Abstract
Background and aim: Recent studies have indicated that prior thermal stress causes upregulation of heat shock protein 70 (HSP70) expression in the pancreas and protects against secretagogue induced pancreatitis. The mechanisms responsible for the protective effect are not known. Similarly, the effects of prior non-thermal stress on HSP70 expression and pancreatitis are not known. The current studies were designed to specifically address these issues.
Methods: In the current studies pancreatitis was induced by administration of a supramaximally stimulating dose of caerulein 12 hours after thermal stress and 24 hours after non-thermal (that is, β adrenergic stimulation) stress.
Results: Both thermal and non-thermal stresses caused pancreatic HSP70 levels to rise and resulted in increased expression of HSP70 in acinar cells. Both forms of stresses protected against caerulein induced pancreatitis and prevented the early intrapancreatic activation of trypsinogen which occurs in this model of pancreatitis.
Conclusions: These results suggest that both thermal and non-thermal stresses protect against pancreatitis by preventing intrapancreatic digestive enzyme activation and that HSP70 may mediate this protective effect.
Keywords: heat shock proteins, hyperthermia, adrenergic, isoproterenol
Recent reports have indicated that prior thermal stress can protect rats from the subsequent development of secretagogue induced pancreatitis.1,2 Thermal stress has been shown to result in intrapancreatic upregulation of heat shock protein 70 (HSP70) expression2 but the location of that HSP70 expression and the mechanism(s) by which either thermal stress or HSP70 might protect against pancreatitis have not been established. Acute pancreatitis is generally believed to be an autodigestive disease in which the pancreas is injured by enzymes that are synthesised and secreted by acinar cells. Under physiological conditions, most of these potentially harmful digestive enzymes are synthesised and secreted as inactive proenzymes or zymogens which are subsequently activated in the duodenum. During the early stages of pancreatitis however digestive enzyme zymogens including trypsinogen are believed to become prematurely activated within the pancreas.3–6 In recently reported time dependence studies,6 we noted that trypsinogen activation, with release of trypsinogen activation peptide (TAP), occurred within intracytoplasmic vacuoles that appeared during the initial 15–30 minutes of supramaximal secretagogue stimulation. We have suggested that this activation of trypsinogen is an early and critical event which leads, subsequently, to acinar cell injury and pancreatitis.7,8
In the present communication, we show that prior β adrenergic stimulation, as well as prior thermal stress, can lead to intrapancreatic upregulation of HSP70 expression and that β adrenergic stimulation, like thermal stress, can protect against secretagogue induced pancreatitis. We show that intrapancreatic activation of trypsinogen after supramaximal secretagogue stimulation is prevented by either prior thermal stress or prior β adrenergic stimulation and that in both cases the upregulated HSP70 expression is primarily localised to the cytoplasmic acinar cell vacuoles which are believed to be the site of trypsinogen activation in this model of pancreatitis. We suggest that HSP70, which is expressed after either thermal or non-thermal (that is, β adrenergic stimulation) stress, protects against pancreatitis by preventing premature trypsinogen activation within acinar cells.
MATERIALS AND METHODS
Male Wistar rats (125–175 g), obtained from Charles River Laboratories, were housed in standard cages in a climate controlled room with an ambient temperature of 23 (2)°C and a 12 hour light/dark cycle. They were fed standard laboratory chow, given water ad libitum, randomly assigned to control or experimental groups, and fasted overnight before each experiment. Caerulein, the decapeptide analogue of the pancreatic secretagogue cholecystokinin, was purchased from Research Plus (Bayonne, New Jersey, USA), collagenase from Worthington Biochemical (Freehold, New Jersey, USA), trypsin substrate (Boc-Gln-Ala-Arg-MCA) from Peptides International (Louisville, Kentucky, USA), Superfrost/Plus slides and Permount from Fisher Scientific (Pittsburg, Pennsylvania, USA), and Vectastain Universal ABC and avidin-biotin blocking kits from Vector Laboratories (Burlingame, California, USA). Antibody used for western blotting and immunolocalisation of inducible HSP70 (cat. No. SPA 810) was obtained from StressGen (Victoria, Canada). Antirabbit TAP antibody was a kind gift from D McNally, Abbott Laboratories. All other chemicals and reagents were purchased from Sigma Chemical (St Louis, Missouri, USA). All experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center.
