisca Kasonde, MD; Louise Kuhn, PhD; Katherine Semrau; Moses Sinkala, MD; Donald M. Thea, MD.
Study implementation and data collection (alphabetical order): Chipepo Kankasa, MD; Prisca Kasonde, MD; Louise Kuhn, PhD; Katherine Semrau; Moses Sinkala, MD; Donald M. Thea, MD.
Data analysis and manuscript preparation (alphabetical order): Chipepo Kankasa, MD; Prisca Kasonde, MD; Louise Kuhn, PhD; Katherine Semrau; Moses Sinkala, MD; Donald M. Thea, MD.
Data management and co-ordination and statistical analysis (alphabetical order): Louise Kuhn, PhD; Katherine Semrau.
Footnotes
Sponsorship: This research was supported by the National Institute for Child Health and Human Development, Bethesda, Maryland (R01 HD39611 and R01 HD 40777) for the Zambia Exclusive Breastfeeding Study. G.A. is an Elizabeth Glaser Pediatric AIDS Scientist.
Conflict of interest: we declare that we have no conflicts of interest.
References
- 1.Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS. How many child deaths can we prevent this year? Lancet. 2003;362(9377):65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 2.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban South Africa: a prospective cohort study. South African Vitamin A Study Group. Lancet. 1999;354(9177):471–476. doi: 10.1016/s0140-6736(99)01101-0. [DOI] [PubMed] [Google Scholar]
- 3.Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 4.Bertolli J, Hu DJ, Nieburg P, Macalalad A, Simonds RJ. Decision analysis to guide choice of interventions to reduce mother-to-child transmission of HIV. AIDS. 2003;17:2089–2098. doi: 10.1097/00002030-200309260-00010. [DOI] [PubMed] [Google Scholar]
- 5.Rollins NC, Filteau SM, Coutsoudis A, Tomkins AM. Feeding mode, intestinal permeability, and neopterin excretion: a longitudinal study in infants of HIV-infected South African women. J Acquir Immune Defic Syndr. 2001;28:132–139. doi: 10.1097/00042560-200110010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Willumsen JF, Newell ML, Filteau SM, Coutsoudis A, Dwarika S, York D, et al. Variation in breastmilk HIV-1 viral load in left and right breasts during the first 3 months of lactation. AIDS. 2001;15:1896–1898. doi: 10.1097/00002030-200109280-00026. [DOI] [PubMed] [Google Scholar]
- 7.Semba RD. Mastitis and transmission of human immunodeficiency virus through breast milk. Ann N Y Acad Sci. 2000;918:156–162. doi: 10.1111/j.1749-6632.2000.tb05484.x. [DOI] [PubMed] [Google Scholar]
- 8.Thea DM, Vwalika C, Kasonde P, Kankasa C, Sinkala M, Semrau K, et al. Issues in the design of a clinical trial with a behavioral intervention-the Zambia exclusive breast-feeding study. Control Clin Trials. 2004;25:353–365. doi: 10.1016/j.cct.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh MK, Kuhn L, West J, Semrau K, Decker D, Thea DM, et al. Quantitation of human immunodeficiency virus type 1 in breast milk. J Clin Microbiol. 2003;41:2465–2470. doi: 10.1128/JCM.41.6.2465-2470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willumsen JF, Filteau SM, Coutsoudis A, Newell ML, Rollins NC, Coovadia HM, et al. Breastmilk RNA viral load in HIV-infected South African women: effects of subclinical mastitis and infant feeding. AIDS. 2003;17:407–414. doi: 10.1097/00002030-200302140-00015. [DOI] [PubMed] [Google Scholar]
- 11.Coutsoudis A, Dabis F, Fawzi W, Gaillard JR, Haverkamp G, Harris DR, et al. Late postnatal transmission of HIV-1 in breastfed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 12.Ekpini ER, Wiktor SZ, Satten GA, Adjorlolo-Johnson GT, Sibailly TS, Ou CY, et al. Late postnatal mother-to-child transmission of HIV-1 in Abidjan, Cote d’Ivoire. Lancet. 1997;349(9058):1054–1059. doi: 10.1016/s0140-6736(96)06444-6. [DOI] [PubMed] [Google Scholar]
- 13.Chung MH, Kiarie JN, Richardson BA, Lehman DA, Overbaugh J, John-Stewart GC. Breast milk HIV-1 suppression and decreased transmission: a randomized trial comparing HIVNET 012 nevirapine versus short-course zidovudine. AIDS. 2005;19:1415–1422. doi: 10.1097/01.aids.0000181013.70008.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, Mwatha A, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183:206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 15.Magder LS, Mofenson L, Paul ME, Zorrilla CD, Blattner WA, Tuomala RE, et al. Risk factors for in utero and intrapartum transmission of HIV. J Acquir Immune Defic Syndr. 2005;38:87–95. doi: 10.1097/00126334-200501010-00016. [DOI] [PubMed] [Google Scholar]
- 16.Abrams EJ, Wiener J, Carter R, Kuhn L, Palumbo P, Nesheim S, et al. Maternal health factors and early pediatric antiretroviral therapy influence the rate of perinatal HIV-1 disease progression in children. AIDS. 2003;17:867–877. doi: 10.1097/00002030-200304110-00012. [DOI] [PubMed] [Google Scholar]
