Abstract
This article provides current information on the potential role of oxidation in relation to age-related macular degeneration (AMD). The emphasis is placed on the generation of oxidants and free radicals and the protective effects of antioxidants in the outer retina, with specific emphasis on the photoreceptor cells, the retinal pigment epithelium and the choriocapillaris. The starting points include a discussion and a definition of what radicals are, their endogenous sources, how they react, and what damage they may cause. The photoreceptor/pigment epithelium complex is exposed to sunlight, is bathed in a near-arterial level of oxygen, and membranes in this complex contain high concentrations of polyunsaturated fatty acids, all considered to be potential factors leading to oxidative damage. Actions of antioxidants such as glutathione, vitamin C, superoxide dismutase, catalase, vitamin E and the carotenoids are discussed in terms of their mechanisms of preventing oxidative damage. The phototoxicity of lipofuscin, a group of complex autofluorescent lipid/protein aggregates that accumulate in the retinal pigment epithelium, is described and evidence is presented suggesting that intracellular lipofuscin is toxic to these cells, thus supporting a role for lipofuscin in aging and AMD. The theory that AMD is primarily due to a photosensitizing injury to the choriocapillaris is evaluated. Results are presented showing that when protoporphyric mice are exposed to blue light there is an induction in the synthesis of Type IV collagen synthesis by the choriocapillary endothelium, which leads to a thickened Bruch’s membrane and to the appearance of sub-retinal pigment epithelial fibrillogranular deposits, which are similar to basal laminar deposits. The hypothesis that AMD may result from oxidative injury to the retinal pigment epithelium is further evaluated in experiments designed to test the protective effects of glutathione in preventing damage to cultured human pigment epithelial cells exposed to an oxidant. Experiments designed to increase the concentration of glutathione in pigment epithelial cells using dimethylfumarate, a monofunctional inducer, are described in relation to the ability of these cells to survive an oxidative challenge. While all these models provide undisputed evidence of oxidative damage to the retinal pigment epithelium and the choriocapillaris that is both light- and oxygen-dependent, it nevertheless is still unclear at this time what the precise linkage is between oxidation-induced events and the onset and progression of AMD.
Oxidative processes have been proposed to play a causative or contributing role in a steadily growing number of diseases, such as heart disease, certain types of cancers, neurodegenerative disorders, cataract, and age-related macular degeneration (AMD). Much has been made in recent years about oxidative stress, free radicals and how oxygen, which is necessary for life in providing aerobic respiration, may also be toxic. Vision loss in AMD occurs through photoreceptor damage in the macula, with abnormalities in the retinal pigment epithelium (RPE) and Bruch’s membrane being the hallmark of the disease [1,2]. Since the original study by Noell et al. [3] showing that exposure of freely moving rats to continuous bright visible (green) light leads to selective degeneration of rod photoreceptor cells, there has been a growing concern that long-term exposure to sunlight may be a contributing factor to the development of AMD [4]. Indeed, it was Noell et al. who first suggested that the mechanism of light damage may involve a photosensitization reaction. The outer retina and, in particular, the outer segments of photoreceptors contain high concentrations of polyunsaturated fatty acids (PUFAs) in the membranes. This region is also exposed to a relatively high oxygen tension which is close to that found in arterial blood. Given the well-known susceptibility of PUFAs to undergo oxidation in the presence of oxygen or oxygen-derived radical species, it is understandable why oxidative steps are thought to be involved in the pathogenesis of AMD. The nature of these radical species and their sources of generation will be described.
As stated by Young [4], “the first sign of senescence in (outer retinal layers) is the appearance of residual bodies (lipofuscin) within the RPE. Progressive engorgement of the RPE cells with lipofuscin is accompanied by abnormal excretions, which accumulate on the basal aspect of the cells and within Bruch’s membrane. This process of cellular impairment may finally culminate in the death of the RPE and visual cells.” Lipofuscin is the generic name given to a heterogeneous group of complex, autofluorescent lipid/protein aggregates present in a wide variety of both neuronal and non-neuronal tissues [5]. In the eye, lipofuscin accumulates within the RPE throughout life eventually occupying up to 19% of cytoplasmic volume by 80 years of age [6]. Unlike other cells in the body, in which lipofuscin occurs through autophagic breakdown of intracellular organelles, the major substrate for lipofuscin formation in the RPE is the undegradable endproducts resulting from the phagocytosis of photoreceptor outer segments. There is considerable evidence linking the formation of lipofuscin to autoxidative tissue damage suggesting that lipofuscin is (at least in part) a product of autoxidation [7]. The major sites of lipid peroxidation damage for most cell types are at the mitochondrial and microsomal membranes which contain relatively large amounts of PUFAs [8]. These peroxidized lipids are thought to act as the principal precursors of lipofuscin damage. In vivo, lipofuscin granules are continually exposed to visible light (400–700 nm) and high oxygen tensions (70 mm Hg), ideal conditions for the formation of reactive oxygen species, with the potential to damage cellular proteins and lipid membranes. The possible role of lipofuscin in the development of AMD will be presented.
A second site where photosensitization reactions may be involved in the development of AMD is the choriocapillaris [9]. It has been proposed that photoactivation of hemoglobin precursors may occur in red blood cells passing through the choriocapillaris. Activation of these precursors may generate reactive oxygen species, e.g., superoxide, hydrogen peroxide, and singlet oxygen, which may damage the RPE and Bruch’s membrane. Evidence supporting the hypothesis that AMD results from a photosensitizing injury to the choriocapillaris will be outlined.
The different types of oxidants produced in cells require that cells have antioxidant defense systems [10–12]. Antioxidants may act at different levels in the oxidation process, for example, by preventing formation of initiating radicals, binding metal ions or removing damaged molecules. The major cellular water soluble antioxidants are ascorbic acid (vitamin C) and glutathione, with their biochemical/antioxidant importance primarily related to their reducing potentials [13], and enzymes such as superoxide dismutase and catalase [12]. The major lipid-soluble antioxidants include vitamin E, retinoids and carotenoids, the latter group including lutein and xeaxanthin which selectively accumulate in the macular and account for the yellow color observed in this region of the retina [14–18]. The health of the RPE and visual cells is dependent on their ability to metabolize free radicals, lipid hydroperoxides and other potentially toxic compounds. Considerable effort has been expended to ascertain the role of these antioxidants in protecting the retina and RPE from oxidative damage [19], and the National Eye Institute has a large ongoing multicenter collaborative clinical trial (AREDS) to assess the potential benefit of antioxidant supplement in AMD. One such focus has been on the potentially beneficial role of glutathione in protecting RPE cells from oxidative damage, and results of these experiments will be summarized.
Figure 1 presents a generic schematic (and admittedly incomplete) diagram of the kinds of reactions (the Damaging Sequence) that may be involved in the development of oxidative damage which may lead to AMD. Figure 1 also shows the armory of protectants, dark sunglasses to minimize exposure to sunlight, antioxidants (glutathione, vitamin C, vitamin E, carotenoids) and antioxidant enzymes (glutathione peroxidase, catalase, superoxide disumutase), and mechanisms for repair and replacement of damaged molecules. The damaging reactions begin with an oxidation, a photooxidation or a photosensitizing event, which increases the production of oxygen-dependent radicals or reactive species. These species alter structures of macromolecules to yield peroxides, oxidized proteins and DNA strand breaks, precursors of disease and cell death. At this time, it remains unclear whether oxidation is a causative factor in the progression of AMD. Nevertheless, evidence will be presented in the following sections suggesting that phototoxic reactions in the RPE and choroid are an ongoing threat to the health and survival of the photoreceptor cells and the RPE, and ultimately to vision.
Figure 1.