Thermal and non-thermal stress
Thermal stress was induced using rats anaesthetised with an intraperitoneal injection of pentobarbital. They were placed on a heating pad and core body temperature was monitored with a rectal probe. Their core body temperature was gradually increased, over approximately 25 minutes, to 42°C and then maintained at that level for 20 minutes. β-Adrenergic stimulation was used to induce non-thermal stress. In this case, conscious animals were given a single intraperitoneal injection of isoproterenol (0.05 mg/kg).
Induction of pancreatitis
Pancreatitis was induced, 12 hours after thermal stress or 24 hours after administration of isoproterenol, by administration of five hourly intraperitoneal injections of caerulein (10 μg/kg). Control animals received comparable injections of saline. One hour after the final injection, animals were sacrificed by administration of a lethal intraperitoneal dose of pentobarbital.
Measurement of hsp70 levels by western blotting
Pancreas samples obtained immediately after sacrifice were homogenised and aliquots (10 μg protein) subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis according to Laemmli.9 The separated proteins were transferred to nitrocellulose membranes as described by Towbin and colleagues.10 Induction of HSP70 was monitored using antibody to inducible HSP70 (1:1000). Labelled proteins were visualised by the enhanced chemiluminescence method using horseradish peroxidase coupled secondary antibody (1:5000) and quantitated using a Scion Image Analysis Program.
Evaluation of pancreatitis severity
Serum amylase activity was quantitated using 4,6-ethylidene (G7)-p-nitrophenyl (G1)-α1D-maltoheptoside, as described by Kruse-Jarres and colleagues.11 Pancreatic oedema was evaluated by measuring pancreatic water content. This was accomplished by weighing blotted fresh pancreas samples before and after desiccation (95°C, 12 hours). Water content—that is, the difference between wet and dry weight—was expressed as a per cent of tissue wet weight. The extent of pancreatic inflammation was evaluated by quantitating neutrophil sequestration within the pancreas. This was accomplished by measuring myeloperoxidase (MPO) activity, as described previously,12 in pancreas homogenates and that activity was expressed as a function of tissue dry weight. Pancreas necrosis and the extent of acinar cell vacuolisation were determined by morphometry, as previously described,13 performed by an experienced observer who was unaware of the sample identity. The activity of trypsin within the pancreas was quantitated using the fluorimetric assay of Kawabata and colleagues14 and expressed as arbitrary units per μg of DNA in the pancreas homogenate. To allow for pooling of results from different experiments, values were calculated and expressed as a per cent of that noted in homogenates obtained from non-stressed animals given caerulein. TAP levels in the pancreas homogenates were quantitated by ELISA using affinity purified rabbit polyclonal anti-TAP antibodies, as previously described.7
Immunolocalisation of hsp70
Pancreas samples were fixed (12 hours) in neutral buffered formalin, embedded in paraffin, sectioned (5 μm), and mounted on Superfrost/Plus slides. After being deparaffinised, endogenous peroxidase activity was quenched with 3% hydrogen peroxide (10 minutes) and non-specific binding was blocked by incubation in phosphate buffered saline containing 1% bovine serum albumin (30 minutes). They were then incubated in avidin and biotin blocking solutions (15 minutes each) with washes in-between. The sections were then incubated with primary antibody (anti-mouse HSP70 5 μg/ml) overnight at 4°C. Negative control sections were incubated similarly but without primary antibody. After rinsing, the sections were sequentially treated with biotinylated universal secondary antibody from Vector Laboratories (1:200) for 30 minutes, and with Vecta ABC reagent for another 30 minutes. The antigen-antibody complex was visualised using the diaminobenzidine (DAB) method (DAB 5 mg/10 ml phosphate buffered saline plus 12 μl of 30% hydrogen peroxide, three minutes) and slides were then counterstained with methyl green (1% in 0.1 M sodium acetate, pH 4.0) for 10 minutes. After rinsing in distilled water and butanol, they were cleared in xylene and coverslipped with Permount.
In vitro amylase secretion
Fresh pancreatic acini were prepared from control thermally and non-thermally stressed rats using collagenase digestion, as previously described,15 and amylase secretion, in response to varying concentrations of caerulein, measured over 30 minutes.16 Amylase secretion was expressed as a per cent of total amylase content.
