Abstract
Background and aims: Screening for colorectal cancer (CRC) by faecal occult blood testing (FOBT) decreases CRC mortality by 15–33%. Compliance remains an obstacle to maximising the benefit of FOBT screening. We tested the hypothesis that individuals offered FOBT screening but refused would have an increased incidence and worse prognosis for CRC compared with those tested and with controls.
Methods: Annual screening was offered to 3548 average risk individuals, ≥40 years of age, from a highly stable population. A total of 2538 agreed to testing (group 1) and 1010 (28%) refused (group 2). Another 1376 individuals were never offered the test and served as controls (group 3). The groups were followed for 11 years: a three year screening period (1985–1987) and an eight year follow up period at the end of the screening programme (1988–1995). Incidence, stage, and mortality were compared. Characterisation of refusers was completed in 188 and 130 subjects of groups 1 and 2, respectively.
Results: In the screening phase, mortality from CRC was significantly lower in group 1 than in groups 2 and 3. The cumulative incidence of CRC in the eight year follow up period was 21 (0.88%), 23 (2.28%), and 13 (0.94%) in groups 1, 2, and 3, respectively. This shows a reduction of 61.4% in group 1 compared with group 2 (relative risk 0.28 (95% confidence interval (CI) 0.19–0.32)) (p<0.001) and 6.4% compared with group 3 (relative risk 0.93 (95% CI 0.93–1.00)) (NS). During follow up, group 1 subjects also demonstrated a decrease in advanced Dukes' stage and mortality rate by 80% and 64%, and 79% and 62%, compared with groups 2 and 3, respectively. Refusers were more likely to be male, of Asian-African descent, and more likely to smoke, consume more coffee, and less tea or dairy foods.
Conclusions: When accepted, FOBT may protect against CRC for prolonged periods. Individuals who refuse FOBT have a significantly higher CRC incidence and mortality rates than those who accept testing.
Keywords: colorectal cancer, polyp, adenoma, screen, faecal occult blood testing
Screening for colorectal cancer (CRC) by faecal occult blood testing (FOBT) has been shown to decrease mortality by 15–33% in three prospective controlled studies in which more than 200 000 participants were enrolled.1–3 A 33% reduction in mortality was achieved by annual testing but this result may have been obtained due to a high rate of colonoscopies performed for false positive tests.1 The results have been less impressive when testing was performed biannually and without rehydration.4 In a recent meta-analysis of four randomised controlled trials and two non-randomised trials of 330 000 and 130 000 subjects, respectively, there was a 23% reduction in mortality for individuals actually screened.5
Based on the results of these large prospective controlled studies, the US Agency for Health Care Policy and Research recommended annual FOBT, or annual FOBT plus flexible sigmoidoscopy, every five years for CRC screening in average risk populations over the age of 50 years.6 The American Cancer Society issued almost identical guidelines but declined to recommend FOBT alone.7 This may reflect concealed anxiety that FOBT alone may not be as effective in preventing mortality from CRC or may be due to differences in efficacy and effectiveness of various CRC screening tests and procedures.
Compliance is an important issue in every screening programme, including FOBT screening for CRC, and has been reported to range from 30% to 80%.3,8–11 The reasons for refusing screening by FOBT have received little attention.12
In this prospective controlled study, we investigated the effect of FOBT performed annually for three years on early detection and survival from CRC in a relatively small population. We also examined the protective effect of the three annual FOB tests during a follow up period of eight years in which no screening procedure was performed. Special attention was given to follow up of individuals who refused to participate in the screening programme (refusers) in order to test the hypothesis that they would have an increased incidence of CRC and a worse prognosis. The refusers were compared with those who participated in the screening programme (screenees), and with controls not offered FOBT.
