Abstract
Background: Different acid and peptic related gastroduodenal diseases are associated with both increased gastric secretion and Helicobacter pylori infection. Patients with H pylori associated gastritis or duodenal ulcer have increased serum pepsinogen levels which decrease after eradication. The mechanisms of H pylori induced gastric mucosal damage are not completely understood.
Aim: To determine the effects of H pylori on pepsinogen secretion from isolated human peptic cells.
Methods: Dispersed human peptic cells were prepared from endoscopically obtained biopsy specimens after collagenase digestion, mechanical disruption, and density gradient centrifugation. H pylori was obtained from gastric biopsies (antrum and body), and cultured in non-selective and selective media. Isolates of H pylori were used at different concentrations (1–20×106 colony forming units (cfu)).
Results: H pylori (106–2×107 cfu) increased basal pepsinogen secretion in a concentration dependent manner. This stimulus was not observed with Escherichia coli. The increased secretion was in addition to that observed with 0.1 mM histamine and 0.1 mM dibutyryl-cyclic adenosine monophosphate. However, H pylori did not affect either carbamylcholine (0.1–10 μM) or cholecystokinin (1 μM) stimulated pepsinogen secretion. Addition of the nitric oxide synthase inhibitor Nw-monomethyl-l-arginine (1 mM) inhibited H pylori induced cGMP generation and pepsinogen secretion, which were also reduced in the absence of extracellular calcium. H pylori induced pepsinogen secretion was not affected by the absence/presence of the cagA gene.
Conclusions: H pylori increases pepsinogen secretion from human peptic cells through a calcium and nitric oxide mediated intracellular pathway. This effect is independent of the H pylori virulent cagA gene, and may be a mechanism of H pylori induced gastric mucosal damage.
Keywords: Helicobacter pylori, pepsinogen, nitric oxide, peptic celss
Helicobacter pylori is now recognised as the main acquired factor in the pathogenesis of duodenal ulcer disease. The infection is found in more than 95% of patients with duodenal ulcer, and numerous studies have shown that eradicating the bacteria markedly decreases the ulcer relapse rate.1–4 The mechanism by which this infection, which predominantly affects the gastric mucosa, predisposes to ulceration of the duodenum has been the subject of much speculation.
The potential mechanisms by which H pylori induces mucosal damage include injury to gastric cells by direct contact of the bacterium through elaboration of enzymes and putative cytotoxins, immune response, and effects of H pylori on the mechanisms that control gastric secretion.5
Patients with duodenal ulcers have increased pepsinogen I levels, which was believed to be of genetic origin.6 However, numerous studies have shown that both duodenal ulcer patients and healthy volunteers with H pylori infection have increased serum pepsinogen I levels which decrease after H pylori eradication.7–10 Different studies have shown that H pylori sonicate and H pylori lipopolysaccharide stimulate pepsinogen release from either isolated rabbit gastric glands11 or guinea pig gastric mucosa,12 suggesting a direct stimulatory effect of H pylori on chief cells. The mechanism whereby H pylori stimulates pepsinogen secretion is not clear and evidence in humans is not available. To address this question, we have examined the effects of H pylori on pepsinogen release from dispersed human peptic cells. The second messenger system involved in H pylori induced stimulation of chief cell pepsinogen secretion was characterised by functional studies.
METHODS
Materials
Chemicals were obtained from the following sources: bovine serum albumin (fraction V), carbamylcholine (carbachol), histamine, dibutyryl-cyclic adenosine monophosphate (db-cAMP), cholecystokinin octapeptide (CCK-OP), HEPES, Percoll, crystalline porcine pepsinogen, soybean trypsin inhibitor, ethylene glycol tetraacetic acid (EGTA), Nw-monomethyl-l-arginine (l-NMMA), 8-bromo (8Br)-cGMP, and glucose from Sigma Chemical Co (St Louis, Missouri, USA); bovine haemoglobin from Gibco Diagnostics (Madison, Wisconsin, USA); and collagenase (type IV) from Worthington Biochemical Corp (Freehold, New Jersey, USA). All reagents for polymerase chain reaction (PCR) (primers, deoxynucleoside triphosphates, recombinant Taq DNA polymerase, and reaction buffer) were purchased from Boehringer Mannheim GmbH (Mannheim, Germany), and agarose was obtained from Bio Rad (California, USA).
