Abstract
Background and aims: Liver donors with serological evidence of resolved hepatitis B virus (HBV) infection (HBV surface antigen (HBsAg) negative, anti-HBV core (HBc) positive) can transmit HBV infection to recipients. In the context of organ shortage, we investigated the efficacy of hepatitis B immunoglobulin (HBIG) to prevent HBV infection, and assessed the infectious risk by polymerase chain reaction (PCR) testing for HBV DNA on serum and liver tissue of anti-HBc positive donors.
Patients: Between 1997 and 2000, 22 of 315 patients were transplanted with liver allografts from anti-HBc positive donors. Long term HBIG therapy was administered to 16 recipients. Four naive and two vaccinated patients received no prophylaxis.
Results: Hepatitis B developed in the four HBV naive recipients without prophylaxis and in none of the vaccinated subjects. Among the 16 recipients receiving HBIG, one patient with residual anti-HBs titres below 50 UI/ml became HBsAg positive. The remaining 15 remained HBsAg negative and HBV DNA negative by PCR testing throughout a 20 month (range 4–39) follow up period. HBV DNA was detected by PCR in 1/22 donor serum, and in 11/21 liver grafts with normal histology. A mean of 12 months post-transplantation (range 1–23) HBV DNA was no longer detectable in graft biopsies from patients remaining HBsAg negative.
Conclusion: Anti-HBs antibodies may control HBV replication in liver grafts from anti-HBc positive donors, without additional antiviral drugs. These grafts are thus suitable either to effectively vaccinated recipients or to those who are given HBIG to prevent HBV recurrence.
Keywords: hepatitis B virus, anti-hepatitis B virus core, liver transplantation, hepatitis B virus infection, liver grafts
Liver transplantation is the major treatment for patients with end stage liver disease or localised hepatocellular carcinoma. Despite the need to increase the donor population,1 many candidates are excluded because of the risk of transmission of infectious diseases. Hepatic allografts from hepatitis B surface antigen (HBsAg) negative and anti-core antibody (anti-HBc) positive donors have been shown to transmit hepatitis B virus (HBV) infection.2–7 The probability of de novo HBV infection depends on the HBV serological status of the recipient: anti-HBc and anti-HBs positive recipients are generally resistant to HBV infection while the rate of de novo infection in naive recipients reaches 70%.6–8 Recently, combination therapy with hepatitis B immunoglobulins (HBIG) and lamivudine was shown to prevent the emergence of HBsAg in anti-HBs negative recipients.9 However, there are unanswered questions concerning the risk of drug induced viral mutations and the scarcity of alternative efficient therapies. Continuous use of high doses of HBIG in liver transplantation for hepatitis B infected patients has been shown to reduce dramatically the incidence of recurrent HBV infection without serious adverse effects.10–12 At our institution, recipients of liver allografts from anti-HBc positive donors have been given HBIG without additional antiviral drugs since 1998 to prevent de novo HBV infection. Here, we report our experience from 1997 to 2000 and our attempt to assess the infectious risk of liver grafts harvested from anti-HBc positive donors by means of HBV polymerase chain reaction (PCR) testing of serum and liver tissue.
METHODS
Patients
Between January 1997 and September 2000, 315 orthotopic liver transplants were performed at our institution. Twenty two patients (7%) received allografts from anti-HBc positive donors. Indications for liver transplantation, clinical condition, and HBV serological status are listed in table 1 ▶. All were negative for serum HBV DNA using a hybridisation technique. HBIG long term prophylaxis was routinely given to HBsAg positive patients (n=4). The HBIG prophylactic regimen consisted of 10 000 IU HBIG intravenously (Laboratoire Français de Biotechnologie, les Ulis, France) daily for seven days after liver transplantation, and then whenever the anti-HBs titre decreased to less than 500 IU/ml. After July 1998, all HBsAg negative recipients of allografts from anti-HBc positive donors were given a modified HBIG prophylaxis protocol, whatever their pretransplant anti-HBs status (n=12): 1–7 infusions of 5000 IU HBIG during the first week post-transplantation and thereafter to maintain anti-HBs titres above 100 IU/ml. Before 1998, four HBV naive (anti-HBs negative, anti-HBc negative) and two vaccinated recipients received no HBIG prophylaxis.