MATERIALS AND METHODS
Study design
All study participants were residents of 24 kibbutz settlements, chosen at random from 32 settlements in the Upper Galilee region of northern Israel. The populations of these communities are small (usually less than 1000 adults) and stable. The population of the Upper Galilee is very similar to the rest of the Israeli population in terms of age, sex, and origin. Asymptomatic individuals at least 40 years of age, with no family history of CRC or adenomas, were invited to participate in a three stage CRC screening programme (table 1 ▶) and were offered FOBT. A preliminary report of the results of this study in 17 kibbutz settlements was published in 1992.11 At that time we believed that screening should begin at the age of 40 years. In order to achieve maximum compliance, an educational phase of three months preceded the screening phase. In each kibbutz, a lecture was given by a gastroenterologist (YN), written material was distributed, and personal explanations were generously given by the family physicians and nurses from the kibbutz clinics. The FOBT kits were offered to eligible persons by the family physician. In the screening phase (1985–1987), annual FOBT testing was offered to residents of each kibbutz and the population was divided into screenees (group 1) and refusers (group 2) on the basis of compliance. Compliance was monitored by one of the authors (YN). After the three year screening phase had been completed, screenees and refusers were followed for a further eight years (follow up phase, 1988–1995) during which no screening procedures were systematically performed. During the screening phase, individuals in groups 2 and 3 were referred for investigation only when there were relevant symptoms such as rectal bleeding or change in bowel habit. A similar policy was operative for all three groups during the follow up phase.
Table 1.
Demographic characteristics of group 1 (screenees), group 2 (refusers), and group 3 (controls)
| Group 1 | Group 2 | Group 3 | |
| n | 2538 | 1010 | 1376 |
| Average settlement eligible population size | 147.5 | 275.2 | |
| Sex (male) | 1175 (46.3) | 636 (62.97)* | 674 (48.98 ) |
| Mean (SD) age (y) | 58.03 (9.30) | 56.22 (3.98) | 58.22 (6.22) |
| Origin | |||
| Asia-Africa | 197 (7.76 )† | 156 (15.45) | 132 (9.61) |
| Europe-America | 1599 (63.0) | 671 (66.44) | 811 (58.96) |
| Israel | 742 (29.24) | 183 (18.11) | 433 (31.42) |
Values are number (%) unless otherwise stated.
*The number of males was significantly greater in group 2 (p<0.01).
†Difference in distribution of ethnic groups in the population was significant (p<0.01).
Data
All relevant medical files in each kibbutz clinic were studied by one of the authors (ML), and data on morbidity, mortality, and medical evaluation were extracted. Between 2% and 3% of individuals from each group left the kibbutz and were not included in the analysis. Data on mortality were confirmed by cross reference with the Central Population Registry of the Ministry of the Interior, and if cancer was diagnosed, by the Cancer Registration of the Ministry of Health. The Israeli Cancer registry is almost complete and includes all areas of Israel. The sources are death certificates, and all endoscopic, surgical, and pathological reports. None of the subjects who left the kibbutz had CRC or died of CRC according to these sources. Hospitalisation and outpatient files, as well as endoscopy and pathological reports of the district hospital, Rebecca Sieff Government Medical Center, were retrieved and studied. The Dukes' stage of each CRC was recorded.
Study groups
There were 3548 average risk individuals (asymptomatic with no family history of CRC and no premalignant state), at least 40 years of age, who were eligible for the three year screening phase. A total of 2538 (71.5%) agreed to testing (group 1, screenees) and 1010 (28%) refused (group 2, refusers). An additional 1376 individuals at least 40 years of age from another five kibbutz settlements, similar in socioeconomic and demographic patterns who were never offered screening, served as controls (group 3). At that time screening for CRC was not a regular procedure in Israel. All of these subjects were followed during both the screening and follow up phases of the study. Only individuals with CRC diagnosed in the screening phase did not enter the follow up phase.
Screening methods
Stool specimens were collected sequentially for three days, and samples from two different sites in the stool were applied to the two windows (Hemoccult II; Smith, Kline and French Diagnostics, USA). The cards were returned to the clinic by personal delivery. The FOBT slides were kept at room temperature and developed within 48 hours, without rehydration, by one of the authors (YN) who was experienced in FOBT processing.13 When FOBT was positive, a total colonoscopy was performed. When a polyp was found, it was removed and retrieved for pathological examination. When CRC was diagnosed, the patient was referred for surgery and oncological treatment appropriate for the Dukes' stage. Screenees who had negative FOBT at each stage continued testing for up to three years.
Health and diet questionnaire
At the end of the study, validated standard nutritional and health questionnaires14 were offered to 240 members of groups 1 and 2, and completed by 188 (78%) and 130 (54%) subjects from groups 1 and 2, respectively (p=0.001). Participants who completed the questionnaires were interviewed by one of the authors (ML) who verified and computed the data.
