Abstract
Background: A decrease in folic acid and subsequent DNA hypomethylation may be involved in gastric carcinogenesis. Epidemiological and nutritional studies have indicated that folate status modulates the risk of developing cancers.
Aims: To investigate whether folic acid plays an important role in the chemoprevention of gastric carcinogenesis induced by N-ethyl-N-nitrosoguanidine (ENNG) in beagles.
Methods: Sixteen male beagles were randomly divided into two groups: folic acid treated group and control group. In both groups beagles were fed ENNG 75 mg per day for eight months and in the treated group 20 mg folic acid was given to beagles for 15 months. Gastroscopy and biopsies were performed before and every 2–3 months after administration of ENNG until the end of the experiment. Histopathological lesions were diagnosed with regard to the criteria for human gastric mucosal biopsies. Serum and gastric mucosal tissue folic acid concentrations were measured.
Results: In the control group, all beagles developed gastric cancer (8/8) compared with only 3/8 in the folic acid treated group (p<0.05). Moreover, serum and gastric mucosal tissue folic acid concentrations were markedly elevated 15 months after folic acid administration. The difference was statistically significant between the two groups (p<0.05).
Conclusions: Our results indicate that high dose folic acid plays an important role in the chemoprevention of gastric carcinogenesis induced by a chemical carcinogen ENNG in beagles.
Keywords: folic acid, gastric cancer, N-ethyl-N-nitrosoguanidine, beagles
Folic acid is one of the micronutrients essential for normal human growth. Large scale epidemiological and nutritional studies have indicated that folate status modulates the risk of developing cancers in selected tissues.1,2 Folic acid depletion appears to enhance carcinogenesis whereas folic acid supplementation above what is presently considered to be the basal requirement appears to convey a protective effect. Studies of folic acid in this aspect have been confined mainly to determination of serum folic acid concentrations and survey on dietary intake of folic acid.3–6 In our previous study,7 we found that a decrease in folic acid and subsequent DNA hypomethylation may be involved in human gastric carcinogenesis. Hence in this study we delivered a high dose of folic acid to beagles, on the basis of our previous studies in a canine model, to observe the effects of folic acid on carcinogenesis of gastric cancer and to study its chemopreventive effect on gastric cancer induced by a chemical carcinogen N-ethyl-N-nitrosoguanidine (ENNG).8,9
MATERIALS AND METHODS
Animal studies
Sixteen healthy male beagles, aged 11–14 months, with a mean body weight of 8.5 kg (purchased from Experimental Animal Centre of Shanghai Medical University) were randomly allocated to one of two groups: folic acid treated group and control group (n=8 in each group). In both groups each dog received ENNG 75 mg/day (Sigma, St Louis, Missouri, USA) from Monday to Saturday, and no drug on Sunday. Concomitantly, in the folic acid treated group, each dog was fed folic acid 20 mg (5 mg/tablet; Shanghai 6th Pharmaceutical Corporation, Shanghai, P R China). ENNG and folic acid were given separately at different times. In the control group, each dog received the same dosage of ENNG without folic acid. Duration of ENNG administration was eight months and that of folic acid 15 months. Animals were then sacrificed and dissected. In addition, 4 ml blood samples and four gastric mucosal biopsies were obtained in all beagles from both groups, respectively, before and after 15 months of the experimental period for measurement of serum and gastric mucosal tissue folic acid concentrations.
Administration of ENNG
Tween-80 200 ml and 1.5 g ENNG were added to 800 ml of distilled water, forming a stock solution of 1500 μg/ml ENNG.8 The mixed solution was stirred for five hours (light protected) until completely dissolved and stored at 4°C. The stock solution was prepared freshly. It remained stable over one week. The working solution was a 1:5 dilution of the stock solution (that is, it contained 300 μg/ml ENNG). Each beagle was given a 250 ml ENNG solution mixed with dietary pellets (purchased from Shanghai Animal Food Factory, Shanghai, P R China) once a day. Their general condition was observed during the experimental period. Gastroscopy was performed before and every 2–3 months after administration of ENNG until the end of the experiment, and two mucosal biopsy specimens were obtained from the gastric antrum and body, respectively, at gastroscopy. Mucosal specimens were fixed in 10% formalin and dehydrated, embedded in wax, sectioned, and stained with haematoxylin-eosin, alcian blue (pH 2.5)-periodic acid-Schiff, and high iron diamine-alcian blue (pH 2.5). Finally, an experienced pathologist examined the tissue sections under a microscope. The histopathological lesions were diagnosed in relation to the criteria for human gastric mucosal biopsies.
