Abstract
Amnesic patients have a well established deficit in remembering their past experiences. Surprisingly, however, the question as to whether such patients can imagine new experiences has not been formally addressed to our knowledge. We tested whether a group of amnesic patients with primary damage to the hippocampus bilaterally could construct new imagined experiences in response to short verbal cues that outlined a range of simple commonplace scenarios. Our results revealed that patients were markedly impaired relative to matched control subjects at imagining new experiences. Moreover, we identified a possible source for this deficit. The patients' imagined experiences lacked spatial coherence, consisting instead of fragmented images in the absence of a holistic representation of the environmental setting. The hippocampus, therefore, may make a critical contribution to the creation of new experiences by providing the spatial context into which the disparate elements of an experience can be bound. Given how closely imagined experiences match episodic memories, the absence of this function mediated by the hippocampus, may also fundamentally affect the ability to vividly re-experience the past.
Keywords: episodic, hippocampus, imagination, memory, construction
Each of us has our own unique personal past, comprising a myriad of autobiographical experiences accrued over a lifetime. Recollection of these rich autobiographical or episodic memories has been likened to mentally traveling back in time and re-experiencing one's past (1). It has long been known that the hippocampus and related medial temporal lobe structures play a critical role in supporting episodic memory (2), and damage to even the hippocampus alone is sufficient to cause amnesia (3, 4). How exactly the hippocampus supports episodic memory (5–7), or indeed whether its involvement is time-limited (5, 8) or permanent (7, 9) is uncertain, however. Numerous studies have attempted to settle this debate by ascertaining the status of remote episodic memory in patients with hippocampal amnesia (10) but without resolution thus far. This is not altogether surprising as studying memory for personal experiences is fraught with methodological issues (11–13), not least of which is how to generalize across individuals when autobiographical memories are unique to each person (9, 14).
We therefore sought to further our understanding of the role of the hippocampus in episodic memory by adopting a different approach. If patients with hippocampal damage are impaired at recollecting past events, we wondered, can they imagine new experiences? While there have been some suggestions that amnesic patients have difficulty envisioning themselves in the future (15–18), surprisingly, the more general question of whether imagining new experiences depends on a functioning hippocampus has not been formally addressed to our knowledge. In fact, episodic memory and imagining or constructing events share striking similarities in terms of the psychological processes engaged (19–21). These include imagery (22), sense of presence (1), retrieval of semantic information and multimodal details (23), and narrative structure (22). Moreover, both episodic memory and construction involve the salient visualization of an experience within a rich spatial setting or context (24), and therefore differ markedly from “simple” visual imagery (e.g., for faces or single objects) (25), which is thought not to depend on the hippocampus (26). Constructions, then, have much in common with episodic memories but have the advantage of being easier to systematize and experimentally manipulate. For example, all patients can be asked to construct the same fictitious situations, and their performances can be compared and contrasted more directly than would be possible in a standard episodic memory recall paradigm.
We therefore tested whether a group of patients (n = 5) with amnesia associated with bilateral hippocampal damage [see Methods and supporting information (SI) Text] and a group of matched control subjects (n = 10) could construct new imagined experiences in response to short verbal cues which outlined a range of simple commonplace scenarios (see Methods). When imagining a new experience participants were explicitly told not to describe a remembered event or any part of one but rather to give free reign to their imaginations and create something new. They were also encouraged to “see the situation and setting in their mind's eye” as if they themselves were physically present and to describe as many sensory and introspective details about the situation as they could. These descriptions were then scored along a range of parameters to address two questions: (i) is the hippocampus critical for imagining experiences, paralleling its vital role in recollecting the past; and if so, (ii) is there a specific hippocampal mechanism underpinning imagining that might also bear on its role in episodic memory?