Analysis of data
Results are reported as mean (SEM) values obtained from multiple determinations in three or more separate experiments. In all figures, vertical bars denote SEM values and the absence of such bars indicates that the SEM was too small to illustrate. The significance of changes was evaluated using the Student's t test when data consisted of only two groups or by analysis of variance (ANOVA) when three or more groups were compared. Differences associated with a p value of less than 0.05 were considered significant.
RESULTS
Effects of prior thermal stress on pancreatic hsp70 expression
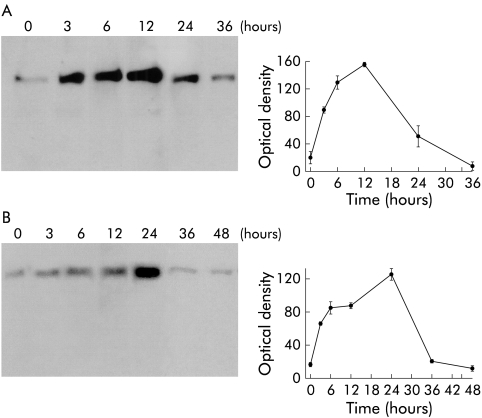
Exposure of rats to thermal stress resulted in time dependent upregulation of HSP70 expression in the pancreas. As shown in fig 1A ▶, HSP70 expression was increased within three hours of thermal stress, reached a peak 12 hours after thermal stress, and returned to basal levels 24–36 hours after thermal stress.
Figure 1.
Effect of thermal stress and β adrenergic stimulation on pancreatic heat shock protein 70 (HSP70) expression. Rats were exposed to either thermal stress (A) or β adrenergic stimulation (B), as described in the text. At the indicated times, the animals were sacrificed, the pancreata were homogenised, and HSP70 expression was evaluated by western blotting, as described in the methods section. Relative optical densities are expressed as mean (SEM) values of at least three animals at each time point.
Effect of β adrenergic stimulation on pancreatic hsp70 expression
Exposure of rats to β adrenergic stimulation, accomplished by administration of a single intraperitoneal dose of isoproterenol, resulted in time dependent upregulated expression of intrapancreatic HSP70. As shown in fig 1B ▶, pancreatic HSP70 levels were increased within one hour of isoproterenol administration, reached a peak 24 hours after administration, and then declined to resting levels over the subsequent 12–24 hours.
Effect of prior thermal stress or β adrenergic stimulation on the severity of caerulein induced pancreatitis
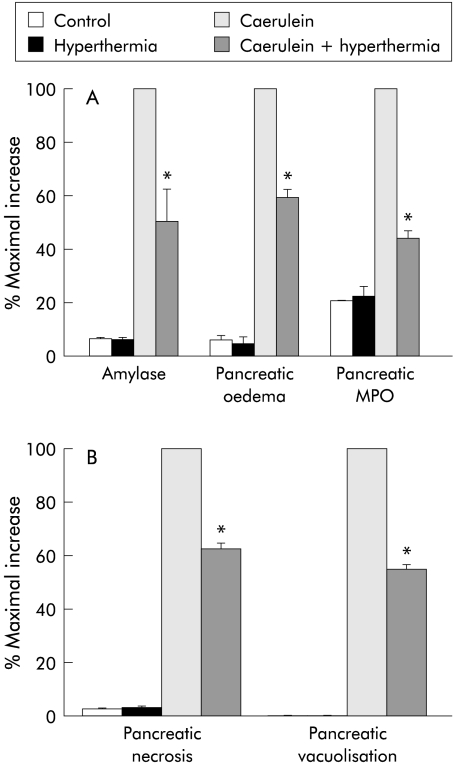
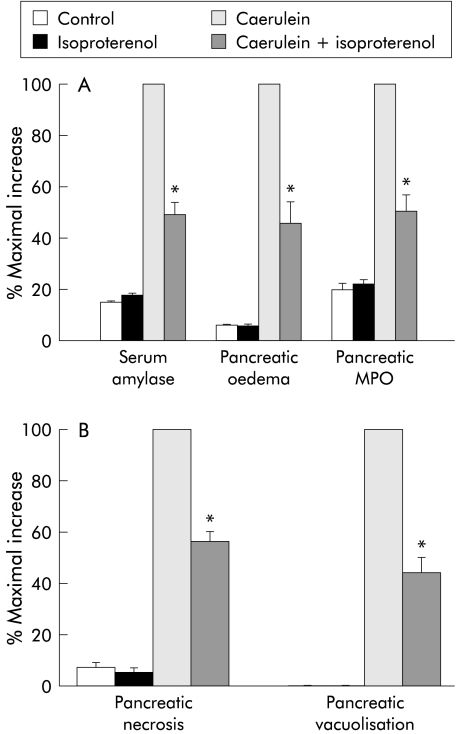
Pancreatitis was induced by administration of five hourly injections of a supramaximally stimulating dose of caerulein begun either 12 hours after thermal stress or 24 hours after administration of isoproterenol. As shown in figs 2 and 3 ▶ ▶, the magnitude of each of the parameters used to evaluate the severity of caerulein induced pancreatitis was reduced by approximately 50% following either thermal stress or β adrenergic stimulation.