Measurement of serum and gastric mucosal tissue folic acid
Serum folic acid concentration was measured by radioimmunoassay. The Solid Phase No Biol Folic Acid kit (Diagnostic Products, Los Angeles, California, USA) is designed for single analytic determination of folic acid. The procedure includes alkaline denaturation of endogenous proteins, competition for purified binder at pH 9.3, and solid phase separation. Mucosal tissue folic acid concentration was measured according to the method by O'Broin and Kelleher.10 Briefly, specimens were mixed with extraction buffer, placed in a boiling water bath, homogenised, and centrifuged. The supernatant was further incubated with Chicken pancrease conjugase and added to 96 well microtitre plates. A working standard solution of folic acid was made by dilution of a stock standard in 0.5% sodium ascorbate. The concentration of folic acid was measured spectrophotometrically using a Beckman spectrophotometer and calculated using SAS software. The protein concentration of each sample was measured.
Statistical analysis
Statistical analysis of matched data was performed using the Student's t test.
RESULTS
Histopathological changes
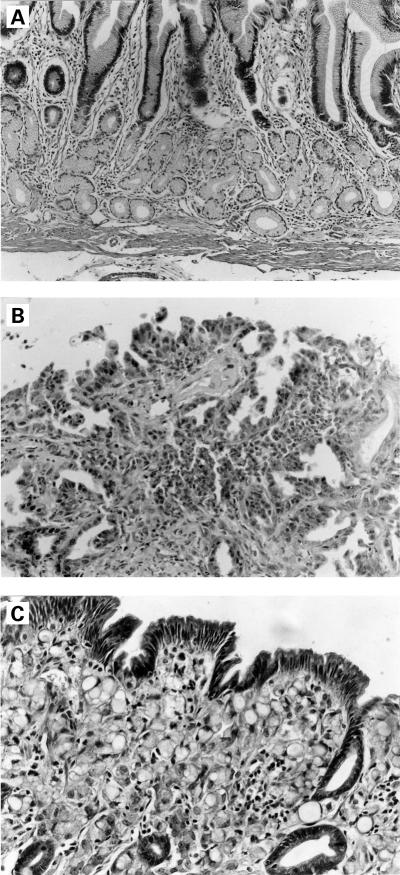
The results of the pathological examination of the gastric mucosa are shown in table 1 ▶. Data showed that only 3/8 beagles developed gastric cancer in the folic acid treated group after 15 months of the experiment. However, all beagles (8/8) in the control group developed gastric cancer. Using the χ2 test and the precise probability method, it was shown that the difference was statistically significant for the rate of development of gastric cancer (p=0.028 or p<0.05). Histopathological changes are shown in fig 1A–C ▶.
Table 1.
Histopathological results in 16 beagles
| Dog | 0 month | 3 months | 6 months | 8 months | 10 months | 12 months | 14 months | 15 months |
| T1 | N | F | D | D | ||||
| T2 | N | Pa, D++ | D++ | S | S, P, Pa, O | |||
| T3 | N | D+∼++ | S | P, S, Pa, T, D | ||||
| T4 | N | D+ | D+ | D++ | F, D++ | |||
| T5 | N | F | Pa, D++ | |||||
| T6 | N | D+ | S, D++ | S | ||||
| T7 | N | D+ | D+ | D+ | ||||
| T8 | N | D+ | D+ | D+ | ||||
| C9 | N | D | T | T | S, P, T, Pa | |||
| C10 | N | S, D++ | D+ | Pa | S, P, T, Pa | |||
| C11 | N | S | S | Pa | S, Pa, T | |||
| C12 | N | D+ | S | F | P, S, T | |||
| C13 | N | D+∼++ | S | F | P, S, T | |||
| C14 | N | S | Pa | S | ||||
| C15 | N | D++ | P, S | P, S, D+ | ||||
| C16 | N | D+ | D++ | S |
N, normal; D, dysplasia (+mild, ++moderate, +++severe); F, fibrotic proliferation; S, signet ring cell carcinoma; Pa, papillary adenoma; P, poorly differentiated adenocarcinoma; O, oesophageal carcinoma; T, tubular adenocarcinoma.
T1–8, folic acid treated beagles; C9–16, control beagles.
Figure 1.
Folic acid treated beagle (T4): (A) normal mucosa before administration of N-ethyl-N-nitrosoguanidine (ENNG). Normal pyloric glands with a few chronic inflammatory cells in the lamina propria (haematoxylin-eosin, ×100); (B) moderate to severe dysplasia after 15 months of folic acid intervention. Dysplastic glands are irregular in shape and size, cell nuclei are large, and deeper staining (haematoxylin-eosin, ×300). Control beagle (C10): (C) signet ring cell carcinoma occurred 11 months after ENNG administration. The superficial and middle of gastric mucosal layers are diffusely infiltrated signet ring cells, the covering epithelium is still intact (haematoxylin-eosin, ×300).