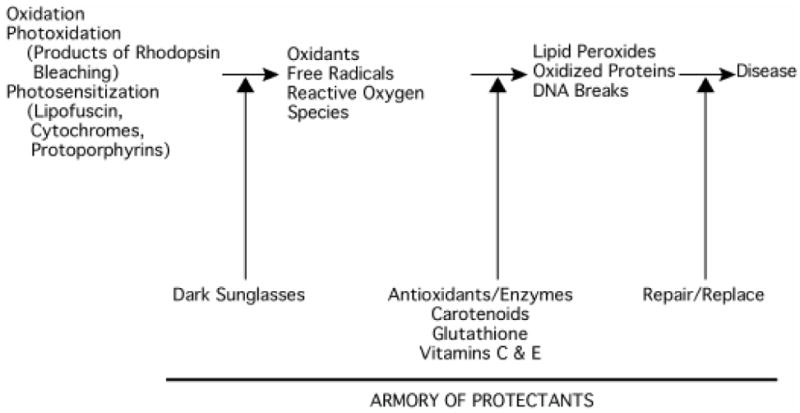
Schematic sequence of damaging reactions. The sequence begins with either an oxidation, a photoxidation or a photosensitization event and ends with a damaging reaction resulting in a disease. It is proposed that oxidants and free radicals produced as a result of these initial events cause lipid peroxidation, the oxidation of critical bonds in proteins and DNA strand breaks. Cells possess an armory of protectants, including antioxidants and enzymes, that serve to quench the oxidants and free radicals, thereby minimizing the damage and the need for repair and replacement. In the case of light-dependent damage to ocular tissues, such as the retina in AMD, dark sunglasses will reduce exposure to sunlight.
BIOCHEMISTRY OF OXYGEN AND ITS COMMON METABOLITES
Figure 2 shows some fundamental features of the chemistry of oxygen and four common oxygen metabolites: superoxide anion, hydrogen peroxide, hydroxyl radical and singlet oxygen. Clearly, there are a number of mechanisms by which oxidants and free radicals are generated. Each mechanism has its own pathway of producing free radicals and of quenching and neutralizing the metabolite. It is important to point out that: (1) a radical is a chemical species with an unpaired electron and it can be neutral or negatively or positively charged, (2) all radicals are not oxidants, and (3) all oxidants are not radicals. Thus, superoxide anion (a radical species) reduces ferric ions (Fe+3) producing ferrous ions (Fe+2), which are more reactive in lipid peroxidation systems, and hydrogen peroxide (H2O2 or HO-OH), an oxidant but not a radical. It can also be seen that the half-lives of these oxygen metabolites vary greatly from nanoseconds to minutes. Moreover, the reactive oxygen species have different abilities to react with molecules. Superoxide anion and hydrogen peroxide are not considered to be as reactive as the hydroxyl radical, which is one of the more reactive of all the free radicals. These reactive oxygen species impairing cell function by readily reacting with lipids in membranes, surface proteins, and transmembrane glycoproteins. Singlet oxygen is a particularly destructive oxygen metabolite. Singlet oxygen is generated by photosensitization reactions wherein a particular molecule (called a sensitizer) absorbs light of a given wavelength, exciting the molecule. The increased energy of the sensitizer, termed the triplet state, can be transferred to molecular oxygen creating singlet oxygen, which can attack a membrane or other cell components. Nature has countered these destructive phenomena with the protective properties of carotenoids found in nearly all plants [20,21]. Although carotenoids can scavenge other free radicals, the primary function of carotenoids is to scavenge singlet oxygen primarily produced by photosensitization. This is believed to be the protective benefit of lutein and zeaxanthin in the macular region of the retina.
Figure 2.
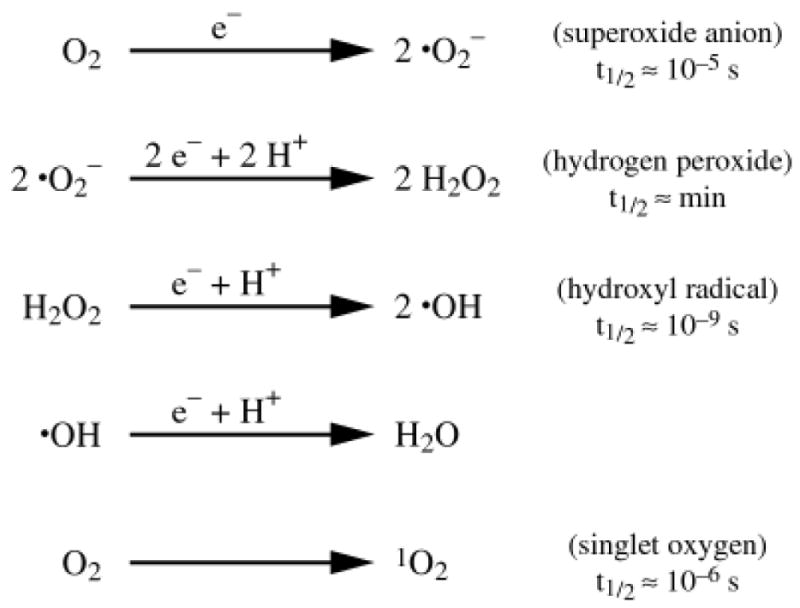
Biochemistry of oxygen derived metabolites. The basic elements and reactions by which oxygen is converted to a variety of oxidants and free radicals are shown. Major oxygen derived metabolites include superoxide anion, hydrogen peroxide, hydroxyl radical and singlet oxygen. Also shown is the approximate half-life (t1/2) for each metabolite.
While the chemistry of oxygen radicals is of interest theoretically, the reality is that many of these molecules are produced as byproducts of normal physiology. Figure 3 provides a short list of endogenous sources of these oxidants and free radicals. Free radicals can be produced by enzymatic generation of reactive oxygen when tissues are subjected to ischemia and then are reoxygenated. The respiratory burst is a well known phenomenon in which inflammatory cells utilize oxygen centered free radicals to kill microorganisms. Even more pertinent to the retina/RPE/choroid complex are the examples highlighted in Figure 3. These endogenous sources include mitochondrial metabolism, rod outer segment phagocytosis, lipofuscin phototoxcity and protoporphryin photosensitization. Indeed, it is well known that there is a high density of mitochondria and a high rate of respiration in the inner segments of rod and cone photoreceptor cells and that outer segment tips, with their high content of PUFAs, are phagocytosed by the RPE [22–26]. It appears that retinal photoreceptors and the RPE live in an environment seemingly primed for oxidant and free radical production, especially during the daylight hours.
Figure 3.
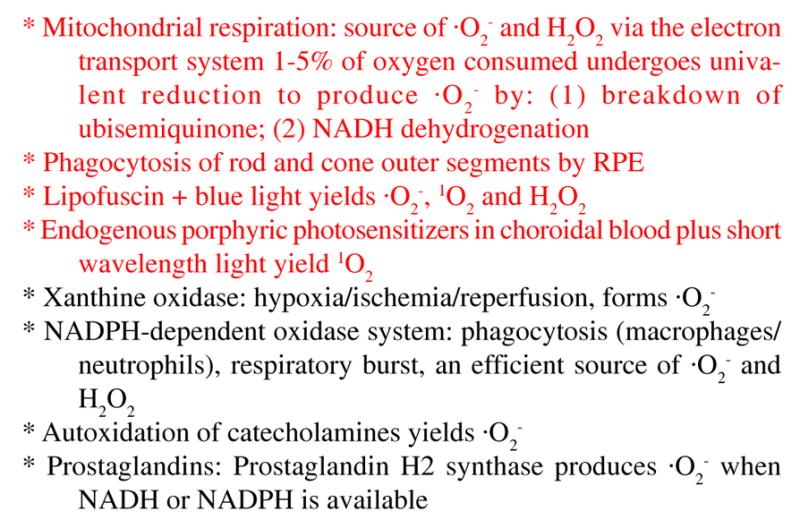
Cellular sources of oxygen radicals. Listed above are a number of enzyme catalyzed reactions and activities in cells which are known to produce oxidants and oxygen free radicals. Particularly relevant to the photoreceptor cell/RPE complex are the four activities highlighted in red.