Statistical analysis
Data are reported as mean (SD). The χ2 test was used to compare the distribution of categorical variables between the three groups at a significance level of p<0.05, as indicated. Proportions were compared using Fisher's exact test (two tailed), and relative risks and 95% confidence intervals (CI) were calculated. Comparisons of survival were made using the Cox proportional hazards model. Survival curves were calculated by the Kaplan-Meier method.
RESULTS
Subject characteristics
The total population of the 24 participating settlements was 13 000. The screening programme was offered to 3548 men and women who were at least 40 years of age and less than 75 years of age. A total of 2538 subjects agreed to participate in the three year annual screening (compliance rate of 71.5%, group 1), and 1010 subjects refused (group 2). In total, 1376 subjects of similar age from five other kibbutz settlements who were not offered screening (group 3) served as controls. The demographic characteristics of the study and control groups are shown in table 1 ▶. Mean age of individuals was similar among the three groups but group 2 contained more men (p<0.01) and more Asian-African born individuals than groups 1 and 3 (p<0.01).
Prevalence
A positive FOBT was found in 4%, 2.6%, and 2.5% of participants (group 1) during years one, two, and three of the screening phase, respectively (three year prevalence 9.1%). Colonoscopy was performed in all of these individuals. CRC was found in 13 cases (0.51%) and adenoma in 35 cases (1.38%). During the same three year period, there were six (0.59%, NS) and seven (0.51%, NS) cases of CRC in groups 2 and 3, respectively (table 2 ▶). As individuals in group 2 and 3 were investigated only when they had symptoms, no information was available concerning the incidence of adenomatous polyps in these groups.
Table 2.
Cumulative incidence of colorectal cancer (CRC) during the screening and follow up phases
| Group | Subjects enrolled (n) | CRC incidence in screening phase | Subjects followed (n) | CRC incidence in follow up phase | CRC incidence in screening and follow up phases |
| 1 | 2538 | 13 (0.51)* | 2525 | 21 (0.88)† | 34 (1.35) |
| 2 | 1010 | 6 (0.59) | 1004 | 23 (2.28) | 29 (2.89) |
| 3 | 1376 | 7 (0.51) | 1369 | 13 (0.94) | 20 (1.46) |
| 1+2 | 3548 | 19 (0.54) | 3529 | 44 (1.25)‡ | 63 (1.78) |
Values are number (%).
*There was no difference in the incidence of CRC between the groups during screening.
†Incidence was higher in group 2 compared with group 1 (p<0.001) and group 3 (p=0.013).
‡There was no significant difference in incidence comparing group 1 or group 1+2 and group 3.
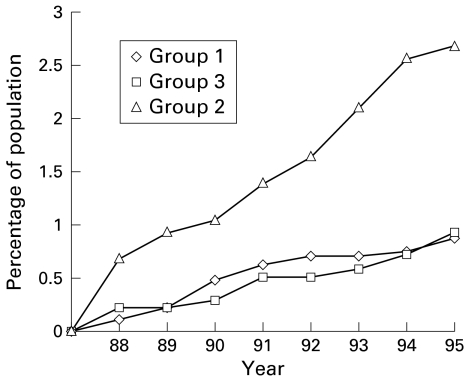
No screening procedures were performed during the eight year follow up phase (1988–1995). Symptomatic individuals were investigated only when the family physician determined that this was required. Colonoscopy, sigmoidoscopy, or barium enema were performed in 700 members: 400 in group 1 (16%), 150 in group 2 (15%), and 178 in group 3 (13%) (NS). The cumulative incidence of CRC in the eight year follow up period was 21 (0.88%), 23 (2.28%), and 13 (0.94%) in groups 1, 2 and 3, respectively (table 2 ▶). This shows a reduction of 61.4% in group 1 compared with group 2 (relative risk 0.28 (95% CI 0.19–0.32)) (p<0.001) and 6.4% compared with group 3 (relative risk 0.93 (95% CI 0.93–1.00)) (NS) (fig 1 ▶). (These were calculated according to the data in table 2 ▶: for group 2 ((2.28%−0.88%)/2.28%)×100=61.4%; for group 3 ((0.94%−0.88%)/0.94%)×100=6.4%.) When groups 1 and 2 were combined, the cumulative incidence of CRC in the same period was 44 (1.25%) which was not statistically different from controls. In the follow up period, the peak incidence of CRC was in the age group 80–89 years for groups 1 and 3, but a decade earlier (age group of 70–79) for group 2. Sex distribution among CRC patients was similar in groups 1 and 3, with a greater number of men in group 2 (p<0.01). In the three groups, most patients were of European-American origin. In group 2 there was an excess of Asian-African born individuals (p<0.01).