Serum and gastric mucosal folic acid concentrations
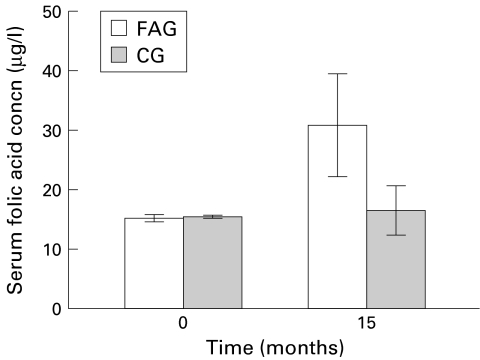
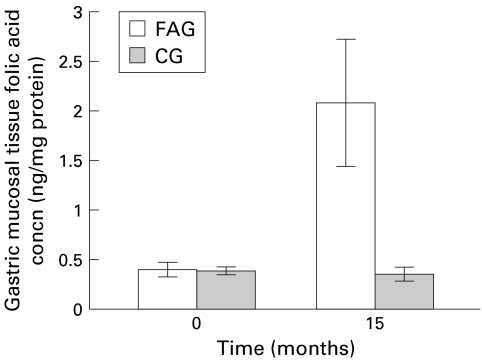
Fifteen months after folic acid supplementation, mean serum folic acid concentrations were markedly increased in the folic acid treated group (from 15.1 (SD 0.61) to 30.7 (8.6) μg/l v 15.4 (0.3) to 16.5 (4.1) μg/l in the control group; p<0.01) (fig 2 ▶). Moreover, mean concentrations of folic acid in the gastric mucosa were significantly increased in the folic acid treated group 15 months after folic acid supplementation (from 0.4 (SD 0.07) to 2.1 (0.64) ng/mg protein v 0.39 (0.04) to 0.38 (0.06) ng/mg protein in the control group) (fig 3 ▶). The difference was statistically significant between the two groups (p<0.01).
Figure 2.
Changes in serum folic acid concentrations after folic acid supplementation. FAG, folic acid treated group; CG, control group.
Figure 3.
Changes in gastric mucosal tissue folic acid concentrations after folic acid supplementation. FAG, folic acid treated group; CG, control group.
DISCUSSION
Vitamins are essential for human life and deficiency of vitamins results in various diseases, including malignant neoplasia.11,12 Recently, attention has been paid to the use of vitamins in the prevention and treatment of cancer. A mixture of multiple antioxidant vitamins such as vitamin C, beta carotene, d-alpha-tocopheryl succinate and retinoic acid was found to be more effective than individual vitamins in reducing the growth of tumorigenic acinar cells.13 However, studies were limited to epidemiological surveys and frequently the vitamins were used in combination.14 Therefore, it is difficult to determine which vitamin plays the key role in the prevention of carcinogenesis. In this study we choose folic acid as the sole agent to explore its preventive effects in the carcinogenesis of gastric cancer in the hope that it might be of both theoretical and practical significance. To date, such studies have not been reported.