Table 1.
Characteristics of the recipients
| Pretransplant HBV serology | Most recent serology | ||||||||||
| Pt No | Liver transplant indication | Blood group | HBsAg | Anti-HBs | Anti-HBc | Passive immunoprophylaxis | HBsAg seroconversion (months post-OLT) | Follow up (months) | HBsAg | Anti-HBs | Anti-HBc |
| 1 | HBV cirrhosis/HCC | A+ | + | − | + | 39 | − | + | + | ||
| 2 | HBV cirrhosis/HCC | A− | + | − | + | Standard HBlg protocol | 31 | − | + | + | |
| 3 | HBV cirrhosis/HCC | O+ | + | − | + | 3 (deceased) | − | + | + | ||
| 4 | HBV cirrhosis | A+ | + | − | + | 4 | − | + | + | ||
| 5 | HCV and alcoholic cirrhosis | A+ | − | − | − | 7 (deceased) | − | + | + | ||
| 6 | Alcoholic cirrhosis | A+ | − | − | − | 17 | 24 | + | − | + | |
| 7 | Symptomatic amyloidosis | O+ | − | − | − | 14 | − | + | + | ||
| 8 | Symptomatic amyloidosis | O+ | − | − | − | 12 | − | + | − | ||
| 9 | Primary biliary cirrhosis | A+ | − | − | − | 6 | − | + | − | ||
| 10 | HCV cirrhosis | A+ | − | + | − | Modified HBlg protocol | 21 | − | + | + | |
| 11 | Symptomatic amyloidosis | A− | − | + | − | 19 | − | + | + | ||
| 12 | HCV cirrhosis | O+ | − | + | − | 9 | − | + | + | ||
| 13 | HCV cirrhosis/HCC | A+ | − | − | + | 36 | − | + | + | ||
| 14 | HCV cirrhosis | O+ | − | − | + | 29 | − | + | + | ||
| 15 | HCV cirrhosis | O+ | − | − | + | 23 | − | + | + | ||
| 16 | HCV cirrhosis | O+ | − | − | + | 17 | − | + | + | ||
| 17 | Symptomatic amyloidosis | A+ | − | + | − | None | 36 | − | + | − | |
| 18 | Hepatic metastases | B+ | − | + | − | None | 36 | − | + | + | |
| 19 | Alcoholic cirrhosis | O+ | − | − | − | None | 15 | 45 | + | − | + |
| 20 | Budd−Chiari syndrome | A+ | − | − | − | None | 9 | 37 | + | − | + |
| 21 | Alcoholic cirrhosis | A+ | − | − | − | None | 8 | 36 | + | − | + |
| 22 | Alcoholic cirrhosis | B+ | − | − | − | None | 11 | 32 | + | − | + |
HCV, hepatitis C virus; HCC, hepatocellular carcinoma; HBc, HBV core; HBs, HBV surface; HBsAg, HBV surface antigen; OLT, orthotopic liver transplantation.
Routine virological testing
Donor HBV serological status was systematically examined at our laboratory. Sera were tested using commercial enzyme immunoassays for HBsAg, anti-HBs (Dade Behring, Marburg, Germany), anti-HBc (Murex Biotech, Dartford, England), and HBV e antigen (HBeAg) (BioMérieux, Marcy l'Etoile, France). After transplantation, recipients were routinely screened at least every four months for HBsAg and HBV-DNA by Quantiplex HBV b-DNA kit (Chiron Corporation, Emeryville, California, USA), except for patient No 19 who was lost to follow up for 10 months.