Figure 1.
Cumulative incidence of colorectal cancer in the three groups (1988–1995). The cumulative incidence of colorectal cancer decreased in group 1 by 61% and 6% compared with groups 2 and 3, respectively.
Dukes' stage
Dukes' stage for CRC in each group during the screening and follow up phases is shown in table 3 ▶. In group 1, 12/13 (92%) CRC cases were Dukes' A or B compared with 0/6 in group 2 (p<0.001) and 2/7 (29%) in group 3 (p=0.016) during the screening phase. During the follow up phase, there were no differences in Dukes' stage between the groups.
Table 3.
Dukes' stage of colorectal cancer in the screening and follow up phases
| Group | Total | Duke's A | Dukes' B | Dukes' C | Dukes' D | Dukes' A+B | Dukes' C+D |
| Screening phase | |||||||
| 1 | 13 | 8 (61.5) | 4 (30.7) | 1 (7.6) | 0 | 12 (92.3)* | 1 (7.6) |
| 2 | 6 | 0 | 0 | 5 (83.3) | 1 (16.7) | 0 | 6 (100.0) |
| 3 | 7 | 1 (14.3) | 1 (14.3) | 3 (42.8) | 2 (28.6) | 2 (28.6) | 5 (71.4) |
| Follow up phase | |||||||
| 1 | 21 | 2 (9.5) | 12 (57.0) | 5 (23.7) | 2 (9.5) | 14 (66.5)† | 7 (33.2) |
| 2 | 23 | 1 (4.3) | 12 (51.6) | 6 (25.8) | 4 (17.2) | 13 (55.9) | 10 (43.0) |
| 3 | 13 | 1 (7.7) | 5 (38.5) | 5 (38.5) | 2 (15.4) | 6 (46.2) | 7 (53.9) |
*The difference between the Dukes' stage of tumours was significant (p<0.001). There were significantly more cases with tumours of Dukes' stage A+B in group 1 than group 2 (p<0.001) and group 3 (p=0.016).
†There was no difference in Dukes's stage between groups during follow up.
Mortality
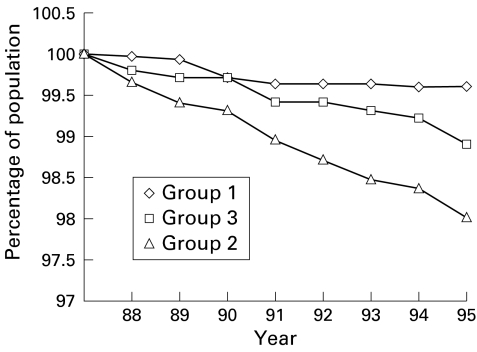
Mortality from CRC during the screening phase was 0.04%, 0.50%, and 0.22% for groups 1, 2, and 3, respectively (p<0.001). During the follow up phase, disease related mortality was 0.42%, 1.99%, and 1.10%. This shows a reduction of 78.9% in group 1 compared with group 2 (relative risk 0.21 (95% CI 0.18–0.23)) (p<0.001) and 61.8% compared with group 3 (relative risk 0.38 (95% CI 0.34–0.40)) (p=0.04) (fig 2 ▶). (These values were calculated according to the data in table 4 ▶: for group 2 ((1.99%−0.42%)/1.99%)×100=78.9%; for group 3 ((1.10%−0.42%)/1.10%)×100=61.8%; for groups 1+2 ((1.10%−0.88%)/1.10%)×100=20.0%.) There was also a 20% reduction in mortality between group 3 and group 1+2 (relative risk 0.80 (95% CI 0.76–0.86)) (p=0.05).