Folic acid plays an important role in DNA methylation and synthesis of DNA and RNA, and it is related to the synthesis of S-adenosylmethionine.15 Rats fed a diet with low folic acid had diminished hepatic S-adenosylmethionine synthesis, resulting in DNA hypomethylation.16 In addition, folic acid has also been implicated in the development of cancer, in particular colorectal cancer. There appear to be two principal mechanisms through which low folate status may increase the risk of malignancy.1 Firstly, folate deficiency, by reducing intracellular S-adenosylmethionine, can alter cytosine methylation in DNA, leading to inappropriate activation of proto-oncogenes and induction of malignant transformation. Secondly, folate is essential for normal DNA synthesis and repair. There were abnormal breakages of chromosomes, incomplete contraction of bone marrow cells, as well as extension of centrosomes in patients deficient in folic acid.17,18 In vitro, DNA strand breakage and uracil misincorporation increased in a time and concentration dependent manner after human lymphocytes were cultured with decreasing amounts of folic acid.19 Such breaks are associated with an increased risk of cancer in humans. Moreover, folate deficiency impairs DNA excision repair in rat colonic mucosa.20 In addition, the presence of the Hprt locus of T lymphocytes was also related to a lower serum folic acid level, and replenishment of folic acid restored these abnormalities to normal.21–23 These data indicate that folic acid deficiency could affect the stability of cellular DNA/RNA at the chromosomal and molecular levels, which may facilitate activation of oncogenes and induce carcinogenesis.24
Despite the attention surrounding its relationship with carcinogenesis, the results of animal experiments were not consistent. Cravo and colleagues25 gave a low folic acid diet to rats with further treatment with dimethylhydrazine compared with rats fed a normal diet. The results showed that there were significant differences in the incidence of colonic neoplasia between the two groups after 20 weeks of dimethylhydrazine exposure: folate deficient rats had a greater incidence of dysplasia and cancer. Also, a significantly greater proportion of folate replete rats than folate deficient rats were free of neoplastic lesions. Moreover, Kim et al found that dietary folate protected against the development of macroscopic colonic neoplasia in a dose responsive manner in rats.26 Kamei et al reported that epithelial hyperplasia and metaplasia of the respiratory tract induced by methylcholanthrene was suppressed by administration of folic acid.27 In our study, only 3/8 beagles developed gastric cancer in the folic acid treated group. However, all eight dogs in the control group who did not receive folic acid developed gastric cancer. The difference was highly significant (p=0.028, <0.05). But some experiments showed that folic acid supplementation had no protective effect on carcinogenesis and that it even enhanced the development and progression of malignant tumour. In contrast, diminution of folic acid levels had an inhibitory effect on the development and growth of tumours.28,29 These conflicting results are probably due to factors affecting the effects of folic acid on tumours under different conditions, including different animal and tumour models used, differences in dosage, timing of folic acid administration, variety of carcinogens, and methods of administration. All of these factors could influence subsequent results.
We used the lactobacilli culture method with concomitant determination of gastric mucosal tissue as well as serum folic acid concentrations in these beagles to reflect mucosal tissue and serum folic acid changes during gastric carcinogenesis induced by ENNG. Our data indicated that serum and gastric mucosal tissue folic acid concentrations were markedly elevated 15 months after folic acid administration. The differences were statistically significant between the two groups 15 months after folic acid administration (p<0.05). It should be noted that the beagles used in our study are regarded as having a normal folate status, as suggested by serum and gastric mucosal tissue folic acid concentrations in both groups. However, our data indicated that high dose folic acid may play an important role in the chemoprevention of gastric carcinogenesis induced by the chemical carcinogen ENNG. Presumably, the consequence of folate status and carcinogenesis depends on the balance between folate and the carcinogens. Folate depletion appears to produce procarcinogenic effects. However, increased intensity of the carcinogen may also lead to carcinogenesis even if folic acid levels in blood and tissue are within the normal range. It is also noteworthy that all beagles in the folic acid treated group developed dysplastic lesions during follow up, and therefore it is possible that high dose folic acid might postpone the development of gastric cancer. Yet it is hard to draw the conclusion that high dose folic acid only postpones but does not prevent the development of gastric cancer. Further study including a normal group of beagles may be informative.
Our study has shown that high dose folic acid has a marked interventional effect on gastric carcinogenesis, although in a small number of animals. Further investigation is needed.
Abbreviations
ENNG,N-ethyl-N-nitrosoguanidine
REFERENCES