THE ANTIOXIDANTS/PROTECTANTS
Cells contain a number of antioxidants, which serve various roles in the protection from the hazardous reactions initiated by light, oxygen, and other stimulators of oxidative injury. The major water-soluble antioxidant metabolites are glutathione (GSH) and vitamin C. They carry out antioxidant activities primarily in the cytoplasm and mitochondria. GSH is a naturally occurring tripeptide that acts as a reductant of peroxides either by a nonenzymatic reaction or by a reaction catalyzed by glutathione peroxidase [27]. The major activity of GSH peroxidase is catalyzed by selenoenzymes that are active on fatty acid hydroperoxides, phospholipid hydroperoxides, cholesterol hydroperoxides and hydrogen peroxide. GSH can also be used to detoxify reactive aldehydes generated from lipid peroxidation. Major properties of GSH include: (1) it is stable in nitrogen; (2) it undergoes metal-catalyzed oxidation; (3) its oxidation product, GSSG (oxidized glutathione), is stable at physiological temperature and pH; (4) neither GSH nor GSSG readily passes through membranes; and (5) GSSG is reduced back to GSH by well understood NADPH-dependent pathways involving glutathione reductase and cell metabolism of glucose (hexose monophosphate shunt pathway) and other substrates. Table 1 shows that both GSH reductase and glucose-6-phosphate dehydrogenase (G6PDH) are present in rat retina, rat rod outer segments, bovine rod outer segments, and cultured human RPE cells. Thus, these cells have an active system for reducing GSSG back to GSH, and maintaining its antioxidant capacity.
Table 1.
Enzymes involved in recycling of glutathione
| Tissue | Glutathione Reductase | Glucose-6-phosphate Dehydrogenase |
|---|---|---|
| Rat retina | 25 | 22 |
| Rat rod outer segments | 30 | 20 |
| Bovine rod outer segments | 25 | 25 |
| Human RPE | 20 | 20 |
Activities expressed as nmoles/min/mg protein. Each value is the average of at least 4 individual experiments, and standard deviations were always less than 15% of the means.
Vitamin C is the second major water-soluble antioxidant. Like GSH, vitamin C is stable in nitrogen and undergoes metal-catalyzed oxidation. The oxidation products are, however, unstable in aqueous solution at physiological temperature and pH [13]. The instability of dehydroascorbic acid (DHA, t1/2=3–5 min at pH=7.4 and 37 °C) is a potential problem since this is the only oxidation product that is reduced back to vitamin C in a GSH-dependent manner [13]. In an extensive series of experiments, Winkler et al. [13] showed that the spontaneous degradation of DHA limits the ability of cultured human RPE cells to regenerate vitamin C following an oxidative challenge, unless vitamin C (or DHA) is included in the incubation media. The results suggested that transmembrane transport of vitamin C (or DHA) is necessary for maintaining its level and antioxidant capacity in these cells.
In addition to these metabolites which serve as antioxidants in cells, cells contain several water-soluble enzymes that also act as antioxidants. As already mentioned, glutathione peroxidase catalyzes the reaction between GSH and H2O2 to produce GSSG and water. Also, H2O2 can be converted to water and oxygen by catalase. Glutathione peroxidase has a lower Km for H2O2 than does catalase, but the specific activity of catalase can be many-fold higher than the specific activity of glutathione peroxidase, especially at concentrations of peroxide greater than 1 mM. Though perhaps less well known, hydrogen peroxide can also be converted to water by direct reaction with vitamin C; this reaction also results in the formation of DHA. Superoxide anion is reduced to hydrogen peroxide by enzymatic dismutation involving superoxide dismutase. The H2O2produced by the action of superoxide dismutase can then be converted to water by the several reactions discussed above.
The principal lipid-soluble antioxidants are vitamin E and the carotenoids. Vitamin E is the major chain-breaking lipid-soluble antioxidant in membranes, and thus is expected to play the most important role in minimizing effects of oxidation of PUFAs. Both vitamin E and the carotenoids scavenge free radicals, particularly hydroxyl radical and singlet oxygen. The salient characteristics and structures of vitamin E and selected carotenoids are shown, respectively, in Figure 4 and Figure 5. Both types of compounds are stable in nitrogen, but unstable in oxygen. Vitamin E is recycled by redox coupling with vitamin C. The macular carotenoids, lutein and zeaxanthin, absorb blue light and protect against short wavelength damage to the RPE. Under “resting” conditions, the reduced forms of the antioxidants typically account for 95–99% of their total intracellular content. This is a tribute to the remarkable capacity of cells to maintain an appropriate redox status, even in the face of ongoing oxidative challenges. When the capacities of the respective defense systems are overcome by a heightened or prolonged oxidative challenge, the resultant change to a more oxidized state is generally viewed as a hallmark of incipient damage, and this is a critical factor in the models of oxidative damage and AMD.
Figure 4.
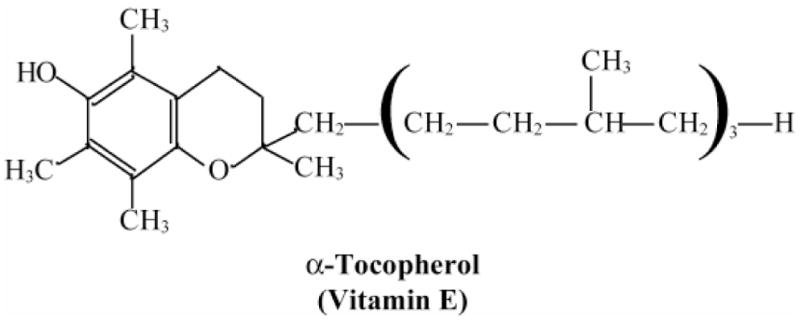
Basic properties and structure of vitamin E. Keys facts regarding vitamin E and its role as a scavenger of free radicals; the structure of vitamin E.
* A group of eight fat-soluble compounds
* α-tocopherol is the biologically most active form
* Absorbed into lymphatics from the intestines
* Protects lipids from peroxidative damage
* A chain-breaking antioxidant that reacts with, ·O2− 1O2, peroxyl (ROO·), and alkoxyl (RO·) radicals
* Major lipid-soluble antioxidant protecting membranes and lipoproteins from injury
* Vitamin E· (radical form) is reduced back to Vitamin E by Vitamin C
Figure 5.

Basic properties and structures of carotenoids. Basic properties of the macular carotenoids lutein and zeaxanthin; the structures of the macular carotenoids are shown with other carotenoids for comparison.
* Absorb blue light, protective against short wavelength visible light
* Quench singlet oxygen
* Quench triplet state of photosensitizers
* Inhibit autoxidation of lipids
* β-carotene, an effective antioxidant at low P O2; assume same for macular carotenoids
* Undergo autoxidation
* Small amount of oxidation products detected in normal human and monkey retinas
PHOTOTOXICITY OF LIPOFUSCIN IN VITRO
The progressive lifelong accumulation of lipofuscin in highly metabolic, postmitotic cells is thought to contribute to a wide variety of age-related and pathological conditions in man [5,7], however, there has been considerable debate as to how lipofuscin affects cell function. Some consider lipofuscin to be an inert substance that acts directly by congesting the cytoplasm (lipofuscin can occupy up to 30% of cell volume in certain tissues [7]), while others propose that lipofuscin is toxic, acting as a source of reactive oxygen species [5,28–30] or releasing lysosomotropic amines [31]. There are also several lines of evidence suggesting that vitamin A metabolites (major components of photoreceptor outer segements) are also important in the development and photoreactivity of lipofuscin [31–33]. First, the accumulation of lipofuscin in rats is dependent on the dietary level of vitamin A [34]. Second, analysis of the major solvent extractable fluorophore of lipofuscin is thought to arise as a Schiff’s base reaction product of retinaldehyde and ethanolamine [31,35]. Thus, lipid peroxides and vitamin A are both likely to be major substrates in lipofuscin formation in the RPE.