Results
Descriptions of the newly constructed experiences provided by participants were recorded and later scored across a number of ratings by using a similar method to the autobiographical interview (13) that has been used to assess the richness of episodic memories. We also developed additional ratings and measures to fit the requirements of our task (see Methods and SI Text). A composite score, the experiential index, measuring the overall richness of the imagined experience and ranging from 0 to 60 (0, not experienced at all; 60, extremely richly experienced), was calculated from four subcomponents: information content (content, classified into four categories), ratings of sense of presence and perceived vividness (participant ratings), a subindex generated from participant feedback (spatial coherence index), and a scorer rating of overall quality of the constructed experience (quality judgment). Table 1 shows the mean scores on these measures for patients and controls. Examples of scenario cues, excerpts from patients' constructions, and those of matched control subjects are shown in Fig. 1(see SI Fig. 4).
Table 1.
Performance on the construction task
| Measure | Mean (SD) |
|
|---|---|---|
| Patients, n = 5 | Controls, n = 10 | |
| Overall richness: Experiential index | 27.54 (13.12) | 45.06 (4.02) |
| Subcomponents | ||
| Content | ||
| Spatial references | 2.38 (1.82) | 5.28 (1.15) |
| Entities present | 4.94 (1.26) | 6.49 (0.42) |
| Sensory descriptions | 4.12 (1.03) | 5.64 (0.72) |
| Thought/emotion/actions | 2.76 (1.77) | 5.52 (0.64) |
| Participant ratings | ||
| Sense of presence | 3.46 (1.15) | 3.65 (0.49) |
| Perceived salience | 3.52 (1.19) | 3.88 (0.48) |
| Spatial coherence index | 0.10 (3.21) | 3.68 (1.30) |
| Scorer rating: quality judgment | 3.88 (2.70) | 7.13 (0.96) |
Fig. 1.
Examples of imagined experiences. Representative excerpts from transcriptions relating to two of the scenarios, with the cue at the top, followed by an excerpt from a patient's transcription, followed by that of a control subject who was age-, education-, and IQ-matched to that patient. See SI Fig. 4 for an additional example. Interviewer's probing comments are in italics. Relevant background is information noted in square brackets.
Each participant was tested on 10 scenarios covering a range of themes. Seven were standard commonplace scenarios (involving a beach, museum, pub, port, market, forest, and castle setting). We also examined the effect of scenarios that were explicitly self-relevant and potentially plausible in the future, so-called “episodic future thinking” (17, 18) (possible Christmas event, possible event over next weekend, possible future meeting with a friend). Performances on the two scenario types were initially analyzed separately. However, both had identical patterns of results, and so for clarity we present the results collapsed across scenarios.
Experiential Index.
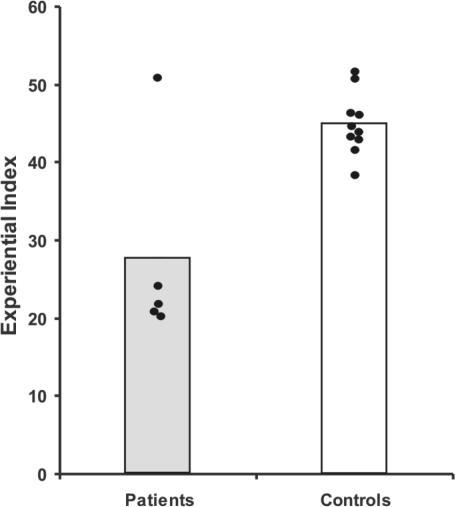
The patient group scored significantly lower on the overall experiential index than the control group (P = 0.002) (Fig. 2), thus revealing that the ability to richly imagine new experiences is compromised in the context of bilateral hippocampal damage.
Fig. 2.
Scores on the experiential index. The data points for every patient and control subject are shown. Vertical bars represent group means.
In the first instance, we wondered whether the impairment might be because patients found the task more difficult than the control subjects. After imagining each new experience, subjects rated how difficult they found this task on a scale of 1–5 (1, very easy; 5, very difficult). There was no significant difference between patients and controls in perceived difficulty of imagining scenarios [patient mean 2.20 (1.07), controls 2.13 (0.64), P = 0.87]. We next considered the extent to which imagined experiences were derivatives of actual memories in the control subjects, despite the instruction to create something new. After each construction we asked participants to rate on a scale of 1–5 its similarity to an actual memory, in whole or in part (1, nothing at all like any memories; 5, exactly like a memory). The purpose of this measure was to verify that control subjects had adhered to the instructions to create something new. The low overall mean of the control subjects [2.03 (0.62)] confirmed that this was the case, and therefore that successful construction of rich new experiences did not depend on recalling real memories. For completeness, we report that the patient mean was 2.37 (0.90) and there was no significant difference between groups (P = 0.40).