Histology
Surgical wedge biopsies of the grafted livers were routinely performed before and on day 0 post-transplantation. Transcutaneous liver biopsies were performed after transplantation if liver test abnormalities occurred or HBsAg emerged. Paraffin embedded 4 μm thick sections were stained with haematein-eosin-saffron, Perls, and Sirius red. All slides were reviewed for this study. Immunostaining was performed on sections of fixed liver biopsy specimens with a commercial three step streptavidin-biotin technique according to the manufacturer's instructions (Ventana Medical Systems, Strasbourg, France). The primary monoclonal antibodies were anti-HBsAg, anti-HBcAg (Dako, Copenhagen, Denmark), and anti-pre-S1, which is described in detail elsewhere.13
Detection of serum and liver HBV DNA by PCR
HBV-DNA was detected with the Amplicor HBV Monitor Test (Roche Diagnostics, Branchburg, New Jersey, USA) on sera stored at −20°C and on paraffin embedded liver biopsy specimens. Five 4 μm sections of each biopsy were deparaffinised in xylene at 65°C for 10 minutes, followed by two washes in absolute ethanol. Overnight tissue digestion and DNA extraction were performed with the QIAmp tissue kit (Qiagen GmbH, Germany) according to the manufacturer's instructions. Extracted DNA was suspended in 100 μl of sterile water. A volume of 25 μl was used for HBV DNA detection. To check the DNA conservation and extraction steps, a 261 bp sequence of the human β globin gene was amplified from 1 μl of the extract with the following primers: 5′-GGTTGGCCAATCTACTCCCAGG-3′ and 5′-TGGTCTCCTTAAACCTGTCTTG-3′.
RESULTS
Outcome of patients on HBIG prophylaxis
Standard long term HBIG prophylaxis was given to four patients (table 1 ▶). On day 0, patient Nos 2 and 4 had detectable HBV DNA in serum by PCR: 4×105 and 4×104 copies/ml, respectively. Patient No 4 died three months post-transplantation from recurrent hepatocellular carcinoma. The remaining three patients remained HBsAg negative and serum HBV DNA negative by PCR testing throughout a mean follow up period of 24 months (range 4–39).
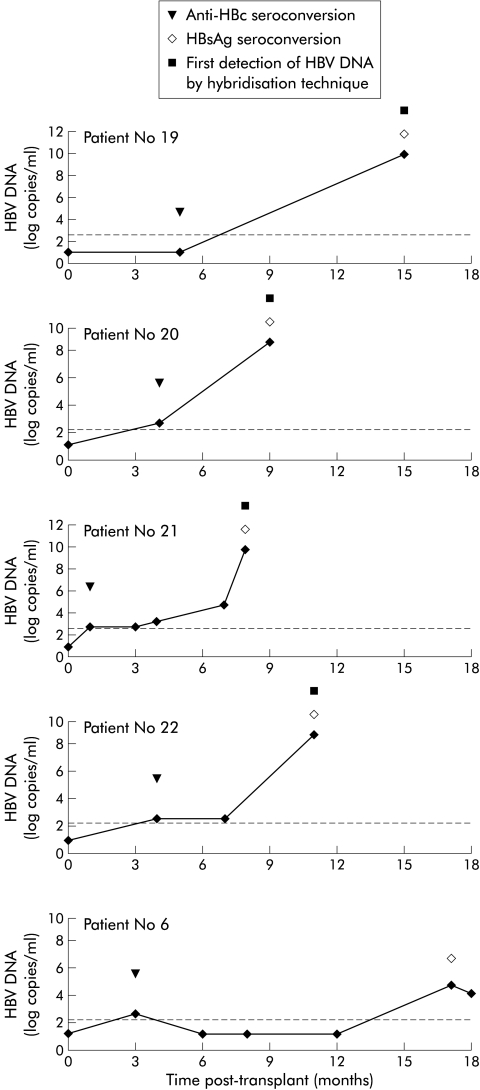
A modified HBIG protocol was given to 12 HBsAg negative recipients of liver grafts from anti-HBc positive donors (table 1 ▶). Five were HBV naive (anti-HBs and anti-HBc negative), three were anti-HBs positive after vaccination, and four had isolated anti-HBc positivity. All were serum HBV DNA negative by PCR before transplantation. The serological follow up evidenced anti-HBc seroconversion in 6/8 anti-HBc negative recipients a mean of two months post-transplantation. Anti-HBc positivity persisted throughout follow up. Patient No 5 died after seven months from a HBV unrelated cause. Patient No 6 was found to be HBsAg positive 17 months post-transplantation. At this time HBeAg and anti-HBe antibodies were negative and serum HBV DNA was negative by a hybridisation technique but PCR was positive. This patient had been lost to follow up for a few months before HBsAg seroconversion. The last available anti-HBs residual titre was below 50 UI/ml. Retrospective analysis on stored sera showed that HBV DNA was detectable at HBc seroconversion (fig 1 ▶). He developed lamivudine resistance after a seven month course with hybridisation detectable HBV DNA. The remaining 10 patients remained HBsAg negative and serum HBV DNA negative by PCR testing throughout a mean follow up period of 18 months (range 6–36). Monthly surveillance of anti-HBs titres determined the frequency of HBIG infusions. HBV naive and anti-HBc positive patients received no more than four infusions per year and vaccinated recipients no more than two; the prevention of HBV recurrence in HBsAg positive recipients requires 6–12 infusions per year.