Boulton et al. [36] demonstrated RPE lipofuscin to be a photoinducible free radical generator. White light irradiation of RPE lipofuscin granules results in the production of superoxide anions, their rate of production increasing with increasing light intensity. The effect is wavelength dependent; superoxide anion generation is greatest in granules exposed to blue light (400–520 nm) as compared to red light (660–730 nm) or full white light. Subsequent studies found that under aerobic conditions, in addition to superoxide anion, lipofuscin is also capable of photo-generating significant quantities of a variety of reactive oxygen species including singlet oxygen, hydrogen peroxide and lipid hydroperoxides [37–39]. Using laser flash photolysis, it was shown [31] that both lipofuscin and a synthetic fluorophore (A2E) produce excited triplet states and radical species with lifetimes sufficiently long to allow interaction with other molecules of biological significance including ground state singlet oxygen (3O2) [40]; this is in agreement with Gaillard et al. [39]. This reaction has been observed with the formation of excited singlet oxygen (1O2) which is well established as a strong oxidizing and damaging species that can react with DNA, protein and lipids, compromise cell function, and contribute to cellular aging. Furthermore, the action spectrum of singlet oxygen formation by hydrophobic components of lipofuscin indicate that this process is strongly wavelength-dependent and its efficiency decreases with increasing wavelength by a factor of ten, comparing 420 nm and 520 nm [38].
The light-induced generation of reactive oxygen species by lipofuscin supports the hypothetical relationship between light irradiation, aging changes in the retina and retinal degeneration [41]. While it is difficult to compare the effect of chronic low level light (less than 0.1 mW/cm2) with short intense exposures, it is clear that both may contribute to the formation of reactive oxygen species from lipofuscin. Under the conditions used (light exposure=1 mW/cm2) it is possible to make the following extrapolations with respect to superoxide anion [36]: (1) that one lipofuscin granule can produce 8 × 10−19 mol superoxide anion/min; (2) since 1 mol = 6.02 × 1023 molecules then a single granule is capable of producing 4.8 × 105 superoxide molecules/min; and (3) if the average volume of the cell is 2000 μm3 and up to 19% of that volume is occupied with lipofuscin granules [6] of 1 μm in diameter then each RPE cell has the capacity of generating 3.5 × 108 superoxide anions/cell/min. This high level of free radical production may explain why the RPE contains a high concentration and wide variety of antioxidants [5,13].
The spectral dependence of reactive oxygen species generation by lipofuscin (production being greatest in granules exposed to blue light as compared to other regions of the visible spectrum) may explain the so-called “blue light hazard” to the retina. At wavelengths below 550 nm, extended irradiances produce actinic or photochemical lesions but are too low to produce thermal effects [42]. These photochemical lesions are prominent at the level of the RPE and it has been noted that the action spectrum for “blue light damage” is grossly similar to the broad band absorption spectra of both melanin [41] and lipofuscin [5,43]. Analysis of blue light photoreactivity in freshly isolated human RPE cells demonstrates a marked increase in the rate of oxygen photo-uptake as donor age increases and that this photo-uptake is predominantly due to lipofuscin [37]. These observations suggest an adverse functional role for lipofuscin in the cell and may explain the association between high levels of lipofuscin and AMD. The RPE is particularly rich in antioxidants and these may be sufficient to detoxify any reactive oxygen species [5,13,28]. Conversely, the antioxidants may be insufficient to detoxify all the radicals and there may be an insidious buildup of oxidative damage throughout life that only manifests itself in the aged.
It has also been observed that lipofuscin-photosensitization reactions lead to enhanced intragranular lipid peroxidation as measured by accumulation of lipid hydroperoxides and malondialdehyde in illuminated pigment granules [37,44]. This peroxidation correlates with “bleaching” whereby the chloroform soluble fluorophores normally associated with lipofuscin are no longer present. More importantly, lipofuscin is also capable of extragranular peroxidation of lipids and enzyme inactivation. Freshly isolated lipofuscin granules incubated with visible light induce a 30% increase in lipid peroxidation compared to controls. Incubation of granules with antioxidant (catalase) and lysosomal (acid phosphatase) enzymes, in the presence of light, produce a 50% and 30% decrease in activity, respectively. Lipid peroxidation and loss of enzyme activity could be prevented by antioxidants confirming that the photodamage caused by lipofuscin is due to reactive oxygen species.
Studies of the phototoxic potential of lipofuscin, uncovered using photophysical assays, were subsequently extended to a cellular system. Two approaches were used; the first to generate lipofuscin-like granules in cultured RPE cells by feeding rod outer segments to the cells [45] and the second to repigment cultured RPE cells by feeding them isolated human lipofuscin granules [46]. The first approach was deemed inappropriate once it was determined that the accumulated autofluorescent granules, while having some spectral similarities with native RPE lipofuscin, exhibit major differences in solubility and chromatographic mobility of the constituent fluorophores [33]. Thus, all further studies were undertaken on repigmented RPE cells.
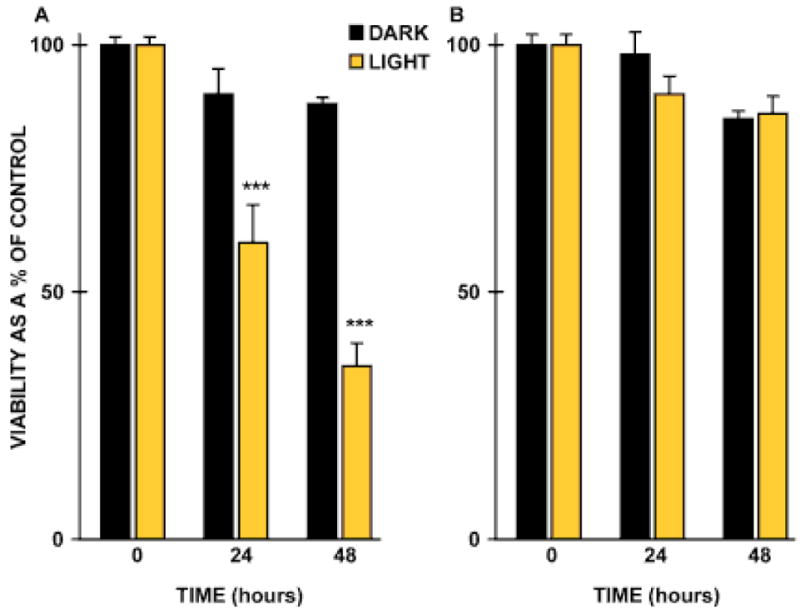
In brief, cultured human RPE cells fed isolated lipofuscin granules (300 granules/cell) and control cells lacking granules were either maintained in the dark or exposed to “blue” light (400–550 nm) or “amber” light (550–800 nm) at irradiances of 0.1–2.8 mW/cm2 at 37 °C for up to 14 days. Cells were then assessed for alterations in morphology, cell viability, lysosomal stability and lipid peroxidation. Exposure to both blue light and lipofuscin led to a time-dependent loss of cells from the monolayer with the remaining cells exhibiting a change in cell morphology, increased vacuolation and membrane blebbing [47]. Cell viability was decreased in a time-dependent manner as compared to control cells; a 1.5-fold (p=0.01) and 2.5-fold (p=0.001) reduction in cell viability was observed at 24 and 48 h, respectively, together with an associated loss of lysosomal stability (Figure 6). This was associated with a two-fold increase in the lipid peroxidation endproducts, malondialdehyde and 4-hydroxynonenal. Morphological changes in compromised cells were indicative of apoptosis. Control cultures were unchanged. Cells exposed to lipofuscin and “amber” light remained viable throughout the experimental duration, indicating that the phototoxic effect was wavelength specific.
Figure 6.

Histogram demonstrating the phototoxicity of lipofuscin. Lipofuscin-fed RPE cells were either maintained in the dark (black bars) or exposed to light (gold bars): A. blue light (400–550 nm); B. amber light (550–800 nm) for up to 48 h. Cell viability was determined using the MTT assay [47]. Vertical bars represent standard error of the mean.
In conclusion, there is now compelling evidence that lipofuscin is a photoinducible generator of reactive oxygen species that can compromise lysosomal integrity, induce lipid peroxidation, and cause RPE cell atrophy. These observations support a role for lipofuscin in RPE aging and the development of AMD.