We next sought to investigate the source of the patients' experiential index deficit further by analyzing the subcomponents that comprised this composite score.
Subcomponents.
Content.
Each scenario description given by the participant was segmented into a set of statements. Every statement was then classified as belonging to one of four main categories: spatial reference, entity presence, sensory description, or thought/emotion/action. The spatial reference category encompassed statements regarding the relative position of entities within the environment, directions relative to the participant's vantage point, or explicit measurements (e.g., “behind the bar” or “to my left I can see” or “the ceiling is about 40 ft high”). The entity category was a simple count of how many distinct entities (e.g., objects, people, animals) were mentioned (e.g., “I can see some birds”). The sensory descriptions category consisted of any statements describing (in any modality) properties of an entity (e.g., “the chair I'm sitting on is made of wood”) as well as general weather and atmosphere descriptions (e.g., “it is very hot” or “the room is very smoky”). Finally, the thought/emotion/action category covered any introspective thoughts or emotional feelings (e.g., “I have a sense of being alone”) as well as the thoughts, intentions, and actions of other entities in the scene (e.g., “he seems to be in a hurry” or “the barman is pouring a pint”) (see SI Text and SI Data Set for an example categorization).
For each category, patients produced fewer details than controls: spatial references (P = 0.002), entities present (P = 0.003), sensory descriptions (P = 0.005), and thought/emotion/actions (P = 0.001). Although impaired, all patients performed above floor and were able to produce at least some associations and even substantial descriptions relevant to the scenario in question, as can be appreciated from Fig. 1 (and SI Fig. 4). None had deficits in semantic retrieval. Nevertheless, we next asked whether an inability to retrieve relevant semantic information might underpin their construction deficit. We selected one patient at random and tested him in the same way on additional scenarios. However, on this occasion, before starting to imagine, the patient was provided with pictures of objects, as well as sounds and smells relevant to the scenario (see Methods). This information, which remained present during the task, thus eschewed the need for retrieval from memory of relevant semantic information, and using the provided materials appropriately could produce a reasonable score. Despite this assistance, however, performance did not improve, with no difference in experiential index scores between original and “assisted” scenarios (P = 0.96), the overall content score (P = 0.50), or the spatial coherence index (P = 0.85).
Participant ratings.
Perhaps their imagined experiences lacked some key experiential qualities. We tested whether patients were explicitly aware of these qualities by asking them to rate each construction on a scale of 1–5 for sense of presence (1, did not feel like I was there at all; 5, felt strongly like I was really there). There was no significant difference between patients and controls in perceived sense of presence [patient mean 3.46 (1.15), controls 3.65 (0.49), P = 0.65]. We also asked subjects to rate the perceived salience of each imagined experience on a scale of 1–5 (1, couldn't really see anything; 5, extremely salient). As with sense of presence, there was no significant difference between patients and controls in perceived salience [patient mean 3.52 (1.19), controls 3.88 (0.48), P = 0.41]. Thus while patients were impaired in terms of the amount of content included in their imagined experiences, their subjective feelings of salience and sense of presence were no different from control subjects, indicating that the deficit is subtle in nature. Although the feedback received from patients was confidently and promptly given, we cannot completely exclude the possibility that these subjective ratings are somehow systematically different in patients compared with controls. Patients might remember less accurately their immediate task performance because of their amnesia, or because they do not have the same volume of rich experiences available to draw on. However, if these participant ratings were somehow inaccurately scored by patients, and they should have scored themselves lower, resulting in their experiential index being further decreased.
We therefore next considered another factor fundamental to episodic experiences, namely the extent to which the patients felt like the imagined experiences were taking place in an integrated and coherent spatial context as opposed to merely being a fragmented collection of images.
Spatial Coherence Index.