Figure 2.
Effect of prior thermal stress on the severity of caerulein induced pancreatitis: 12 hours after exposure to thermal stress, pancreatitis was induced by caerulein administration and its severity was quantitated, as described in the text, in control animals, in control animals exposed to hyperthermia, in rats given caerulein, and in rats given caerulein after prior exposure to hyperthermia. Results are expressed as a per cent of the increase noted in non-stressed animals given caerulein (that is, maximal increase). Results represent mean (SEM) values for at least three animals at each time point. *Significantly different (p<0.05) from non-stressed caerulein treated rats. MPO, myeloperoxidase.
Figure 3.
Effect of β adrenergic stimulation on the severity of caerulein induced pancreatitis: 24 hours after administration of isoproterenol, pancreatitis was induced by caerulein administration and its severity was quantitated, as described in the text, in control animals, in control animals injected with isoproterenol, in rats given caerulein, and in rats given caerulein after administration of isoproterenol. Results are expressed as a per cent of the increase noted in non-stressed animals given caerulein (that is, maximal increase). Results represent mean (SEM) values for at least three animals at each time point. *Significantly different (p<0.05) from non-stressed caerulein treated rats. MPO, myeloperoxidase.
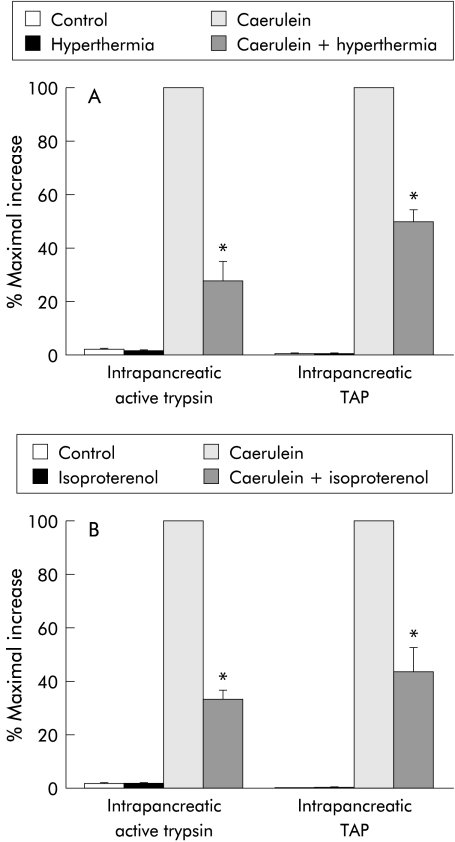
effect of prior thermal stress or β adrenergic stimulation on intrapancreatic trypsinogen activation during caerulein induced pancreatitis
Intrapancreatic activation of trypsinogen can be detected during the early stages of caerulein induced pancreatitis (fig 4 ▶). Trypsinogen activation is manifested in two ways—that is, by the appearance of trypsin activity in the pancreas homogenate and by the rise in TAP levels in the homogenate. Both manifestations of trypsinogen activation were reduced by approximately 50% after either prior thermal stress (fig 4A ▶) or β adrenergic stimulation (fig 4B ▶). In contrast with its effect on trypsinogen activation, neither prior thermal stress nor prior β adrenergic stimulation altered pancreatic trypsinogen or cathepsin B content (not shown).
Figure 4.