Figure 1.
Kinetics of de nova hepatitis B virus (HBV) infection determined by polymerase chain reaction on serum samples from patient Nos 19–22 and No 6. Anti-HBc, anti-HBV core; HBsAg, HBV surface antigen.
Outcome of patients without HBIG prophylaxis
Six HBsAg negative recipients were not given HBIG prophylaxis: two were anti-HBs positive after efficient vaccination (Nos 17 and 18) and four were HBV naive (Nos 19–22). All were serum HBV DNA negative by PCR before transplantation. Anti-HBc seroconversion was detected in 5/6 recipients a mean of three months post-transplantation. None of the vaccinated recipients developed HBV infection. In contrast, the four naive patients developed de novo HBV infection 8–15 months after transplantation, with high levels of serum HBV DNA measurable by a hybridisation technique. Aminotransferases levels were normal or moderately elevated. Liver biopsies showed mild lobular inflammation. The kinetics of HBV infection were investigated by PCR on all available stored sera. HBV DNA was detectable in 3/4 patients from anti-HBc seroconversion (fig 1 ▶). All de novo infected patients were treated with 100 mg/day lamivudine. After a 12–16 month course of antiviral therapy, all developed resistance, with HBV DNA returning to pretreatment levels.
Virological and histological assessment of anti-HBc positive donors
Sera and pre-reperfusion liver graft biopsies were available from all 22 donors. All were HBsAg negative and anti-HBc positive, and 19 were also anti-HBs positive. Serum HBV DNA detection by PCR was negative in all but one donor. Immunostaining for HBs, HBc, and pre-S1 antigens was negative on all biopsy specimens. The liver β globin gene was amplified in 21/22 biopsy specimens. HBV DNA was positive in 11 (52%) of these 21 biopsy specimens (table 2 ▶). Five grafts showed mild inflammation and/or fibrosis while the remaining 17 were normal.
Table 2.
Characteristics of the donors
| Serum day 0 | Liver biopsy day 0 | ||||
| Donor | Anti-HBs | Anti-HBc | HBV DNA (PCR) | HBV DNA (PCR) | β globin |
| 1 | + | + | − | − | + |
| 2 | + | + | − | + | + |
| 3 | + | + | − | − | + |
| 4 | + | + | − | − | + |
| 5 | + | + | − | − | + |
| 6 | + | + | − | + | + |
| 7 | + | + | − | − | + |
| 8 | + | + | − | − | + |
| 9 | + | + | − | − | + |
| 10 | − | + | − | + | + |
| 11 | − | + | − | − | + |
| 12 | − | + | + | − | + |
| 13 | + | + | − | + | + |
| 14 | + | + | − | NI | − |
| 15 | + | + | − | + | + |
| 16 | + | + | − | + | + |
| 17 | + | + | − | + | + |
| 18 | + | + | − | + | + |
| 19 | + | + | − | − | + |
| 20 | + | + | − | + | + |
| 21 | + | + | − | + | + |
| 22 | + | + | − | + | + |
| 1/22 | 11/21 | ||||
| (4.5%) | (52%) | ||||
HBV, hepatitis B virus; HBc, HBV core; HBs, HBV surface; PCR, polymerase chain reaction; NI, not interpretable.