AMD AND PHOTOSENSITIZATION
Photosensitization is a mechanism that can destroy tissue by utilizing artificial photosensitizers to treat certain types of cancers and vascular formations. But does photosensitization cause disease in humans? In certain porphyrias, photosensitization occurs that can cause damage in sun exposed areas, such as the skin [48]. It has recently been proposed [9] that AMD results from a photosensitizing injury to the choriocapillaris; chronic low level exposure of reactive oxygen to the choriocapillary endothelium induces Type IV collagen synthesis which in turn thickens Bruch’s membrane and choriocapillary septa. Compromised blood supply to the retina has been postulated by others to play a role in drusen formation and the development of AMD [49].
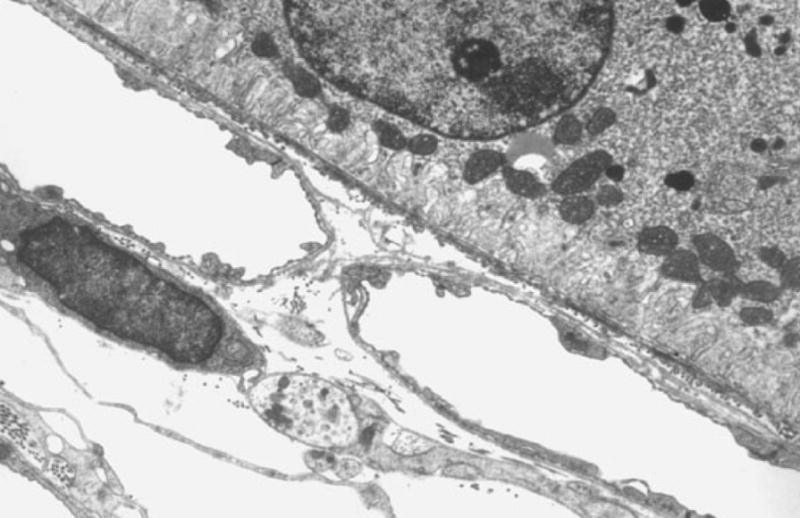
Gottsch et al. [50] have developed an animal model of a chronic low level photosensitizing injury to the choriocapillaris. A mouse model of protoporphyria was used for the development of thickening of Bruch’s membrane and the choriocapillary endothelial basement membrane [51]. In the mouse model of protoporphyria, with an approximately 10-fold increase in protoporphyrin IX and exposure to blue light (380–430 nm, 14μW/cm2), a time and light dependent increase in choriocapillary and subretinal RPE basal laminar-like deposits was demonstrated (Figure 7). At seven months protoporphyric mice exposed to blue light exhibited a 100% thickening of Bruch’s membrane when compared to controls (Figure 8). This thickening extended around the entire basement membrane of the choriocapillary endothelium. A thick band of homogeneous electron-dense material was seen at the level of the choriocapillary basement membrane as were electron-dense fibrillogranular deposits of varying sizes along the inner aspect of Bruch’s membrane (Figure 9). Importantly, the ultrastructure of the RPE and the rod outer segments demonstrated no evidence of light-induced degeneration or other abnormalities in experimental animals or the light and dark controls.
Figure 7.
Choriocapillaris in a mouse model of protoporphyria. In the mouse model of protoporphyria with approximately a 10-fold increase in protoporphyrin IX and exposure to blue light (380–430 nm, 14 μW/cm2), a time and light dependent increase in choriocapillary and subretinal pigment epithelial basal laminar-like deposits are demonstrated (see arrows).
Figure 8.
Normal choriocapillaris in mouse. In dark controls, no increase of the choriocapillary basement membrane was noted.
Figure 9.
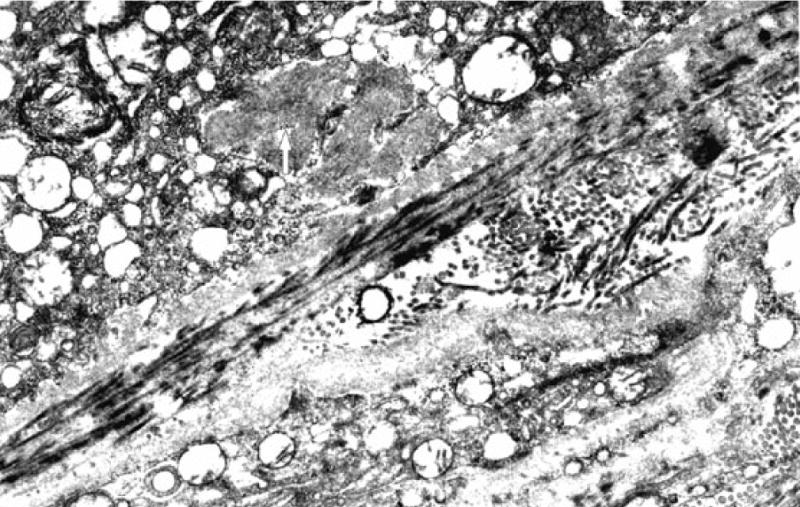
Sub-retinal pigment epithelial deposits in a mouse model of protoporphyria. Protoporphyric mouse model exposed to blue light demonstrates sub-retinal pigment epithelium fibrillogranular deposit (white arrow) with fibrils measuring up to 16 nm with a periodicity of 13 nm.
Using pre-embedding electron-immunocytochemical staining to demonstrate Type IV collagen, the basement membrane of the choriocapillaris and RPE of light-treated protoporphyric mice was compared with that of light-treated control animals. The data showed intense Type IV collagen specific staining of basement membrane of the choriocapillaris with intense staining around choriocapillary endothelial fenestrations (Figure 10). Only very weak staining of the RPE basement membrane occurred in protoporphyric mice. The Type IV collagen of basement membrane of the capillaris thickened with more irregularities suggestive of excessive Type IV collagen deposition. However, there was no significant change in the staining intensity of Type IV collagen in basement membrane of the RPE of light-treated protoporphyric mice in comparison to light-treated control animals on standard diet.
Figure 10.

Type IV collagen staining of choriocapillaris in a mouse model of protoporphyria. Intense Type IV collagen specific staining of basement membrane of the choriocapillaris especially notable around chroiocapillary endothelial fenestrations.
Additional experiments were conducted using longer-term light exposures of cells in culture. Fetal bovine aortic endothelial and RPE cells were incubated with the photosensitizer, protoporphyrin IX (PP IX) with 116 × 10−3 lumens/cm2 in a light irradiating incubator with feedback control to maintain the temperature at 37 °C. 3H-proline and L-proline (1 mM) were added to the media for a consistent labelling environment. A physiologic concentration of PP IX of 1.5 μg/ml was used at which cell growth was minimally retarded and cell culture morphologic appearance was unchanged. Separate cell pellets were processed for total and non-collagen proteins. Blue light filtration experiments were performed utilizing a steep-cut off color filter with a band width of 480–900 nm. β-carotene was obtained as beadlets and suspended in deionized water. Collagen synthesis was increased with light and PP IX when compared to cells exposed to PP IX and dark (p=0.0004), controls in light (p=0.003), and controls in dark (p=0.003); comparisons use a two-tailed Student’s t-test (Figure 11). The use of a blue light filter eliminated radiation beneath 450 nm. Total energy delivered remained the same by increasing the irradiation of longer wavelengths.
Figure 11.