- 17.Embree JE, Njenga S, Datta P, Nagelkerke NJ, Ndinya-Achola JO, Mohammed Z, et al. Risk factors for postnatal mother-child transmission of HIV-1. AIDS. 2000;14:2535–2541. doi: 10.1097/00002030-200011100-00016. [DOI] [PubMed] [Google Scholar]
- 18.Fleet IR, Peaker M. Mammary function and its control at the cessation of lactation in the goat. J Physiol. 1978;279:491–507. doi: 10.1113/jphysiol.1978.sp012358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stelwagen K, Farr VC, McFadden HA, Prosser CG, Davis SR. Time course of milk accumulation-induced opening of mammary tight junctions, and blood clearance of milk components. Am J Physiol. 1997;273(1 Pt 2):R379–R386. doi: 10.1152/ajpregu.1997.273.1.R379. [DOI] [PubMed] [Google Scholar]
- 20.Neville MC, Allen JC, Archer P, Casey CE, Seacat J, Keller RP, et al. Studies in human lactation: milk volume and nutrient composition during weaning and lactogenesis. Am J Clin Nutr. 1991;54:81–92. doi: 10.1093/ajcn/54.1.81. [DOI] [PubMed] [Google Scholar]
- 21.Garza C, Johnson CA, O’Brian Smith E, Nichols BL. Changes in the nutrient composition of human milk during gradual weaning. Am J Clin Nutr. 1983;37:61–65. doi: 10.1093/ajcn/37.1.61. [DOI] [PubMed] [Google Scholar]
- 22.Dumont M. Milk fever. Rev Fr Gynecol Obstet. 1989;84:451–453. [PubMed] [Google Scholar]
- 23.Willumsen JF, Filteau SM, Coutsoudis A, Uebel KE, Newell ML, Tomkins AM. Subclinical mastitis as a risk factor for motherinfant HIV transmission. Adv Exp Med Biol. 2000;478:211–223. doi: 10.1007/0-306-46830-1_19. [DOI] [PubMed] [Google Scholar]
- 24.del Pozo E, Brownell J, Prolactin I. Mechanisms of control, peripheral actions and modification by drugs. Horm Res. 1979;10:143–174. doi: 10.1159/000178998. [DOI] [PubMed] [Google Scholar]
- 25.Lincoln DW, Paisley AC. Neuroendocrine control of milk ejection. J Reprod Fertil. 1982;65:571–586. doi: 10.1530/jrf.0.0650571. [DOI] [PubMed] [Google Scholar]
- 26.Leake RD, Waters CB, Rubin RT, Buster JE, Fisher DA. Oxytocin and prolactin responses in long-term breast-feeding. Obstet Gynecol. 1983;62:565–568. [PubMed] [Google Scholar]
- 27.Lucas A, Drewett RB, Mitchell MD. Breast-feeding and plasma oxytocin concentrations. BMJ. 1980;281(6244):834–835. doi: 10.1136/bmj.281.6244.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNeilly AS, Robinson IC, Houston MJ, Howie PW. Release of oxytocin and prolactin in response to suckling. BMJ (Clin Res Ed) 1983;286(6361):257–259. doi: 10.1136/bmj.286.6361.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston JM, Amico JA. A prospective longitudinal study of the release of oxytocin and prolactin in response to infant suckling in long term lactation. J Clin Endocrinol Metab. 1986;62:653–657. doi: 10.1210/jcem-62-4-653. [DOI] [PubMed] [Google Scholar]
- 30.Uvnas-Moberg K, Widstrom AM, Werner S, Matthiesen AS, Winberg J. Oxytocin and prolactin levels in breast-feeding women. Correlation with milk yield and duration of breast-feeding. Acta Obstet Gynecol Scand. 1990;69:301–306. doi: 10.3109/00016349009036151. [DOI] [PubMed] [Google Scholar]
- 31.Rojkittikhun T, Uvnas-Moberg K, Einarsson S, Lundeheim N. Effects of weaning on plasma levels of prolactin, oxytocin, insulin, glucagon, glucose, gastrin and somatostatin in sows. Acta Physiol Scand. 1991;141:295–303. doi: 10.1111/j.1748-1716.1991.tb09084.x. [DOI] [PubMed] [Google Scholar]
- 32.Collazos J, Ibarra S, Martinez E, Mayo J. Serum prolactin concentrations in patients infected with human immunodeficiency virus. HIV Clin Trials. 2002;3:133–138. doi: 10.1310/QAQQ-XTCJ-8AL4-6F5P. [DOI] [PubMed] [Google Scholar]
- 33.Croxson TS, Chapman WE, Miller LK, Levit CD, Senie R, Zumoff B. Changes in the hypothalamic-pituitary-gonadal axis in human immunodeficiency virus-infected homosexual men. J Clin Endocrinol Metab. 1989;68:317–321. doi: 10.1210/jcem-68-2-317. [DOI] [PubMed] [Google Scholar]
- 34.Tennekoon KH. Maternal prolactin concentrations and lactational behaviour in the early postpartum period in women with lactational amenorrhoea. Ceylon Med J. 2001;46:6–10. [PubMed] [Google Scholar]
- 35.Tay CCK, Glasier AF, McNeilly AS. Twenty-four hour patterns of prolactin secretion during lactation and the relationship to suckling and the resumption of fertility in breast-feeding women. Hum Reprod. 1996;11:950–955. doi: 10.1093/oxfordjournals.humrep.a019330. [DOI] [PubMed] [Google Scholar]
- 36.Ram S, Acharya S, Fernando JJ, Anderson NR, Gama R. Serum prolactin in human immunodeficiency virus infection. Clin Lab. 2004;50:617–620. [PubMed] [Google Scholar]