HBV DNA in liver biopsy specimens after transplantation
Liver biopsy was available in 15/22 patients a mean of 12 (7) months post-transplantation (range 1–23). Liver biopsies from patient Nos 19, 20, and 22, performed after HBsAg seroconversion, showed positive immunostaining for HBs, HBc, and pre-S1 antigens, and HBV DNA was detectable by PCR in liver tissue. The remaining 12 liver biopsy specimens showed negative immunostaining and were PCR HBV DNA negative, including biopsies from patient Nos 6 and 21 obtained 12 and five months post-transplantation, respectively, a few months before HBsAg seroconversion. It is noteworthy that HBV DNA was detectable in eight of these 12 grafts on day 0.
DISCUSSION
A significant percentage of organ donors—7% in our series—had serological evidence of apparently resolved HBV infection, as manifested by absence of HBsAg and the presence of circulating anti-HBc and anti-HBs antibodies. Several reports on HBV reactivation in immunosuppressed individuals,2–6,14 as well as observations on the high risk of HBV transmission by liver allografts from anti-HBc positive donors,5–8 support the conclusion that occult HBV DNA in a donor liver may be a significant source of HBV infection. Our study confirms previous estimations of the infectious risk based on the donor or recipient's HBV serological status7: naive recipients are highly susceptible to de novo infection regardless of the donor's anti-HBs status and patients with pretransplant vaccine immunity appear to be resistant. In this series, four HBV naive recipients developed infection while pretransplant vaccination appeared to be protective in two patients. This well recognised infectious risk suggests discarding or directing liver grafts from anti-HBc positive donors to selected recipients, such as HBsAg positive patients. However, due to organ shortage, HBsAg negative patients may occasionally receive these grafts because of a critical condition, rare blood group, or failure in the screening of the donor's anti-HBc status. The efficacy of long term high dose HBIG in lowering the rate of recurrence or the severity of the recurrent infection in HBsAg positive recipients10–12 and the apparent resistance to infection of patients with vaccine immunity prompted us to extend HBIG prophylaxis to HBV naive recipients of latently HBV infected liver grafts. The modified HBIG prophylaxis protocol was less stringent than that given to prevent HBV recurrence as we assumed that there were no (or less) circulating viral particles. Combination therapy with HBIG and lamivudine has been shown to prevent both HBV recurrence after transplantation in pretransplant HBV DNA negative HBsAg positive recipients15 and the emergence of HBsAg in anti-HBs negative recipients of liver grafts from anti-HBc positive donors.9 However, the risk of lamivudine induced mutations and the scarcity of alternative efficient therapies prompted us to adopt HBIG monotherapy. In this series, five naive patients underwent HBIG prophylaxis and anti-HBs titre surveillance. The development of de novo infection in one patient with low anti-HBs titres appears to be a surveillance failure and underlines the importance of achieving high levels of neutralising antibodies in naive patients. HBIG prophylaxis was also given to four patients with pretransplant natural immunity (anti-HBc positive and anti-HBs negative). These patients are at risk of HBV reactivation from donor liver but also from their own infection,5 although the rate of de novo HBV infection has been reported to be lower than that in naive patients.7 Indeed, none developed infection. Over the three year period of the study, three patients with pretransplant vaccine immunity were given HBIG at the perioperative period. The rationale for HBIG infusions was maintenance of high anti-HBs titres in a context where vaccine booster injections are poorly efficient. The decline of anti-HBs however was very slow and none developed infection.