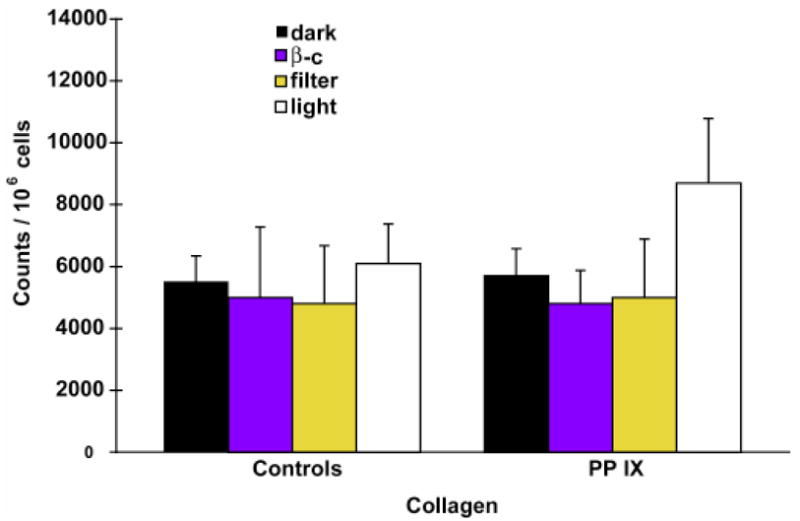
Enhancement of collagen synthesis following photosensitization. Collagen synthesis was increased with light and PP IX exposure when compared to cells exposed to PP IX and dark (p=0.0004), controls in light (p=0.003) and controls in dark (p=0.003; Figure 10). The use of a blue light filter eliminated radiation below 450 nm. Total energy delivered remained the same by increasing intensity irradiation of longer wavelengths.
Certain predictions can be made about this model. The model predicts that AMD is related to sunlight exposure. The accumulative amount of total sunlight exposure may not be important, but the amount of exposure of photosensitizing wavelengths may be. If porphyrins are involved as the photosensitizing agents, then the Soret band (390–440 nm) in the blue light region would necessarily be involved. Although the literature for sunlight exposure and AMD is conflicting, there is evidence by Taylor et al [52] which indicates that in a study of Chesapeake Bay Waterman, there was a correlation of advanced AMD and blue light exposure. Thus, light, particularly in a certain band, may be important in the etiology of this disease.
The model predicts that carotenoids would be protective in AMD. In a multicenter study sponsored by the NEI of risk factors for the development of neovascular AMD, a decrease of serum carotenoids was found to be highly significant for patients with this disease [53]. As already mentioned, carotenoids are best known for their ability to scavenge singlet oxygen, and photosensitization reactions are a major source of singlet oxygen. The model also predicts that the disease would be more prevalent in lightly pigmented individuals where the RPE has fewer melanosomes. This pigment, presumably, would block the penetration of the photosensitizing wavelengths to the choriocapillary endothelium. Racial differences have been observed between blacks and whites in the prevalence of AMD [54], and AMD is less frequently observed in darkly pigmented eyes versus lightly pigmented eyes.
The model also predicts that the site of the primary injury is not the RPE, but is the choriocapillaris. Histopathologic studies of the aging retina demonstrate thickening of Bruch’s membrane with choking of the choriocapillary network, pathologic changes preceding the development of ophthalmoscopic evidence of AMD. Most importantly, in a recent study of Bruch’s membrane and the choriocapillaris comparing aged maculae with those with advanced AMD, those patients with advanced AMD had significantly greater shrinkage of the choriocapillary network with a decrease in choriocapillary density [55]. This resultant compromise of blood flow to the retina has been suggested by a number of investigators to be involved in drusen formation and the development of AMD.
Finally, the model predicts that Type IV collagen would be found in the thickened Bruch’s membrane of patients with AMD. Interestingly, in the mouse model of skin photosensitization, Type IV collagen formation around dermal capillaries was demonstrated [56]. In recent studies of immunolabelling for Type IV collagen and laminin in aged human macula, Type IV collagen is strongly positive in basement membranes of the choriocapillaris [57]. In another study, gross thickening of the choriocapillaris basement membrane was attributed to the deposition of Type IV collagen [58]. The study concluded that only the deposition of Type IV collagen contribute to age-related thickening of Bruch’s membrane.
In summary, a model of the pathogenesis of AMD is proposed based on a photosensitizing injury to the choriocapillaris. The model was able to produce certain pathological features found in AMD such as a thickened basement membrane and fibrillar basal laminar-like deposits in the RPE.
GLUTATHIONE, OXIDATIVE INJURY, AND THE RPE
Sternberg et al. [59] have assessed the effects of incubating cultured human RPE cells with t-butylhydroperoxide (t-BHP). Their results indicated that GSH and its amino acid precursors both protect against oxidative injury. Protection afforded by the amino acids appears to be mediated through GSH because the individual amino acids do not protect and inhibition of GSH synthesis eliminates the protection. Additional studies showed that exogenous GSH also acts against t-BHP-induced injury, apparently by a mechanism different from that provided by the amino acids. This is based upon the lack of effect of inhibitors of GSH synthesis and degradation on the protection by GSH. In addition, intact GSH is not taken up by RPE cells and the concentration of GSH required for protection is only one-tenth of the concentration of amino acids that is needed [60].
The data suggest that exogenous GSH may function by protecting the extracellular surface of cells from oxidants. One possible mechanism for this protection involves stabilization of the cell membrane and protection of membrane proteins such as in transport systems. However, experiments have failed to demonstrate that GSH has any effect on the activity of the Na-K ATPase, with or without treatment of RPE cells with t-BHP. Further, GSH did not protect against oxidative injury by increasing the rate of peroxide elimination from human RPE cells (Sternberg, unpublished observations).
Samiec et al. [61] evaluated the concentration of GSH-related thiol levels in plasma. While plasma GSH decreases with age, there is a concomitant increase in the content of GSSG. There was also a strongly significant age-dependent change in the redox status (Eh) of the ratio of GSH to GSSG (Figure 12), and an increase in plasma cystine levels with age, suggesting that the overall redox status of GSH/GSSG tended to become more oxidized. The levels of GSSG and total glutathione (GSH + GSSG) were measured in two age-matched cohorts of patients: one group with AMD and the second group with retinal diseases other than AMD. A significantly lower level of plasma GSH was found in older individuals (AMD, diabetes, controls) than in younger individuals. Total glutathione was significantly lower only in diabetics.
Figure 12.

Shift in glutathione redox status with aging, AMD, and diabetes. Calculated redox potential (Eh) for GSH pool in blood plasma of younger and older controls and patients with AMD or diabetes. Values for Eh were calculated as Eh = Eo + RT/ZF ln ([GSSG]/(2[GSH])) where Eo was taken as 0.24 V [61], R is the universal gas constant, T is the absolute temperature, Z is the valence, and F is the Faraday constant.
Attempts have also been made to manipulate the GSH content in RPE cells. When administered orally, GSH is poorly absorbed into the blood. When administered in tissue culture, GSH is not transported into human RPE cells. Preliminary studies with the monofunctional inducer, dimethylfumarate (DMF), have shown that this agent stimulates GSH synthesis and elevates intracellular GSH levels in a dose dependent manner. GSH levels in RPE cells rose in concert with an increase in the expression of mRNA for the enzyme γ-glutamyl cysteine ligase, the rate limiting enzyme in GSH synthesis. DMF also protected cultured RPE cells from oxidative injury in the absence of GSH (Figure 13). However, when the time course of GSH stimulation was examined, there appeared to be an initial decrease in intracellular GSH that preceded the elevation in concentration. More recently, Sternberg et al. (unpublished observations) have used another inducer, the antischistomal agent, oltipraz. This compound has been shown to increase intracellular levels of GSH in other cell systems and it also appears to induce an increase in GSH content in RPE cells. Initial indications are that oltipraz does not cause an early decline in GSH content, as was found with DMF. These studies suggest a potential role for these inducers in promoting GSH antioxidant activity without direct supplementation with GSH or amino acid precursors required for the synthesis of GSH.
Figure 13.

Dimethylfumarate protects cultured RPE cells from oxidative injury. A 24 h pretreatment with 0.1 mM dimethylfumarate (DMF) protects cultured human RPE cells from oxidative injury associated with a toxic dose of tertiary butyl hydroperoxide (tBHP). Viability of cells was determined by measuring the extent of leakage of lactic acid dehydrogenase from the cells into the incubation media.