Unless otherwise stated, the standard Ringer solution contained 82.2 mM NaCl, 4.0 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 0.8 mM NaSO4, 17.8 mM NaHCO3, 0.8 mM NaH2PO4, 11.5 mM glucose, and 0.2% bovine serum albumin. The medium was equilibrated with 95% O2–5% CO2, and pH adjusted to 7.25. Ca++ low medium contained 0.1 mM CaCl2 and no MgCl2, and Ca++ free medium contained no CaCl2 or MgCl2.
Human material
Gastric biopsy specimens from the oxyntic area were obtained from 76 patients undergoing upper gastrointestinal endoscopy (39 men/37 women). Mean age of this population was 47.1 (21–72) years. In 21 of these patients, endoscopy was normal, 16 had an active duodenal ulcer, four a healed duodenal ulcer, eight oesophagitis, three a gastric ulcer, and 24 duodenitis or antritis without ulcer. Most were or had recently been receiving H2 receptor antagonists, and the rest were receiving antacids or were free from any treatment. H pylori status was determined by histological examination and/or the urease test (CLO test; Delta West LTD, Canning Vale, Bentley, Australia) in 68 patients (positive in 46). No biopsy specimens were obtained from patients who had recently used non-steroidal anti-inflammatory drugs. The study was approved by the Institutional Review Board and written informed consent was obtained from all patients.
Study design
Cell isolation
Dispersed peptic cells were obtained from endoscopically acquired biopsy specimens after collagenase digestion, filtration, and density gradient centrifugation, according to a method previously described.13,14 Eight to 10 regular sized punch biopsy specimens yielded approximately 106 cells which were suspended in Ringer's solution under an atmosphere of 95% O2–5% CO2 and refrigerated overnight. The next morning, cells were allowed to warm at room temperature before they were used in experiments. No experiments were performed if cell viability was lower than 90% and experiments with a low total pepsinogen content were discarded.
Culture of H pylori
H pylori were obtained from gastric biopsies (antrum and body of the stomach) of different patients (table 1 ▶). Specimens were placed in a sterile tube with 0.5 ml physiological serum, rapidly processed, and cultured in a non-selective medium (blood agar) or a selective H pylori medium (Dent medium; Oxoid, Madrid, Spain) with antibiotics (vancomycin, trimethoprim, cefsulodin, and amphotericin). Plates were incubated in a microaerobic atmosphere (5–10% CO2, 5–20% O2, and 80–90% N2) at 37°C with a relative humidity of 95% for up to seven days. Identification of the bacteria was performed by Gram stain and oxidase, catalase, and urease activity. Different H pylori strains were prepared in saline solution to obtain a heavy suspension (McFarland standard 6) of growth from plates. Isolates of H pylori were used at different concentrations (1–20×106 colony forming units (cfu)). Sonication of the bacteria was performed by ultrasonic homogenisation at 6 kHz for one minute (Bandelin Sonopuls, Berlin, Germany).
Table 1.