An interesting finding was the marked fall in liver HBV DNA after transplantation. While liver HBV DNA was detected in eight of 12 grafts on day 0 of transplantation, it was no longer detectable in post-transplant specimens from the same grafts. Pre- and post-transplant PCR testing was performed on paraffin embedded liver biopsy sections, so the sample size and risk of DNA degradation were the same for both assays. In addition, each liver sample was checked by amplification of cellular DNA control. From an immunological viewpoint, this finding is paradoxical because the studied donors, who were presumably immunocompetent and had neutralising antibodies to HBsAg in most cases, had failed to clear liver HBV DNA. After recovery from acute infection, HBV may persist in liver tissue either integrated or in a replication competent form referred to as covalently closed circular DNA. “Clearance” of graft HBV DNA may be possible when HBV persists in a non-integrated form,16,17 and may be linked to regeneration following transplantation. Thus HBIG or anti-HBs antibodies acquired through vaccination may protect newly formed hepatocytes from HBV infection. From our data, anti-HBs neutralising immunity alone may be sufficient to control HBV replication as no antiviral therapy was given. However, maintenance of anti-HBc after seroconversion suggests persistent antigenic stimulation from core epitopes, probably expressed at low levels,17–19 and appears to be a marker of HBV latent infection in these liver recipients. Furthermore, in two patients who experienced de novo infection, liver HBV DNA was transiently undetectable before HBsAg seroconversion, showing that HBV replication may be reduced beyond detectable levels but not be turned off. These observations support the conclusion that anti-HBs prophylaxis must be given indefinitely.
Our study shows that the current anti-HBc testing is required to identify potential latent HBV carriers and that HBV molecular testing on serum or liver with the currently available techniques does not provide a definitive measure of the infectious risk of anti-HBc positive donors. As reported in previous studies on blood donors and in countries with a low seroprevalence of HBV infection,20 only one of 22 donor serum samples was positive for HBV DNA. Similarly, the persistence of HBV DNA in the liver of healthy donors with no evidence of liver dysfunction has been reported previously.5,16 In this series, HBV DNA was detected in 11/21 liver grafts (52%). It is of note that PCR was positive in 4/5 grafts of patients who developed de novo infection and in 7/16 grafts of patients who did not. This difference was not significant. Furthermore, the HBV detection rate may be underestimated due to low viral load, insufficient sensitivity of the PCR assay, or focal distribution of HBV infection. Additional studies are necessary to develop effective assays for the detection of latently infected donors.
In conclusion, circulating anti-HBs antibodies, without additional antiviral therapy, appear to control HBV replication in recipients of previously infected liver grafts and lead to the apparent clearance of liver HBV DNA. Further studies are needed to assess combined therapy with antiviral drugs, which raises concern about the lack of alternatives in cases of reactivation. Liver grafts from anti-HBc positive donors may be suitable for recipients with high anti-HBs titres before transplantation and for those who are given long term high dose HBIG to prevent HBV recurrence. The use of such livers in non-vaccinated HBV naive recipients should be carefully weighted. Indeed, HBIG are a human blood product and their administration is associated with increased costs, occasional adverse events, and time consuming protocols. On the other hand, the mortality rate among patients awaiting a liver graft is particularly high in those with rare blood groups or hepatocellular carcinoma. The risk of waiting longer for a suitable liver graft has to be taken into consideration. Finally, all HBV naive candidates for liver transplantation should be immunised against HBV, despite the low response rate of patients with end stage liver disease. When successful, such immunisation will provide an adequate anti-HBs response in situations where a liver from an HBsAg negative/anti-HBc positive donor is available.