The spatial coherence index is a measure of the contiguousness and spatial integrity of the imagined scene. After each scenario, participants were presented with a set of statements, each providing a possible qualitative description of the imagined experience. They were instructed to indicate which statements they felt accurately described their construction. They were free to identify as many or as few as they thought appropriate. The spatial coherence index was then derived from the statements identified (see SI Text). Some of the statements indicated that aspects of the scene were integrated (e.g., “I could see the whole scene in my mind's eye”), whereas others indicated that aspects of the scene were fragmented (e.g., “it was a collection of separate images”). Participants were blind to the purpose of the statements and indeed the concept of coherence.
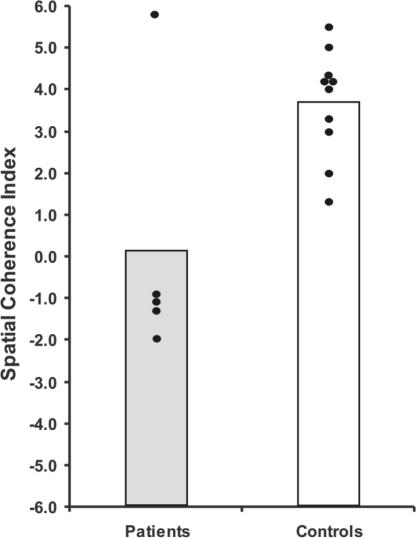
Compared with controls, the feedback from the patients indicated that their imagined experiences were fragmentary and lacking in coherence (P = 0.007; see also Fig. 3). Although the spatial coherence index encompasses a range of concepts, to ensure that the patient feedback was reliable we performed a correlation analysis between the spatial coherence scores and the data from the spatial reference content category, the most closely related (although narrower) objective measure. A strong trend was observed (P = 0.07), which, although not significant, indicates that it is likely that something concrete and real was captured by the derived spatial coherence measure. Notably, when performing the spatial coherence task, several of the patients identified fragmentation as a relevant difficulty with the task at hand, but also pertinent to their memory problems in general.
Fig. 3.
Scores on the spatial coherence index. The data points for every patient and control subject are shown. Vertical bars represent group means.
Quality Judgment.
One final scoring component, the quality judgment, was the scorer's assessment of the overall quality of each construction (see SI Text). This component was included to provide a measure of the range, diversity, and quality of the details described and to ensure that short, succinct, but nonetheless salient, descriptions were not unfairly penalized because of their brevity. Scorers were instructed to rate on a scale of 0–10 how well they felt the description evoked a detailed “picture” of the experience in their own mind's eye (0, no picture at all; 10, vivid, extremely rich picture). The imagined experiences of the patients were judged as significantly poorer in quality than those of the controls (P = 0.004). The pattern of quality judgment scores matched the pattern of the overall experiential indices very closely, and, in fact, when an analysis was done without the contribution of the quality judgments to the experiential index, the patients still scored significantly lower than controls (P = 0.001).
P01.
While overall the patient group was impaired at imagining new experiences compared with the control group, examination of Figs. 2 and 3 shows that one of the five patients (P01; see Methods and SI Text) appeared to be unimpaired on the task. This finding was confirmed when his performance was compared with his two exactly matched control subjects. A separate analysis of the data excluding P01 and his two matched control subjects, however, made no difference to the statistical significance of any of the results reported above. The fact that the patient group were so impaired even when P01 was included illustrates the extent of the deficit in imagining new experiences. Furthermore, the other four patients were remarkably homogenous in their performances (see Figs. 2 and 3), despite differences in age, IQ, and memory profiles (see Methods and SI Text). Possible reasons for P01's atypical performance compared with the other patients are considered in Discussion (see also SI Text).