In a final set of preliminary experiments (Sternberg, unpublished observations), cultured human RPE cells were examined for morphological and biochemical changes associated with apoptosis under oxidant conditions. These studies showed that t-BHP causes a number of changes in RPE cells including caspase activation, nuclear condensation, TUNEL-positive staining, and the appearance of phosphatidylserine on the cell surface. All of these findings are consistent with an oxidant-induced apoptotic mechanism of cell death. Mitochondria recently have been found to play an important role in signaling apoptosis [62]. With aging, mitochondria in post-mitotic cells such as the RPE are known to accumulate large deletions and rearrangements in mitochondrial DNA [63]. In this regard, RPE cells treated with t-BHP show an early decline in the mitochondrial membrane potential, suggesting the involvement of a transition in mitochondrial permeability [64]. Furthermore, cytochrome c is released in association with the loss of the mitochondrial membrane potential, and caspase activation occurs over a time course consistent with cleavage of proteins which may contribute to the morphological picture of apoptosis [65]. These findings suggest that oxidant-induced apoptosis may be initiated as a consequence of a permeability transition in the mitochondria.
In summary, at this time there is no clearly established understanding of the etiology or pathogenesis of AMD and no effective treatment for the vast majority of patients. Experiments have been designed to evaluate the mechanisms whereby GSH protects RPE cells against oxidative damage. One interesting approach is to use dietary inducers to stimulate GSH synthesis, as a means to elevate the level of this antioxidant in targeted cells. Finally, studies on human plasma show that with aging there is a shift in the GSH redox potential to a more oxidized state, increasing the ratio of GSSG to GSH, and placing all tissues at risk for age-related diseases, such as AMD. Current work on this theme is focused on evaluating the best way to manipulate the thiol redox state to enhance protection against oxidative injury.
SUMMARY
Reactive oxygen species cause oxidative damage to cytoplasmic and nuclear elements of cells and cause changes to the extracellular matrix. The degree of oxidative damage is restricted by a range of potent antioxidants and the repair of damaged elements. However, some oxidative damage will occur and accumulation of this damage throughout life is believed to be a major contributory factor in tissue aging. The retina is a typical example in which oxidative damage manifests in what we term “retinal aging” and includes loss of retinal cells, accumulation of lipofuscin within the RPE, drusen formation, accumulation of degradative products in Bruch’s membrane and changes in choroidal capillaries. Once these changes become excessive they are believed to contribute to the onset of AMD. This article supports the hypothesis that there is a link between oxidation-induced events and the onset of AMD with the demonstration that: (1) the “age pigment” lipofuscin can be phototoxic to RPE cells, (2) both endogenous and exogenous glutathione play a major role in protective RPE cells against oxidative injury, (3) carotenoids protect against oxidative damage, and (4) oxidative damage to the choriocapillaris of mice leads to pathological changes similar to those seen in AMD. Unequivocal proof is difficult due to the complex pathobiology of AMD and its restriction to human primates; it is hoped that ongoing clinical trials with antioxidants will clarify the issue in the near future.
Acknowledgments
This work was supported by the National Eye Institute, National Institutes of Health, Public Health Service Grants EY 10015 (BSW), EY 07892 (PS), EY06360 (PS) and Core Grant EY 05230 (BSW), by Research to Prevent Blindness (PS), and by the Wellcome Trust (MEB) and Research into Ageing (MEB), United Kingdom. Dr. S. Davies and Mr. S. Ellis contributed to the lipofuscin experiments. The authors thank Mr. Matthew J. Arnold for his assistance in preparing the figures and the manuscript in final form for electronic publication.
References
- 1.Young RW. Pathophysiology of age-related macular degeneration. Surv Ophthalmol. 1987;31:291–306. doi: 10.1016/0039-6257(87)90115-9. [DOI] [PubMed] [Google Scholar]
- 2.Bressler NM, Bressler SB, Fine SL. Age-related macular degeneration. Surv Ophthalmol. 1988;32:375–413. doi: 10.1016/0039-6257(88)90052-5. [DOI] [PubMed] [Google Scholar]
- 3.Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966;5:450–73. [PubMed] [Google Scholar]
- 4.Young RW. Solar radiation and age-related macular degeneration. Surv Ophthalmol. 1988;32:252–69. doi: 10.1016/0039-6257(88)90174-9. [DOI] [PubMed] [Google Scholar]
- 5.Boulton ME. Ageing of the retinal pigment epithelium. Prog Retin Eye Res. 1991;11:125–51. [Google Scholar]
- 6.Feeney-Burns L, Hilderbrand ES, Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984;25:195–200. [PubMed] [Google Scholar]
- 7.Sohal RS, editor. Age Pigments. New York: Elsevier/North Holland Biomedical Press; 1981. [Google Scholar]
- 8.Brunk U, Collins V. Lysosomes and age pigments in cultured cells. In: Sohal RS, editor. Age Pigments. New York: Elsevier/North Holland Biomedical Press; 1981. pp. 243–265. [Google Scholar]
- 9.Gottsch JD, Pou S, Bynoe LA, Rosen GM. Hematogenous photosensitization. A mechanism for the development of age-related macular degeneration. Invest Ophthalmol Vis Sci. 1990;31:1674–82. [PubMed] [Google Scholar]
- 10.Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987;1:358–64. [PubMed] [Google Scholar]
- 11.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of the aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frei B, editor. Natural Antioxidants in Human Health and Disease. San Diego: Academic Press; 1994. [Google Scholar]
- 13.Winkler BS, Orselli SM, Rex TS. The redox couple between glutathione and axcorbic acid: a chemical and physiological perspective. Free Radic Biol Med. 1994;17:333–49. doi: 10.1016/0891-5849(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 14.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–9. [PubMed] [Google Scholar]
- 15.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamin A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272:1413–20. [PubMed] [Google Scholar]
- 16.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62:1448S–61S. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 17.Mayne ST. Beta-carotene, carotenoids, and disease prevention in humans. FASEB J. 1996;10:690–701. [PubMed] [Google Scholar]
- 18.Khachik F, Bersnstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38:1802–11. [PubMed] [Google Scholar]
- 19.Organisciak DT, Winkler BS. Retinal light damage: Practical and theoretical considerations. Prog Ret Eye Res. 1994;13:1–29. [Google Scholar]
- 20.Krinsky NI. Antioxidant functions of carotenoids. Free Radic Biol Med. 1989;7:617–35. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 21.Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9:1551–8. [PubMed] [Google Scholar]
- 22.Young RW. Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci. 1976;15:700–25. [PubMed] [Google Scholar]
- 23.Sjostrand FS. The ultrastructure of the inner segments of the retinal rods of the guinea pig eye as revealed by electron microscopy. J Cell Comp Physiol. 1953;42:45–70. doi: 10.1002/jcp.1030420104. [DOI] [PubMed] [Google Scholar]
- 24.De Robertis E. Electronmicroscopic observations of the submicroscopic organization of the retinal rods. The Journal of Biophysical Biochemical Cytology. 1956;2:319–29. doi: 10.1083/jcb.2.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986;88:521–42. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed J, Braun RD, Dunn R, Jr, Linsenmeier RA. Oxygen distribution in the macaque retina. Invest Ophthalmol Vis Sci. 1993;34:516–21. [PubMed] [Google Scholar]