Abbreviations
HBV, hepatitis B virus
HBc, HBV core
HBsAg, HBV surface antigen
HBeAg, HBV e antigen
HBIG, HBV immunoglobulin
PCR, polymerase chain reaction
REFERENCES
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745. [DOI] [PubMed] [Google Scholar]
- 2.Chazouillères O, Mamish D, Kim M, et al. “Occult” hepatitis B virus as a source of infection in liver transplant recipients. Lancet 1994;343:142–6. [DOI] [PubMed] [Google Scholar]
- 3.Radomski JS, Moritz MJ, Armenti VT, et al. Hepatitis B transmission from a liver donor who tested negative for hepatitis B surface antigen and positive for hepatitis B core antibody. Liver Transpl Surg 1996;2:130–1. [DOI] [PubMed] [Google Scholar]
- 4.Wachs ME, Amend WJ, Ascher NL, et al. The risk of transmission of hepatitis B from HBsAg(−), HBcAb (positive), HBc IgM (−) organ donors. Transplantation 1995;59:230–4. [PubMed] [Google Scholar]
- 5.Roche B, Samuel D, Gigou M, et al. De novo and apparent de novo hepatitis B virus infection after liver transplantation. J Hepatol 1997;26:517–26. [DOI] [PubMed] [Google Scholar]
- 6.Dickson RC, Everhart JE, Lake JR, et al. Transmission of hepatitis B virus by transplantation of livers from donors positive for antibody to hepatitis B core antigen. Gastroenterology 1997;113:1668–74. [DOI] [PubMed] [Google Scholar]
- 7.Dodson SF, Issa S, Araya V, et al. Infectivity of hepatic allografts with antibodies to hepatitis B virus. Transplantation 1997;64:1582–4. [DOI] [PubMed] [Google Scholar]
- 8.Douglas DD, Rakela J, Wright TL, et al. The clinical course of transplantation-associated de novo hepatitis B infection in the liver transplant recipient. Liver Transpl Surg 1997;3:105–11. [DOI] [PubMed] [Google Scholar]
- 9.Dodson SF, Bonham CA, Geller DA, et al. Prevention of de novo hepatitis B infection in recipients of hepatic allografts from anti-HBc positive donors. Transplantation 1999;68:1058–61. [DOI] [PubMed] [Google Scholar]
- 10.Samuel D, Muller R, Alexander G, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med 1993;329:1842–7. [DOI] [PubMed] [Google Scholar]
- 11.Kruger M. European hepatitis B immunoglobulin trials: prevention of recurrent hepatitis B after liver transplantation. Clin Transpl 2000;14(suppl 2):14–19. [PubMed] [Google Scholar]
- 12.Pruett TL, McGory R. Hepatitis B immune globulin: the US experience. Clin Transpl 2000;14(suppl 2):7–13. [PubMed] [Google Scholar]
- 13.Petit MA, Dubanchet S, Capel F. A monoclonal antibody specific for the hepatocyte receptor binding site on hepatitis B virus. Mol Immunol 1989;26:531–7. [DOI] [PubMed] [Google Scholar]
- 14.Lowell J, Howard T, White H, et al. Serological evidence of past hepatitis B infection in the liver donor and hepatitis B infection in the liver allograft. Lancet 1995;345:1084–5. [DOI] [PubMed] [Google Scholar]
- 15.Dodson SF, de Vera ME, Bonham CA, et al. Lamivudine after hepatitis B immune globulin is effective in preventing hepatitis B recurrence after liver transplantation. Liver Transpl 2000;6:434–9. [DOI] [PubMed] [Google Scholar]
- 16.Marusawa H, Uemoto S, Hijikata M, et al. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology 2000;31:488–95. [DOI] [PubMed] [Google Scholar]
- 17.Reherman B, Ferrari C, Pasquinelli C, et al. The hepatitis B virus persists for decades after patients recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med 1996;10:1104. [DOI] [PubMed] [Google Scholar]
- 18.Penna A, Artini M, Cavalli A, et al. Long lasting memory T-cell responses following self-limited acute hepatitis B. J Clin Invest 1996;98:1185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalak TI, Pasquinelli C, Guilhot S, et al. Hepatitis B virus persistence after recovery from acute viral hepatitis. J Clin Invest 1994;93:230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douglas DD, Taswell HF, Rakela J, et al. Absence of hepatitis B virus DNA detected by polymerase chain reaction in blood donors who are hepatitis B surface antigen negative and antibody to hepatitis B core antigen positive from a United States population with low prevalence of hepatitis B serologic markers. Transfusion 1993;33:212–16. [DOI] [PubMed] [Google Scholar]