Discussion
In this experiment, we devised a paradigm where participants, rather than recollecting the past, had to imagine new experiences. Our results demonstrate that amnesic patients with bilateral hippocampal damage were significantly impaired on this task. From this study, we are able to draw several conclusions. To begin with, this systematic study formally documents that patients with hippocampal amnesia have a deficit in richly imagining new experiences. Second, in revealing this we show that the role of the hippocampus extends beyond reliving past experiences, encompassing not only imagining plausible self-relevant future events, but also more generally the construction of fictitious experiences. Third, our findings offer some insight into a mechanism whose absence could underpin all of these deficits. The patients' imagined experiences were strikingly deficient in spatial coherence, resulting in their constructions being fragmented and lacking in richness. The hippocampus, therefore, may make a critical contribution to the creation of new experiences by providing the spatial context or environmental setting into which details are bound (6, 7, 24, 27). Given how closely imagined experiences match episodic memories, the absence of this function mediated by the hippocampus may fundamentally affect the ability to re-experience or reconstruct past events.
Whenever one examines patients with amnesia, and in particular using a novel task that takes more than a few seconds, it could be argued that the results merely reflect their anterograde memory impairment. However, patients had access to a reminder cue during each trial, and the examiner verified throughout that they had not forgotten the task, the instructions, or their own constructions. There were no instances of confusion, requests for clarification, and, in particular, no evidence of repetition, which one might have expected if patients were forgetting recently generated scene elements and then constructing them anew (see excerpts in Fig. 1 and SI Fig. 4).
According to the traditional view of memory (5, 28–30) the role of the hippocampus in episodic memory is time-limited, with these memories consolidated to the neocortex over time. Within this framework it is held that the neocortex contains generalized representations for spatial contextual (e.g., a beach, a market) and nonspatial (e.g., object) memories, and therefore supports remote memory independently of any contribution from the hippocampus/medial temporal lobe (5). According to this view then, successful imagination of experiences would be expected to occur in the presence of hippocampal lesions, by the coordination of activity in multiple neocortical areas, perhaps mediated by the goal-directed temporary or online binding capacities of the prefrontal cortex in working memory (31–33). The demonstration that patients with hippocampal amnesia are impaired at generating new imagined experiences poses a challenge to the traditional model.
It could be argued, however, that the creation of imagined new experiences relies on retrieval of recent episodic memories, a process severely disrupted in hippocampal amnesics. Although we cannot entirely exclude this possibility, we feel it to be unlikely for several reasons. First, commonplace, everyday scenarios were specifically selected to increase the dependence of constructions on generalized semantic memory representations formed from numerous prior experiences, and thus minimize any possible contribution of recent (or even remote) episodic memories. Therefore, an inability to use recent episodic memories to aid their constructions is likely to have caused, at most, a mild impairment on the construction task, rather than the devastated performance that we observed. Second, we explicitly instructed participants not to recount an actual memory or any part of one. That our instructions were adhered to is evidenced by control subjects reporting a low dependence on episodic memories to produce rich constructions. Finally, the fact that one densely amnesic patient was unimpaired on the construction task is further evidence against intact episodic retrieval capabilities being a necessary prerequisite for imagining new experiences.
Although our findings are inconsistent with the traditional view (5), they accord well with suggestions that the hippocampus plays a critical role in imagining experiences through the provision of spatial context, in perpetuity, either through its ability to process spatial information (7, 24, 27) or to bind together disparate elements of the imagined scene (6, 34–36). Given the striking similarities between the process of imagining new experiences and reliving past memories (19–21) our findings may also have implications for the status of remote episodic memory after hippocampal damage. It has been suggested that discrepancies between studies of remote episodic memory in hippocampal patients (8) might be accounted for by differences in the quality or richness of the recollective experience, a feature that is not always captured by existing scoring systems (7, 13, 37). Indeed, recent evidence suggests that the hippocampus may be critical for recollecting vivid, detailed episodic memories, regardless of their age (7, 38, 39). Our results are consistent with this perspective and moreover suggest the critical attribute determining whether internally generated experiences, either real or imaginary, are hippocampal-dependent may be the extent to which they are vividly (re-)experienced.
One caveat to our findings is that P01 was unimpaired on the construction task. Whereas the other four patients, despite variations in age, education, IQ, and memory profiles, were found to be strikingly homogenous in their performances, P01 is clearly distinct. Although the reason for his spared performance is uncertain, it may relate to a degree of residual hippocampal function. Indeed, this patient retains the ability to acquire new semantic information (40). Moreover, in a separate functional MRI study, P01's spared capacity for encoding new semantic information (retained even when tested a few months later) was associated with significant activation in residual hippocampal tissue (see SI Text and SI Fig. 5). We suggest, therefore, that this patient may have sufficient functional hippocampal tissue to allow him to perform successfully on the construction task.