Characteristics of patients whose Helicobacter pylori were isolated
| Age (y) | Sex (M/F) | Endoscopy features | Strain | cagA gene |
| 59 | F | Antritis | HP25 | |
| 63 | M | Duodenitis | HP31 | |
| 48 | F | Duodenal ulcer | HP32 | |
| 36 | M | Duodenal ulcer | HP33 | Negative |
| 62 | F | Duodenal ulcer | HP34 | |
| 60 | F | Normal | HP42 | |
| 73 | M | Normal | HP43 | |
| 39 | F | Normal | HP46 | |
| 47 | F | Duodenal ulcer | HP50 | |
| 66 | M | Antritis | HP52 | Negative |
| 42 | F | Normal | HP58 | |
| 52 | F | Normal | HP59 | Negative |
| 27 | F | Normal | HP60 | |
| 39 | M | Duodenal ulcer | HP61 | Positive |
| 67 | M | Duodenal ulcer | HP62 | Positive |
| 40 | F | Duodenal ulcer | HP63 | Positive |
| 50 | M | Duodenal ulcer | HP65 | Positive |
| 44 | F | Normal | HP66 | Negative |
Detection of the cagA gene
The presence of the cagA gene was determined from eight of 18 different H pylori strains used in this study (table 1 ▶). Genomic DNA was extracted from microbial cultures or gastric biopsy specimens according to standard proteinase K digestion and phenol/chloroform/isoamilalcohol extraction methods. DNA was amplified by PCR using previously described specific oligonucleotide primers.15 DNA samples were subjected to a previous three minute denaturation step at 95°C followed by 30 cycles comprising one minute of denaturation at 95°C, one minute of annealing at 55°C, and one minute of elongation at 72°C. After 30 cycles, a final 10 minute elongation step at 72°C was performed. The PCR product was subjected to gel electrophoresis (1.5% agarose) and ethidium bromide staining, revealing a 400 bp band when the cagA gene was present.
Experiments and calculations
Warmed cells were resuspended in fresh Ringer's solution and counted (haematocytometer). Aliquots of 105 cells were then placed in 1.5 ml polypropylene test tubes containing different concentrations of H pylori and the appropriate agents, and incubated in a shaking water bath under an atmosphere of 95% O2–5% CO2 at 37°C for different periods of time. In general, cells from a single patient were sufficient for 9–11 aliquots (including duplicates). The experiments ended by centrifuging the tubes for 17 seconds at 14 000 g and pipetting off the supernatants. An aliquot of the supernatant was assayed for pepsinogen activity using a modified Anson-Mirsky method using acid denatured haemoglobin as the substrate, as previously described.16 Secretory responses to stimuli were calculated as output per unit time with basal output subtracted. Mean (SEM) basal rate of pepsinogen secretion in the experiments was 0.18% (0.007) of total pepsinogen content per minute.
In some experiments carried out to study the role of calcium in basal or stimulated pepsinogen secretion, cells were washed once in calcium free Ringer's solution, resuspended in the same solution containing 0.5 mM EGTA for three minutes to remove extracellular Ca++, washed again, and finally resuspended in calcium free Ringer's solution plus the drugs to be tested. When the nitric oxide synthase inhibitor l-NMMA was used, this was added 15 minutes before the other agents.17
For determination of cGMP, chief cells (aliquots of 105) were incubated alone or with 1 mM l-NMMA and then stimulated with H pylori or other agonists. After centrifugation at 14 000 g for 30 seconds, the supernatants were aspirated and the pellets were mixed with 0.5 ml iced 65% ethanol. cGMP was measured in 100 μl of supernatant by specific radioimmunoassay kit (Amersham Pharmacia Biotech, Barcelona, Spain).
Unless otherwise specified, pepsinogen secretion obtained after different stimuli was expressed as percentage of total cell pepsinogen content with basal secretion subtracted. Data are reported as mean (SEM) and the “n” in each experiment is equal to the number of separate cell preparations. The Student's t test for paired or unpaired data was used for statistical analysis and applied where appropriate.
RESULTS
Effects of H pylori on basal pepsinogen secretion from isolated human peptic cells
No differences were found in basal pepsinogen secretion from isolated cells in subjects with normal endoscopy or those with abnormal findings (see methods). Most patients were H pylori positive (46 of 68 in whom the status was determined) but no differences in basal pepsinogen secretion were found in experiments performed with cells obtained from H pylori positive and H pylori negative patients (basal secretion 5.5 (0.2)% v 5.8 (0.2)% of total pepsinogen content). Similarly, no differences were found in experiments performed with cells obtained from duodenal ulcer and non-duodenal ulcer patients (basal secretion 5.5 (0.3)% v 5.4 (0.3)% of total pepsinogen content).