Effect of hyperthermia and β adrenergic stimulation on caerulein induced intrapancreatic trypsinogen activation: pancreatitis was induced in rats after prior stress induced by hyperthermia (A) or administration of isoproterenol (B), as described in the text. Trypsinogen activation was monitored either by quantitation of trypsin activity or by measurement of trypsinogen activation peptide (TAP), as described in the text. Results are expressed as a per cent of the increase noted in non-stressed animals given caerulein (that is, maximal increase). Results represent mean (SEM) values for at least three animals at each time point. *Significantly different (p<0.05) from non-stressed caerulein treated rats.
Immunolocalisation of hsp70 in the pancreas
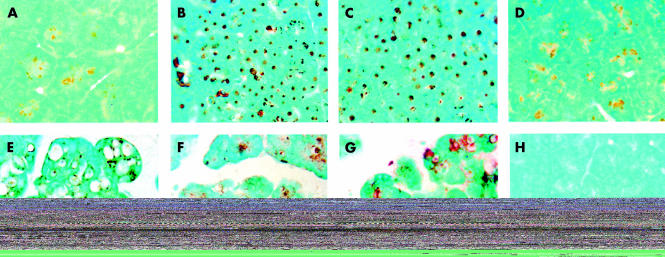
As shown in fig 5 ▶, there was virtually no HSP70 in the pancreas samples obtained from control animals (fig 5A ▶). However, 12 hours after either thermal stress (fig 5B ▶) or 24 hours after β adrenergic stimulation (fig 5C ▶), when the level of HSP70 was at its peak in the respective models, as assessed by western blotting (fig 1A, B ▶), there was profound upregulation of HSP70 expression in the pancreatic acini. Under these conditions, HSP70 expression was confined to acinar cells and localised to the nuclear/perinuclear region. Thirty six hours after thermal stress, HSP70 levels returned to control values (fig 1A ▶) and the immunostaining pattern was similar to that seen in pancreas samples taken from control rats (fig 5D ▶). Administration of caerulein to naïve animals (fig 5E ▶) resulted in modest expression of HSP70 but profound vacuolisation of acinar cells and interstitial oedema. In pancreas samples taken from rats given caerulein 12 hours after prior thermal stress (fig 5F ▶) or 24 hours after isoproterenol injection (fig 5G ▶), the extent of both vacuolisation and oedema were reduced. Under these conditions, HSP70 was localised primarily within those cytoplasmic vacuoles which are noted in acinar cells. No immunostaining was seen when the primary antibody was omitted during the immunostaining protocol (fig 5H ▶).
Figure 5.
Immunolocalisation of HSP70 in the pancreas. Pancreatic tissues obtained from control rats (A), from rats 12 hours after exposure to hyperthermia (B), from rats 24 hours after β adrenergic stimulation (C), from rats 36 hours after prior thermal stress (D), or from rats given caerulein alone (E) or caerulein after prior thermal or non-thermal stress (F, G) were processed for heat shock protein 70 (HSP70) localisation, as described in the text. While there was very little HSP70 expression in control pancreas (A), exposure of rats to hyperthermia (B) or β adrenergic stimulation (C) resulted in significant upregulation of HSP70 expression. HSP70 expression was similar to that seen in control samples 36 hours after prior thermal stress (D). Administration of caerulein to control animals (E) resulted in modest expression of HSP70 with profound vacuolisation and oedema. Prior thermal stress (F) or isoproterenol treatment (G) significantly decreased caerulein induced vacuolisation and oedema. No immunostaining was seen in sections where the primary antibody was omitted (H). Results shown are representative of three different experiments for each experimental condition.
Effect of prior thermal stress on in vitro secretagogue stimulated amylase secretion from acini
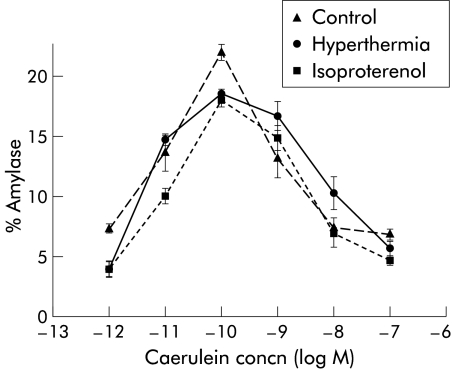
As shown in fig 6 ▶, prior thermal stress did not alter the biphasic dose dependence which characterises caerulein stimulation of amylase secretion from acini in vitro. Low concentrations of caerulein stimulated amylase secretion while high concentrations inhibited amylase secretion from acini prepared from control, prior thermally, or non-thermally stressed rats.