Figure 2.
Survival curve from colorectal cancer in the three groups (1988–1995). The mortality rate decreased in group 1 by 79% and 62% compared with groups B and C, respectively.
Table 4.
Mortality for colorectal cancer (CRC) during the screening and follow up phases
| Group | Individuals enrolled (n) | CRC mortality in screening phase | Individuals followed (n) | CRC mortality in follow up phase |
| 1 | 2538 | 1 (0.04)* | 2525 | 11 (0.42)† |
| 2 | 1010 | 5 (0.50) | 1004 | 20 (1.99) |
| 1+2 | 3548 | 6 (0.17) | 3529 | 31 (0.88)‡ |
| 3 | 1376 | 3 (0.22) | 1369 | 15 (1.10) |
Values are number (%).
*The difference in mortality between the groups during the screening phase was significant (p<0.001).
†Mortality in group 2 was significantly higher than that in groups 1 and 3 (p<0.001 in each case).
‡Mortality in group 1+2 was lower than that in group 3 (p=0.05).
Non-compliant individuals
Individuals who refused FOBT were more likely to be male, of Asian-African descent, and to have undergone fewer investigations in the follow up phase than compliant individuals. Thirty seven per cent (48/130) of patients in group 2 were smokers in comparison with 15% (29/188) in group 1 (p=0.004) (tables 1, 5 ▶ ▶). These individuals also consumed more coffee (p=0.001), fried food (p=0.052), and red meat (NS), and less fibre (p=0.087), tea (p=0.04), and dairy products (p=0.013).
Table 5.
Results of the questionnaire comparing members of group 1 and group 2, eight years after screening
| Group | ||||
| 1 | 2 | Ratio B/A | p Value | |
| n | 2538 | 1010 | ||
| Offered questionnaire | 240 | 240 | ||
| Returned questionnaire | 188 (78%) | 130 (54%) | 0.7 | 0.001 |
| Age (y) (mean (SD)) | 63.1 (10.6) | 61.6 (10.3) | NS | |
| Cancer in the family | 93 (49%) | 26 (20%) | 0.4 | 0.045 |
| Colonic investigation | 106 (56.4%) | 42 (32.3%) | 0.6 | 0.001 |
| Smokers | 29 (15%) | 48 (37%) | 2.5 | 0.004 |
| Coffee* (mean (SD)) | 2.3 (2.0) | 13.5 (2.4) | 5.9 | 0.001 |
| Fried food (mean (SD)) | 1.6 (1.4) | 1.9 (1.3) | 1.2 | 0.052 |
| Red meat (mean (SD)) | 1.2 (1.4) | 1.4 (1.4) | 1.2 | 0.181 |
| Dairy food (mean (SD)) | 4.0 (2.0) | 3.5 (1.9) | 0.9 | 0.013 |
| Tea (mean (SD)) | 1.9 (1.9) | 1.4 (2.4) | 0.7 | 0.040 |
| Fibre (mean (SD)) | 0.5 (1.2) | 0.3 (1.0) | 0.6 | 0.087 |
Values are number (%) or mean (SD).
*For food and beverages, average number of servings per day is compared.