In this study, we demonstrate that patients with hippocampal amnesia are impaired on a novel task that shares many similarities with episodic memory, namely the construction task. As such we were able to provide insights into the nature of essential neural mechanisms carried out by the hippocampus. Our current task was optimized to examine spatial deficits within the more general context of rich experiencing. In the future, it will be interesting to examine whether the lack of coherence observed extends to other nonspatial aspects of an imagined event. Furthermore, it will be important to clarify the precise relationship between construction and episodic memory and establish whether the hippocampus plays a similar role in mediating both of these functions.
Methods
Participants.
Five patients (all male, one left-handed) each with primary damage to the hippocampi bilaterally and concomitant amnesia took part. All but one patient have been reported (40–44) (see also SI Text for summaries of each case and SI Figs. 6–10). Hippocampal damage in three of the cases resulted from limbic encephalitis associated with voltage-gated potassium channel antibodies (VGKC-Ab), meningeoencephalitis and then recurrent meningitis in one case, and limbic encephalitis (not associated with VGKC-Ab) in one case. The mean age of the patients was 52.8 years (SD 18.5, range 24–70), years of education was 14.0 years (SD 3.7, range 11–19) and verbal IQ was 103.2 (SD 11.7, range 90–116). All of the patients had significant impairment of anterograde memory; some were deficient on both recognition and recall tests, others on recall tests alone. Retrieval of premorbid semantic memory was intact in all cases, while retrograde memory for episodic experiences was impaired, with the amnesic period ranging from 10 years to a complete lifetime. Language, perceptual, verbal fluency, and executive functioning were within the normal range in all cases.
Lesions were confirmed by structural MRI scans and appeared to implicate the hippocampi with no evidence of damage in adjacent medial temporal areas. It is notoriously difficult, if not impossible, to be certain in vivo that lesions are selective to a particular brain region. Even scrupulous measurements or ratings of tissue volume from MRI scans cannot provide a definitive indication as to whether the tissue is functioning or not (43, 45, 46) (see also SI Text) or the functional effect of a lesion on wider brain systems. In the patients we tested, the primary area of damage in every case seemed restricted to the hippocampi, which was the only area of overlap. Their neuropsychological profiles suggested an isolated memory impairment, and their performance on the experimental task was remarkably homogenous (excluding P01; see Results, Discussion, and SI Text). Notwithstanding the difficulties inherent in the field, we therefore feel that our data permit conclusions to be drawn regarding the hippocampus.
Ten healthy control participants also took part (all male, one left-handed). The mean age of the control subjects was 52.2 years (SD 16.9, range 25–76), years of education was 14.1 years (SD 2.8, range 11–17), and verbal IQ was 104.3 (SD 6.3, range 94–112). There was no significant difference between the patients and controls on these background characteristics (age P = 0.95; education P = 0.95; IQ P = 0.81). In addition to comparing the two groups, we ensured that each patient was matched exactly to two of the control subjects on age, education, and IQ. All participants gave informed written consent to participation in the study in accordance with the local research ethics committees.
Task and Procedure.