Figure 6.
Caerulein stimulated amylase secretion from rat pancreatic acini. Dispersed pancreatic acini were prepared from control rats, rats pre-exposed to hyperthermia, or rats stimulated with isoproterenol and were incubated with various concentrations of caerulein for 30 minutes. Amylase release into the medium was determined as described in the text. Results are expressed as per cent of total amylase content in acini. Results represent mean (SEM) values obtained from three separate experiments.
DISCUSSION
Our studies have confirmed and extended the findings of Wagner and colleagues2 who noted that prior thermal stress protected rats from caerulein induced pancreatitis. In accord with their observations, we also noted that thermal stress resulted in upregulation of pancreatic HSP70 levels and suggest that the protection against pancreatitis which is afforded by prior thermal stress is mediated by HSP70. This conclusion must remain tentative however as thermal stress may result in other changes17,18 which effect the severity of subsequent pancreatitis. Thus a cause-effect relationship between HSP70 expression and protection against pancreatitis can only be proved by studies which either more specifically induce HSP70 (for example, transgenic animals overexpressing HSP70) or interfere with HSP70 induction (for example, HSP70 knockout animals). However, in support of our conclusion that the protection against pancreatitis which follows thermal stress is mediated by HSP70 are the facts that: (a) β adrenergic stimulation which also resulted in upregulated pancreatic HSP70 expression also protected against pancreatitis; (b) the similar level of HSP70 achieved after either thermal stress or β adrenergic stimulation was associated with a similar degree of protection against pancreatitis; and (c) both thermal stress and β adrenergic stimulation caused HSP70 levels to rise within the acinar cells of the pancreas where a protective effect could occur. Additional support for our conclusion that both thermal and non-thermal stress induced HSP70 expression mediates protection against pancreatitis in vivo comes from our recent in vitro studies using the antisense oligonucleotide approach where we showed that culture stress induced upregulated expression of HSP70 prevented caerulein induced trypsinogen activation and acinar cell injury.19
Heat shock proteins were first described nearly 40 years ago by Ritossa20 who noted that raising the temperature of Drosophila from 25°C to 30°C resulted in a puffing pattern in certain chromosomal locations and that a brief sublethal period of thermal stress protected the Drosophila from a subsequent otherwise lethal exposure to heat. Since then, HSPs of varying molecular weights (HSP27, HSP60, HSP70, HSP90, etc.) have been identified and HSP induction by a variety of different stresses (oxygen derived free radicals, heavy metals, ischaemia/reperfusion, water immersion, etc.) has been observed.21,22
The HSP70 family of HSPs is perhaps the most well studied. It is known to include the constitutively expressed HSC70 (cognate HSP70/73), the inducible HSP70 (HSP70 or HSP72), and glucose regulated protein (GRP78) which is also referred to as heavy chain immunoglobulin binding protein (BiP). The inducible HSP70, which is the form recognised by the antibodies used in this study, has been found in pancreas as well as in other tissues, to be primarily localised in the nucleus and cytoplasm. Some reports23,24 have indicated that it may also be expressed in the endoplasmic reticulum. It is believed to act in a chaperone-like manner aiding in the passage of proteins across membrane barriers,25 preventing misfolding of newly synthesised proteins,26 and facilitating sequestration as well as elimination of improperly assembled, misfolded, or aggregated proteins.27–29
Caerulein induced pancreatitis follows exposure of the pancreas to high doses of the secretagogue and interaction of caerulein with the low affinity cholecystokinin receptors which mediate inhibition of acinar cell secretion.30 Thus one potential mechanism for protection against pancreatitis that follows either thermal stress or β adrenergic stimulation (and, presumably, HSP70 expression) would be alteration in acinar cell responsiveness to caerulein—that is, downregulation of low affinity receptors or loss of the inhibitory response to supramaximally stimulating doses of caerulein. As shown in fig 6 ▶, that is not the case. The in vitro acinar cell biphasic response to caerulein stimulation was unaltered, even in acini prepared from animals previously exposed to thermal stress.