DISCUSSION
We studied the efficacy of annual FOBT in reducing mortality from CRC in a relatively small stable population. We then followed participants for a further eight years to determine if there was an effect beyond the years of testing. To the best of our knowledge, this is the first time that screening with FOBT has proved to be successful in reducing mortality for CRC in a relatively small but well defined population. In the studies reported from Minnesota, Funen, and Nottingham, 46 551, 61 993, and 15 0251 subjects were enrolled, respectively, compared with 5222 in the present study.1–3 We offered annual FOBT for three consecutive years to asymptomatic individuals between 40 and 75 years of age. Non-rehydrated Hemoccult II slides were used to avoid the reduced specificity associated with rehydration. Winawer et al calculated that for every case of cancer detected, 6–10 patients need to undergo colonoscopy using the non-hydrated test compared with 17–50 patients for the rehydrated test.6
There was no difference in the incidence of CRC in the FOBT group compared with refusers and controls during the three year screening phase, which is in agreement with the findings in the Minnesota study.1 However, the tumours that were diagnosed and removed were more likely to be Dukes' stage A or B in screenees compared with the other groups, and mortality was significantly decreased. During the eight year follow up period, subjects were only referred for investigation of symptoms and there was no significant difference in the rate of colonic investigations performed in each group. Therefore, it is not surprising that there was no difference in Dukes' stage for CRC diagnosed during this period. However, the incidence and disease related mortality in screenees was significantly lower compared with refusers. The reduced incidence of CRC in screenees during follow up may be due to removal of polyps found during the screening phase and early diagnosis of CRC. The reduced mortality in screenees during follow up may be explained by the detection and early removal of carcinomas found during the screening period. However, as the number of patients studied in our protocol was relatively small, our data should be interpreted with caution. In other large studies, more than 10 years of follow up were needed to show any impact on incidence of cancer due to the removal of polyps.6
When participants and refusers were taken together (group 1+group 2) and compared with controls (group 3), no significant difference was demonstrated in the cumulative incidence of CRC. This was true for the screening as well as for the follow up period. As CRC was diagnosed in earlier Dukes' stages in the participants, in the study period, this is not surprising. Screening may not change the prevalence of CRC but may save lives due to early detection.
The prolonged beneficial effect of FOBT suggests that this type of screening programme may be more effective than previously recognised. In a case control study, Selby et al determined the frequency of FOBT performed in individuals dying from CRC during the five years prior to diagnosis compared with matched controls.15 An odds ratio of 0.69 was found for exposure to at least one FOBT during that period, after adjusting for confounding factors such as screening sigmoidoscopy or rectal examination. The protective effect of FOBT decreased from the first to the third year, when the odds ratio reached 1.0. The risk of CRC was reduced by 10–30%. In our study, in which data were obtained prospectively, we found that compliance was an important determinant in mortality. Refusers had an increased mortality rate compared with the other groups. In the study by Selby et al, it is not clear how many of the CRC patients in the study groups refused FOBT. However, CRC patients were noted to have undergone fewer health checks than controls. We also found that refusers had undergone fewer investigations than the FOBT group (table 5 ▶). These data support the concept of a refuser population which is at increased risk.
We believe that our ability to demonstrate benefit in this relatively small population is due to the high rate of compliance and the persistent educational effect of our programme. We had a compliance rate of 71.5% for FOBT and 100% for colonoscopy (when the test was positive). In the Nottingham trial, the compliance rate for all FOBT was 38.2%, and in Funen 46%.2,3 This raises the question of why subjects refuse the test with its potential benefits. In most studies compliance rates tended to decline with each additional year of participation.8–11 Younger participants are more likely to abandon the programme.9 Other factors linked to higher rates of compliance include higher educational level,16 family history of CRC,10 belief in health checks,17,18 and female sex.10,18
Education and an enthusiastic family physician may increase compliance.19–23 Reasons for avoiding FOBT include absence of symptoms, unpleasant nature of the test, disinterest in health issues, or technical difficulties in performing the test.6,18 In our study the refuser group was characterised by an excess of males of Asian-African origin who had lifestyle habits that included higher rates of smoking and coffee drinking and a significantly lower consumption of tea and dairy products. These results should be interpreted with caution as 78% of the acceptor group completed the health questionnaires compared with only just over 54% of refusers.
In conclusion, we have shown that FOBT, when accepted, has long term benefit in lowering the incidence and mortality of CRC. Individuals who are non-compliant have a higher incidence, more advanced disease, with higher mortality during follow up. This group has characteristics that can be identified and a special effort should be made to convince them to participate in screening programmes for CRC.
Acknowledgments
We are indebted to Professors D Alqhuist, CR Boland, RS Bresalier, SB Ho, and S Itzkowitz for their support.