Each participant was tested individually and sat facing the examiner. Testing sessions were digitally recorded to enable transcription and later scoring of participants' responses. The requirements of the imagining new experiences task were explained, and several examples were provided. During this practice phase we also established that patients could remember the instructions and the cues throughout a construction trial. Commonplace, ordinary settings were chosen as scenarios to minimize the difficulty level and to be as independent from a participant's innate creative ability as possible. The scenarios also purposely encompassed a wide variety of different subject matters from the manmade to the natural and the busy to the isolated to ensure there were no content biases. For each scenario a short description was read out loud by the interviewer from a prepared script (e.g., “Imagine you're lying on a white sandy beach in a beautiful tropical bay;” see also Fig. 1 and SI Fig. 4), and the participant was instructed to vividly imagine the situation from the cue and describe it in as much (multimodal) detail as possible. Participants were explicitly told not to recount an actual memory or any part of one but rather create something new. A printed text card was placed on the desk in front of the participant summarizing the main concept of the scenario to act as a reminder if needed. Participants were allowed to continue with their descriptions until they came to a natural end or they felt nothing else could be added. A probing protocol dictated the appropriate use of statements used by the examiner during the session. These mostly took the form of general probes encouraging further description (e.g., “can you see anything else in the scene?”), or asking for further elaboration on a theme introduced by the subject (e.g., “can you describe the fishing boat in more detail?” in response to the subject saying “I can see a small fishing boat gently rocking out in the sea”). It was strictly prohibited for the examiner to introduce any concept, idea, detail, or entity that had not already been mentioned by the subject. After each scenario, participants were asked to rate their constructions across a number of different categories (see Results and SI Text). At various points during a trial, and before the postscenario ratings, the examiner verified that the participant still recalled the task instructions, the scenario in question, and the scenario he had created.
In the assisted version of the task (see Results), the scenarios were similar in nature and the tasks were identical except that before commencing imagination, four-color photographs of objects relevant to the scenario, a relevant sound was played, and a relevant smell was provided. These were available to the subject throughout the imagination task, with the sound periodically played by the examiner, and the subject invited to smell the relevant odor.
During the pilot studies we attempted to contrast the first-person perspective of the main task with a third-person or observer perspective. We found, however, that healthy pilot subjects effectively adopted a first-person perspective in any case. Of course, this does not rule out the possibility there could be an effect of a different perspective provided a first-person perspective could be definitely excluded. This is a potentially interesting issue for future studies.
Scoring.
A composite score, the experiential index, ranging from 0 to 60, measuring the overall richness of the imagined experience, was calculated from the four subcomponents: content, participant ratings, spatial coherence index, and quality judgments. Full details of scoring system are provided in SI Text. Similar to the approach used in studies of autobiographical memory (13), the primary scorer was not blind to subject status. Therefore to assess interrater reliability and scoring system robustness, two subjects (one patient and one control) were selected at random and their constructions (representing 13.3% of all constructions) were assessed by a second trained scorer who was blind to subject identity. A two-way ANOVA was performed with scorer and subject as factors. There was no significant main effect of scorer on experiential index ratings [F(1,9) = 0.117; P = 0.74] indicating a high degree of scorer reliability.
Statistical Analysis.
The two groups (patients and controls) were compared by using two-tailed independent t tests with a significance threshold of P < 0.05. For comparison of a patient's performance on standard compared with assisted scenarios, paired two-tailed t tests were used with the same statistical threshold.
Supplementary Material
Acknowledgments
We thank the patients and their families for giving of their time so generously; Brian Levine for information about the autobiographical interview; John Welch for details of P03's retrograde memory; Dennis Chan for similar details about P04; Jason Warren and the Dementia Research Centre at the Institute of Neurology, Tim Griffiths and the Cognitive Neurology Clinic at Newcastle General Hospital (Newcastle upon Tyne, U.K.), John Aggleton at the School of Psychology, Cardiff University, and Geoffrey Schott at the National Hospital (London) for patient referrals; and Peter Aston, Katherine Woollett, Hugo Spiers, and Neil Burgess for assistance and helpful discussions. This work was funded by the Wellcome Trust and the Brain Research Trust.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610561104/DC1.