- 1.Duthie SJ. Folic acid deficiency and cancer: mechanisms of DNA instability. Br Med Bull 1999;55:578–92. [DOI] [PubMed] [Google Scholar]
- 2.Kim YI. Folate and cancer prevention: a new medical application of folate beyond hyperhomocysteinemia and neural tube defects. Nutr Rev 1999;57:314–21. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth CE. A study of folate absorption and metabolism in man utilizing carbon-14-labled polyglutamates synthesized by the solid phase method. J Clin Invest 1969;48:1131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glynn SA, Albanes D. Folate and cancer: a review of the literature. Nutr Cancer 1994;22:101–19. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Stamper MJ, Colditz GA, et al. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst 1993;85:875–84. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Hunter DJ, Hankinson SE, et al. A prospective study of folate intake and the risk of breast cancer. JAMA 1999;281:1632–7. [DOI] [PubMed] [Google Scholar]
- 7.Fang JY, Xiao SD, Zhu SS, et al. Relationship of plasma folic acid and status of DNA methylation in human gastric cancer. J Gastroenterol 1997;32:171–5. [DOI] [PubMed] [Google Scholar]
- 8.Xiao SD, Jiang SJ, wang RN, et al. N-ethyl-N′-nitro-N-nitrosoguanidine induced gastric carcinoma in wolfdogs—useful animal model for tracing gastric malignancy. Chin Med J 1986;99:903–7. [PubMed] [Google Scholar]
- 9.Zhu RM, Xiao SD, Jiang SJ, et al. Cytophotometric DNA analysis on canine stomach carcinogenesis induced by ENNG. Chin Med J 1990;103:1019–23. [PubMed] [Google Scholar]
- 10.O'Broin S, Kelleher B. Microbiological assay on microtitre plate in serum and red cells. J Clin Pathol 1992;45:344–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jennings E. Folic acid as a cancer-preventing agent. Med Hypotheses 1995;45:297–303. [DOI] [PubMed] [Google Scholar]
- 12.Shunshi Zhu S, Yunbiao Hu, Shanjun Yan, et al. Changes of cellular vitamins in diseased gastric mucosa and intervention with folic acid during the process of carcinogenesis induced by ENNG in dogs. Pro Jap Res Soc Gastroenterol 1992;4:291–4. [Google Scholar]
- 13.Prasad KN, Kumar R. Effects of individual and multiple antioxidant vitamins on growth and morphology of human nontumorigenic and tumorigenic parotid acinar cell in culture. Nutr Cancer 1996;26:11–19. [DOI] [PubMed] [Google Scholar]
- 14.Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention traile in Linxian, China: supplementataion with specific vitamin/mineral combination, cancer incidence, and disease-specific mortality in general population. J Natl Cancer Inst 1993;85:1483–92. [DOI] [PubMed] [Google Scholar]
- 15.Gregory JF. Chemical and nutritional aspects of folate research: analytical procedures, methods of folate synthesis, stablity and bioavailability of dietary folates. Adv Food Nutr Res 1989;33:1–101. [DOI] [PubMed] [Google Scholar]
- 16.Huennekens FM, Duffy TH, Vitols KS, et al. Folic acid metabolism and its disruption by pharmacologic agents. Natl Cancer Inst Monogr 1987;5:1–8. [PubMed] [Google Scholar]
- 17.Heath CW. Cytogenic observations in vitamin B12 and folate deficiency. Blood 1966;27:800–15. [PubMed] [Google Scholar]
- 18.Chen AT, Reidy JA, Annest JL, et al. Increased chromosome fragility as a consequence of blood folate levels, smoking status, and coffee consumption. Environ Mol Mutagen 1989;13:319–24. [DOI] [PubMed] [Google Scholar]
- 19.Duthie SJ, Hawdon A. DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB J 1998;12:1491–7. [PubMed] [Google Scholar]
- 20.Choi SW, Kim YI, Weitzel JN, et al. Folate depletion impairs DNA excision repair in the colon of the rat. Gut 1998;43:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branda RF, O'Neill JP, Jacobson-Kram D, et al. Factors influencing mutation at the hprt locus in T-lymphocytes: studies in normal women and women with benign and malignant breast masses. Environ Mol Mutagen 1992;19:274–81. [DOI] [PubMed] [Google Scholar]
- 22.Branda RF, Lafayette AR, O'Neill JP, et al. Effect of folate deficiency on mutations at the hprt locus in Chinese hamster ovary cells exposed to monofunctional alkylating agents. Cancer Res 1997;57:2586–8. [PubMed] [Google Scholar]
- 23.Herbert V. The 1986 Herman Award Lecture. Nutrition science as a continually unfolding story: the folate and vitamin B12 paradigm. Am J Clin Nutr 1987;46:387–402. [DOI] [PubMed] [Google Scholar]
- 24.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr 2000;130:129–32. [DOI] [PubMed] [Google Scholar]
- 25.Cravo ML, Mason JB, Dayal Y, et al. Folate deficiency enhances the development of clonic neoplasia in dimethyhydrazine treated rats. Cancer Res 1992;52:5002–6. [PubMed] [Google Scholar]
- 26.Kim YI, Salomon RN, Graeme-Cook F, et al. Dietary folate protects against the development of macroscopic colonic neoplasia in a dose responsive manner in rats. Gut 1996;39:732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamei T, Kohno T, Ohwada H, et al. Experimental study of the therapeutic effects of folate, vitamin A, and vitamin B12 on squamous metaplasia of the bronchial epithelium. Cancer 1993;71:2477–83. [DOI] [PubMed] [Google Scholar]
- 28.Bills ND, Hinrichs SH, Morgan R, et al. Delayed tumor onset in transgenic mice fed a low-folate diet. J Natl Cancer Inst 1992;84:332–7. [DOI] [PubMed] [Google Scholar]
- 29.Huang RF, Ho YH, Lin HL, et al. Folate deficiency induces a cell cycle-specific apoptosis in HepG2 cells. J Nutr 1999;129:25–31. [DOI] [PubMed] [Google Scholar]