Abbreviations
CRC, colorectal cancer
FOBT, faecal occult blood testing
REFERENCES
- 1.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 1993;328:1365–71. [DOI] [PubMed] [Google Scholar]
- 2.Kronberg O, Fenger C, Olsen J, et al. Randomized study of screening for colorectal cancer with fecal occult blood test. Lancet 1996;348:1467–71. [DOI] [PubMed] [Google Scholar]
- 3.Hardcastle JD, Chamberlain JO, Robinson MHE, et al. Randomized controlled trial of fecal occult blood screening for colorectal cancer. Lancet 1996;348:1472–7. [DOI] [PubMed] [Google Scholar]
- 4.Lang CA, Ransohoff DF. Fecal occult blood screening for colorectal cancer. JAMA 1994;271:1011–13. [PubMed] [Google Scholar]
- 5.Towler B, Irwig L, Glasziou P, et al. A systematic review of the effects of screening for colorectal cancer using the fecal occult blood test, Hemoccult. BMJ 1998; 317:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: Clinical guidelines and rationale. Gastroenterology 1997;112:594–42. [DOI] [PubMed] [Google Scholar]
- 7.Byers T, Levin B, Rothenberger D, et al. American Cancer Society guidelines for screening and surveillance for early detection of colorectal polyps and cancer. Update 1997. CA Cancer J Clin 1997;47:154–60. [DOI] [PubMed] [Google Scholar]
- 8.Morris JB, Stellato TA, Guy BB, et al. A critical analysis of the largest reported mass fecal occult blood screening program in the United States. Am J Surg 1991;161:101–5. [DOI] [PubMed] [Google Scholar]
- 9.Myers RE, Balshem AM, Wolf TA, et al. Adherence to continuous screening for colorectal neoplasia. Med Care 1993;31:508–19. [DOI] [PubMed] [Google Scholar]
- 10.Bat L, Pines A, Ron E, et al. A community based program of colorectal screening in an asymptomatic population: evaluation of screening tests and compliance. Am J Gastroenterol 1986;81:647–51. [PubMed] [Google Scholar]
- 11.Niv Y. Does a risk questionnaire add anything to a colorectal screening project? Report of a 3-year screening experience. J Clin Gastroenterol 1992;15:33–6. [DOI] [PubMed] [Google Scholar]
- 12.Simon JB, Fletcher RH. Should all people over the age of 50 have regular fecal occult blood tests? N Engl J Med 1998;338:1151–3. [DOI] [PubMed] [Google Scholar]
- 13.Niv Y. Fecal occult blood test—the importance of proper evaluation. J Clin Gastroenterol 1990;12:393–5. [DOI] [PubMed] [Google Scholar]
- 14.Howat P, Mohan R, Champange C, et al. Validity and reliability of reported dietary intake data. J Am Dietetic Assos 1994;94:169–73. [DOI] [PubMed] [Google Scholar]
- 15.Selby JV, Friedman GD, Quesenberry CP, et al. Effect of fecal occult blood testing on mortality from colorectal cancer. A case-control study. Ann Intern Med 1993;118:1–6. [DOI] [PubMed] [Google Scholar]
- 16.Polednak AP. Knowledge of colorectal cancer and use of screening tests among higher-risk persons. J Cancer Educ 1990;5:115–24. [DOI] [PubMed] [Google Scholar]
- 17.Blalock SJ, DeVellis BM, Sandler RS. Participation in fecal occult blood screening: a critical review. Prev Med 1987;16:9–18. [DOI] [PubMed] [Google Scholar]
- 18.Dent OF, Bartrop R, Goulston KJ, et al. Participation in fecal occult blood screening for colorectal cancer. Soc Sci Med 1983;17:17–23. [DOI] [PubMed] [Google Scholar]
- 19.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst 1997; 89:1406–22. [DOI] [PubMed] [Google Scholar]
- 20.Brown ML, Potosky AL, Thompson GB, et al. The knowledge and use of screening tests for colorectal and prostate cancer: data from the 1987 National Health interview Survey. Prev Med 1990;19:562–74. [DOI] [PubMed] [Google Scholar]
- 21.Neale AV, Deiners RY, Hennan S. Compliance with colorectal cancer screening in a high-risk occupational group. J Occup Med 1989;31:1007–12. [DOI] [PubMed] [Google Scholar]
- 22.Myers RE, Ross EA, Wolf TA, et al. Behavioral interventions to increase adherence in colorectal cancer screening. Med Care 1991;29:1039–50. [DOI] [PubMed] [Google Scholar]
- 23.Struewing JP, Pape DM, Snow DA. Improving colorectal cancer screening in a medical residents' primary care clinic. Am J Prev Med 1991;7:75–81. [PubMed] [Google Scholar]