References
- 1.Tulving E. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- 2.Scoville WB, Milner B. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zola-Morgan S, Squire LR, Amaral DG. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. J Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squire LR, Stark CE, Clark RE. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 6.Eichenbaum H. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. J Anat. 2005;207:35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayley PJ, Gold JJ, Hopkins RO, Squire LR. Neuron. 2005;46:799–810. doi: 10.1016/j.neuron.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. Curr Opin Neurobiol. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Spiers HJ, Maguire EA, Burgess N. Neurocase. 2001;7:357–382. doi: 10.1076/neur.7.5.357.16245. [DOI] [PubMed] [Google Scholar]
- 11.Maguire EA. Philos Trans R Soc London B. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayley PJ, Hopkins RO, Squire LR. Neuron. 2003;38:135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- 13.Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Psychol Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- 14.Burgess N. Q J Exp Psychol A. 2002;55:1057–1080. doi: 10.1080/02724980244000224. [DOI] [PubMed] [Google Scholar]
- 15.Tulving E. Elements of Episodic Memory. Oxford: Clarendon; 1983. [Google Scholar]
- 16.Rosenbaum RS, Kohler S, Schacter DL, Moscovitch M, Westmacott R, Black SE, Gao F, Tulving E. Neuropsychologia. 2005;43:989–1021. doi: 10.1016/j.neuropsychologia.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Klein SB, Loftus J, Kihlstrom JF. Soc Cognit. 2002;20:353–379. [Google Scholar]
- 18.Atance CM, O'Neill DK. Trends Cognit Sci. 2001;5:533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg DL, Rubin DC. Cortex. 2003;39:687–728. doi: 10.1016/s0010-9452(08)70860-8. [DOI] [PubMed] [Google Scholar]
- 20.Conway MA, Pleydell-Pearce CW. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- 21.Schacter DL. Searching for Memory: The Brain, the Mind, and the Past. New York: Basic Books; 1996. [Google Scholar]
- 22.Rubin DC, Schrauf RW, Greenberg DL. Mem Cognit. 2003;31:887–901. doi: 10.3758/bf03196443. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler MA, Stuss DT, Tulving E. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- 24.Burgess N, Becker S, King JA, O'Keefe J. Philos Trans R Soc London B. 2001;356:1493–1503. doi: 10.1098/rstb.2001.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosslyn SM, Ganis G, Thompson WL. Nat Rev Neurosci. 2001;2:635–642. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum RS, McKinnon MC, Levine B, Moscovitch M. Neuropsychologia. 2004;42:1619–1635. doi: 10.1016/j.neuropsychologia.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 27.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Oxford Univ Press; 1978. [Google Scholar]
- 28.Alvarez P, Squire LR. Proc Natl Acad Sci USA. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Squire LR, Alvarez P. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 30.Squire LR. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 31.Moses SN, Ryan JD. Hippocampus. 2006;16:43–65. doi: 10.1002/hipo.20131. [DOI] [PubMed] [Google Scholar]
- 32.Prabhakaran V, Narayanan K, Zhao Z, Gabrieli JD. Nat Neurosci. 2000;3:85–90. doi: 10.1038/71156. [DOI] [PubMed] [Google Scholar]
- 33.Fuster JM. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. New York: Lippincott–Raven; 1997. [Google Scholar]
- 34.McClelland JL, McNaughton BL, O'Reilly RC. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 35.Hannula DE, Tranel D, Cohen NJ. J Neurosci. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee AC, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, Gaffan D, Bussey TJ, Davies RR, Kapur N, Hodges JR, Graham KS. Hippocampus. 2005;15:782–797. doi: 10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- 37.Kopelman MD, Wilson BA, Baddeley AD. J Clin Exp Neuropsychol. 1989;11:724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- 38.Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Cereb Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- 39.Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- 40.McKenna P, Gerhand S. Cortex. 2002;38:37–58. doi: 10.1016/s0010-9452(08)70637-3. [DOI] [PubMed] [Google Scholar]
- 41.Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, Yonelinas AP. Neuropsychologia. 2005;43:1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Samarasekera SR, Vincent A, Welch JL, Jackson M, Nichols P, Griffiths TD. J Neurol Neurosurg Psychiatry. 2006 doi: 10.1136/jnnp.2006.093096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maguire EA, Nannery R, Spiers HJ. Brain. 2006;129:2894–2907. doi: 10.1093/brain/awl286. [DOI] [PubMed] [Google Scholar]
- 44.Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, Burgess N. Hippocampus. 2007;17:34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maguire EA, Frith CD, Rudge P, Cipolotti L. NeuroImage. 2005;27:146–152. doi: 10.1016/j.neuroimage.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Maguire EA, Vargha-Khadem F, Mishkin M. Brain. 2001;124:1156–1170. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.