One of the earliest events in secretagogue induced pancreatitis involves intra-acinar cell activation of trypsinogen.7 The events which lead to trypsinogen activation during the early stages of pancreatitis are not entirely understood. In previous reports, we have suggested that it might result from colocalisation of trypsinogen and the lysosomal hydrolase cathepsin B within cytoplasmic vacuoles and the subsequent activation of trypsinogen by cathepsin B.6,7 Theoretically, active trypsin could cause activation of other zymogens within acinar cells and this could lead to both acinar cell injury and pancreatitis.
In the current report, we noted that the rise in pancreatic level of active trypsin and TAP after supramaximal caerulein stimulation was attenuated in animals previously exposed to thermal stress or given isoproterenol. Neither the pancreatic content of trypsinogen nor the pancreatic content of cathepsin B were altered by prior thermal stress or prior administration of isoproterenol. Attenuation of the rise in trypsin activity could reflect either inhibition of the active protease or reduction in activation of the zymogen. Our finding that TAP levels were also lower in animals after prior stress indicates that stress results in reduction of the activation process itself, rather than causing inhibition of the activated zymogen.
The mechanisms by which prior thermal stress or β adrenergic stimulation (and, presumably, HSP70 expression) might interfere with trypsinogen activation in secretagogue induced pancreatitis cannot be determined from our studies. It is tempting however to speculate that those mechanisms might alter either colocalisation of trypsinogen with cathepsin B or the ability of cathepsin B to activate trypsinogen in acinar cells during secretagogue induced pancreatitis. Regardless of the mechanisms involved however our studies add to the growing list of observations which suggest that agents or manipulations which cause upregulation of HSP70 expression might prove helpful in the prevention or treatment of pancreatitis.
Acknowledgments
This work was supported by NIH grant DK-58694 (AKS) and DK-31396 (MLS). JLF was financially supported by grant 81GE-50064 from the Swiss National Science Foundation.
Abbreviations
BiP, heavy chain immunoglobulin binding protein
DAB, diaminobenzidene
GRP, glucose regulated protein
MPO, myeloperoxidase
HSP, heat shock protein
TAP, trypsinogen activation peptide
REFERENCES
- 1.Otaka M, Itoh H, Klwabara T, et al. Induction of heat shock protein and prevention of caerulein induced pancreatitis by water immersion stress in rats. Int J Biochem 1994;26:805–11. [DOI] [PubMed] [Google Scholar]
- 2.Wagner ACC, Weber H, Jonas L, et al. Hyperthermia induces heat shock protein expression and protection against cerulein induced pancreatitis in rats. Gastroenterology 1996;111:1333–42. [DOI] [PubMed] [Google Scholar]
- 3.Figarella CB, Miszczuk-Jamska B, Barrett AJ. Possible lysosomal activation of pancreatic zymogens: activation of both human trypsinogens by cathepsin B and spontaneous acid. Activation of human trypsinogen 1. Biol Chem Hoppe Seyler 1988;369:293–8. [PubMed] [Google Scholar]
- 4.Gudgeon AM, Heath DI, Hurley P, et al. Trypsinogen acitvation peptides assay in the early prediction of severity of acute pancreatitis. Lancet 1990;335:4–8. [DOI] [PubMed] [Google Scholar]
- 5.Lerch MM, Saluja AK, Dawra R, et al. Acute necrotizing pancreatitis in the opossum: Earliest morphological changes involve acinar cells. Gastroenterology 1992;103:205–13. [DOI] [PubMed] [Google Scholar]
- 6.Saluja AK, Donovan EA, Yamanaka K, et al. Caerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology 1997;113:304–10. [DOI] [PubMed] [Google Scholar]
- 7.Hofbauer B, Saluja A, Lerch MM, et al. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol 1998;275 :G352–62. [DOI] [PubMed] [Google Scholar]
- 8.Saluja AK, Bhagat L, Lee HS, et al. Secretagogue-induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol 1999;276:G835–42. [DOI] [PubMed] [Google Scholar]