- 27.Rathbun WB. Glutathione in ocular tissues. In: Dolphin D, Avramovic O, Poulson R, editors. Glutathione: chemical, biochemical, and medical aspects. New York: Wiley; 1989. pp. 469–510. [Google Scholar]
- 28.Handleman G, Dratz E. The role of antioxidants in the retina and retinal pigment epithelium and the nature of pro-oxidant induced damage. Free Radic Biol Med. 1986;2:1–89. [Google Scholar]
- 29.Marshall J. The ageing retina: physiology or pathology. Eye. 1987;1:282–95. doi: 10.1038/eye.1987.47. [DOI] [PubMed] [Google Scholar]
- 30.Boulton M, Docchio F, Dayhaw-Barker P, Ramponi R, Cubeddu R. Age-related changes in the morphology, absorption and fluorescence of melanosomes and lipofuscin granules of the retinal pigment epithelium. Vision Res. 1990;30:1291–303. doi: 10.1016/0042-6989(90)90003-4. [DOI] [PubMed] [Google Scholar]
- 31.Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–6. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- 32.Katz ML, Drea CM, Robison WG., Jr Relationship between dietary retinol and lipofuscin in the retinal pigment epithelium. Mech Ageing Dev. 1986;35:291–305. doi: 10.1016/0047-6374(86)90131-4. [DOI] [PubMed] [Google Scholar]
- 33.Wassell J, Ellis S, Burke J, Boulton M. Fluorescence properties of autofluorescent granules generated by cultured human RPE cells. Invest Ophthalmol Vis Sci. 1998;39:1487–92. [PubMed] [Google Scholar]
- 34.Katz ML, Christianson JS, Gao CL, Handlemen GL. Iron-induced fluorescence in the retina: dependence on vitamin A. Invest Ophthalmol Vis Sci. 1994;35:3613–24. [PubMed] [Google Scholar]
- 35.Sakai N, Decatur J, Nakanishi K, Eldred G. Ocular age pigment “A2E”: an unprecedented pyridinium bisretinoid. J Am Chem Soc. 1996;118:1559–60. [Google Scholar]
- 36.Boulton M, Dontsov A, Jarvis-Evans J, Ostrovsky M, Svistunenko D. Lipofuscin is a photoinducible free radical generator. J Photochem Photobiol B. 1993;19:201–4. doi: 10.1016/1011-1344(93)87085-2. [DOI] [PubMed] [Google Scholar]
- 37.Rozanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, Sarna T. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995;270:18825–30. doi: 10.1074/jbc.270.32.18825. [DOI] [PubMed] [Google Scholar]
- 38.Rozanowska M, Wessels J, Boulton M, Burke JM, Rodgers MA, Truscott TG, Sarna T. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic Biol Med. 1998;24:1107–12. doi: 10.1016/s0891-5849(97)00395-x. [DOI] [PubMed] [Google Scholar]
- 39.Gaillard ER, Atherton SJ, Eldred G, Dillon J. Photophysical studies on human retinal lipofuscin. Photochem Photobiol. 1995;61:448–53. doi: 10.1111/j.1751-1097.1995.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 40.Mulroy L, McGarvey DJ, Truscott TG, Boulton M, Davies S. Age related macular degeneration: understanding the roles of lipofuscin, macular carotenoid pigments and reactive oxygen species. Invest Ophthalmol Vis Sci. 1998;39:S129. [Google Scholar]
- 41.Marshall J. Radiation and the ageing eye. Ophthalmic Physiol Opt. 1985;5:241–63. [PubMed] [Google Scholar]
- 42.Ham WT, Jr, Ruffolo JJ, Jr, Mueller HA, Guerry D., 3d The nature of retinal radiation damage: dependence on wavelength, power level and exposure time. Vision Res. 1980;20:1105–11. doi: 10.1016/0042-6989(80)90047-4. [DOI] [PubMed] [Google Scholar]
- 43.Eldred GE, Katz ML. Fluorophores of the human retinal pigment epithelium: separation and spectral characterization. Exp Eye Res. 1988;47:71–86. doi: 10.1016/0014-4835(88)90025-5. [DOI] [PubMed] [Google Scholar]
- 44.Wassell J, Davies S, Bardsley W, Boulton M. The photoreactivity of the retinal age pigment lipofuscin. J Biol Chem. 1999 doi: 10.1074/jbc.274.34.23828. In press. [DOI] [PubMed] [Google Scholar]
- 45.Boulton M, McKechnie NM, Breda J, Bayly M, Marshall J. The formation of autofluorescent granules in cultured human RPE. Invest Ophthalmol Vis Sci. 1989;30:82–9. [PubMed] [Google Scholar]
- 46.Boulton M, Marshall J. Repigmentation of human retinal pigment epithelial cells in vitro. Exp Eye Res. 1985;41:209–18. doi: 10.1016/0014-4835(85)90026-0. [DOI] [PubMed] [Google Scholar]
- 47.Davies S, Mulroy L, McGarvery D, Truscott TG, Boulton M. The phototoxicity of lipofuscin. Invest Ophthalmol Vis Sci. 1998;39:S129. [Google Scholar]
- 48.Ryan EA, Madill GT. Electron microscopy of the skin in erythropoietic protoporphyria. Br J Dermatol. 1968;80:561–70. doi: 10.1111/j.1365-2133.1968.tb12354.x. [DOI] [PubMed] [Google Scholar]
- 49.Friedman E. A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 1997;124:677–82. doi: 10.1016/s0002-9394(14)70906-7. [DOI] [PubMed] [Google Scholar]
- 50.Gottsch JD, Bynoe LA, Harlan JB, Rencs EV, Green WR. Light-induced deposits in Bruch’s membrane of protoporphyric mice. Arch Ophthalmol. 1993;111:126–129. doi: 10.1001/archopht.1993.01090010130039. [DOI] [PubMed] [Google Scholar]
- 51.Honigsmann H, Gschnait F, Konrad K, Stingi G, Wolff K. Mouse model for protoporphyria. III. Experimental production of chronic erythropoietic protoporphyria-like skin lesions. J Invest Dermatol. 1976;66:188–95. doi: 10.1111/1523-1747.ep12481938. [DOI] [PubMed] [Google Scholar]
- 52.Taylor HR, West S, Munoz B, Rosenthal FS, Bressler SB, Bressler NM. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992;110:99–104. doi: 10.1001/archopht.1992.01080130101035. [DOI] [PubMed] [Google Scholar]
- 53.The Eye Disease Case-Control Study Group. Risk factors for neovascular age-related macular degeneration. The Eye Disease Case-Control Study Group. Arch Ophthalmol. 1992;110:1701–8. doi: 10.1001/archopht.1992.01080240041025. [DOI] [PubMed] [Google Scholar]
- 54.Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt JC, Martone JF, Royall RM, Witt KA, Ezrine S. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Eng J Med. 1991;325:1412–7. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 55.Ramrattan RS, van der Shaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–64. [PubMed] [Google Scholar]
- 56.Wick G, Honigsmann H, Timpl R. Immunofluorescence demonstration of type IV collagen and a noncollagenous glycoprotein in thickened vascular basal membranes in protoporphyria. J Invest Dermatol. 1979;73:335–8. doi: 10.1111/1523-1747.ep12550349. [DOI] [PubMed] [Google Scholar]
- 57.Marshall GE, Konstas AG, Reid GG, Edwards JG, Lee WR. Type IV collagen and laminin in Bruch’s membrane and basal linear deposit in the human macula. Br J Ophthalmol. 1992;76:607–14. doi: 10.1136/bjo.76.10.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marshall GE, Konstas AG, Reid GG, Edwards JG, Lee WR. Collagens in the aged human macula. Graefes Arch Clin Exp Ophthalmol. 1994;232:133–40. doi: 10.1007/BF00176781. [DOI] [PubMed] [Google Scholar]
- 59.Sternberg P, Jr, Davidson PC, Jones DP, Hagen TM, Reed RL, Drews-Botsch C. Protection of retinal pigment epithelium from oxidative injury by glutathione and precursors. Invest Ophthalmol Vis Sci. 1993;34:3661–8. [PubMed] [Google Scholar]
- 60.Davidson PC, Sternberg P, Jr, Jones DP, Reed RL. Synthesis and transport of glutathione by cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1994;35:2843–9. [PubMed] [Google Scholar]
- 61.Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Jr, Reed RL, Jones DP. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 62.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 63.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–8. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 64.Cai J, Wu M, Nelson KC, Sternberg P, Jones DP. Oxidant-induced apoptosis in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1999;40:959–66. [PubMed] [Google Scholar]
- 65.Susin AS, Zamzami N, Kroemer G. Mitochondria as regulators of apoptosis: doubt no more. Biochem Biophys Acta. 1998;1366:151–65. doi: 10.1016/s0005-2728(98)00110-8. [DOI] [PubMed] [Google Scholar]