- 9.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–85. [DOI] [PubMed] [Google Scholar]
- 10.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some aplications. Proc Natl Acad Sci USA 1979;76: 4350–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruse-Jarres JD, Kaiser C, Hafkenscheid JC, et al. Evaluation of a new alpha-amylase assay using 4.6-ethylidene-(G7)-1-4-nitrophenyl-(G1)-alpha-D-maltoheptaoside as substrate. J Clin Chem Clin Biochem 1989;27:105–13. [PubMed] [Google Scholar]
- 12.Gerard C, Frossard JL, Bhatia M, et al. Targeted disruption of the beta-chemokine receptor CCR1 protects against pancreatitis-associated lung injury. J Clin Invest 1997;100:2022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frossard JL, Saluja A, Bhagat L, et al. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology 1999;116:694–701. [DOI] [PubMed] [Google Scholar]
- 14.Kawabata S, Miura T, Morita T, et al. Highly sensitive peptide-4-methylcoumaryl-7 amide substrates for blood clotting proteases and trypsin. Eur J Biochem 1988;172:17–25. [DOI] [PubMed] [Google Scholar]
- 15.Powers RE, Saluja AK, Houlihan MJ, et al. Diminished agonist-stimulated inositol triphosphate generation blocks stimulus-secretion coupling in mouse pancreatic acini during diet-induced experimental pancreatitis. J Clin Invest 1986;77:1668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutledge PL, Saluja A, Leli U, et al. A simple and efficient method of measuring in vitro amylase secretion by dispersed pancreatic acini. Pancreas 1988;3:508–11. [DOI] [PubMed] [Google Scholar]
- 17.Currie RW, Karmazyn M, Kloe M, et al. Heat shock response is associated with enhanced postischaemic ventricular recovery. Circ Res 1988;63:543–9. [DOI] [PubMed] [Google Scholar]
- 18.Yellon DM, Downey JM. Current research views on myocardial reperfusion and reperfusion injury. Cardioscience 1990;1:89–98. [PubMed] [Google Scholar]
- 19.Bhagat L, Singh VP, Hietaranta AJ, et al. Heat shock protein 70 prevents secretagogue-induced cell injury in the pancreas by preventing intracellular trypsinogen activation. J Clin Invest 2000;106:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritossa FM. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experentia 1962;18:571–3. [Google Scholar]
- 21.Bellmann KA, Wenz J, Radons V, et al. Heat shock induces resistance in rat pancreatic islet cells against nitric oxide, oxygen radicals and streptozotocin toxicity in vitro. J Clin Invest 1995;95:2840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong HR, Mannix RJ, Rusnak JM, et al. The heat shock response attenuates lipopolysaccharide-mediated apoptosis in cultured sheep pulmonary artery endothelial cells. Am J Respir Cell Mol Biol 1996;15:745–51. [DOI] [PubMed] [Google Scholar]
- 23.Gething MJ. Role and regulation of the ER chaperone BiP. Semin Cell Dev Biol 1999;10:465–72. [DOI] [PubMed] [Google Scholar]
- 24.Haas IG. BiP (GRP78), an essential hsp70 resident protein in the endoplasmic reticulum. Experentia 1994;50:1012–20. [DOI] [PubMed] [Google Scholar]
- 25.Pelham H. Speculations on the function of the major heat shock and glucose regulated proteins. Cell 1986;46:959–63. [DOI] [PubMed] [Google Scholar]
- 26.Beckman RP, Mizzen LE, Welch WJ. Interaction of HSP70 with newly synthesized proteins: implications for protein folding and assembly. Science 1990;248:850–4. [DOI] [PubMed] [Google Scholar]
- 27.Yost HJ, Lindquist S. RNA splicing is interrupted by heat shock and rescued by heat shock protein synthesis. Cell 1986;45:185–93. [DOI] [PubMed] [Google Scholar]
- 28.Pelham HRB. Activation of heat shock genes in eukaryot cells. Trends Genet 1985;1:31–5. [Google Scholar]
- 29.Amin J, Ananatham J, Voellmy R. Key features of heat shock regulatory elements. Mol Cell Biol 1988;8:3761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saluja AK, Saluja M, Printz H, et al. Experimental pancreatitis is mediated by low-affinity cholecystokinin receptors that inhibit digestive enzyme secretion. Proc Natl Acad Sci USA 1989;86:8968–71. [DOI] [PMC free article] [PubMed] [Google Scholar]