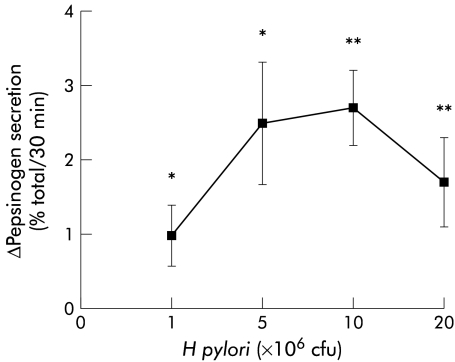
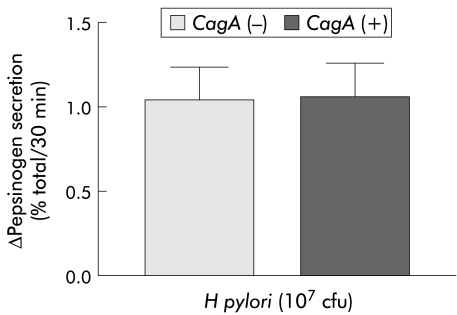
H pylori stimulated basal pepsinogen secretion from isolated peptic cells in a concentration dependent manner (fig 1 ▶). Maximal stimulation was achieved with 107 cfu (Δ secretion 2.7 (0.5)%). This was not observed with other Gram negative bacteria (E coli) at the same concentration (Δ secretion 0.2 (0.2)%). The presence of previous H pylori infection or duodenal ulcer did not influence the effect of the bacterium on pepsinogen secretion (Δ secretion 1.2 (0.1)% in H pylori positive v 1.3 (0.2)% in H pylori negative patients after H pylori 107 cfu (n=35 and 17, respectively) and Δ secretion 1.4 (0.2)% in duodenal ulcer v 1.5 (0.2)% in non duodenal ulcer (n=16 and 15, respectively)). Moreover, H pylori induced pepsinogen secretion was not affected by the absence/presence of the cagA gene because no differences were found with the different strains (fig 2 ▶).
Figure 1.
Effect of different concentrations of Helicobacter pylori on basal pepsinogen secretion from dispersed human peptic cells. H pylori stimulated basal secretion in a concentration dependent manner and maximal stimulation was achieved with 107 cfu (*p<0.05; **p<0.01). Results are presented as mean (SEM) of 4–8 separate experiments. Basal secretion=6.20 (0.29)%. The H pylori strains used in these experiments correspond to strains HP25, HP31, and HP32.
Figure 2.
Effect of Helicobacter pylori on basal pepsinogen secretion from dispersed human peptic cells according to the absence/presence of the cagA gene. Results are presented as mean (SEM) of 14 separate experiments. Basal secretion=5.2 (0.32)%. The H pylori strains used in these experiments correspond to strains HP33, HP52, HP59, HP66, HP61, HP62, HP63, and HP65.
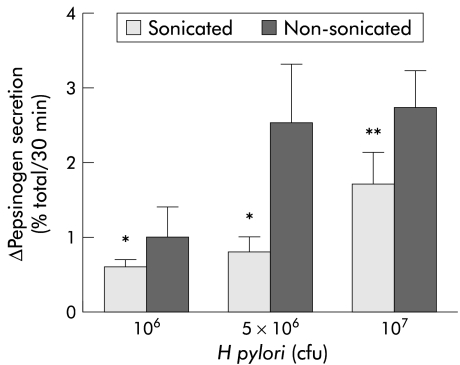
Bacterial sonicates also increased basal pepsinogen secretion but the stimulus was lower than that obtained with the whole bacteria at the same concentration (fig 3 ▶).
Figure 3.
Effect of different concentrations of Helicobacter pylori sonicates on basal pepsinogen secretion from dispersed human peptic cells. H pylori sonicates stimulated basal secretion in a concentration dependent manner but the stimulus was lower than that obtained with the whole bacterium at the same concentration (*p<0.05; **p<0.01). Results are presented as mean (SEM) of six separate experiments. Basal secretion=5.1 (0.20)%. The H pylori strains used in these experiments correspond to strains HP25, HP31, and HP32.
This effect was calcium dependent because removal of extracellular calcium inhibited the stimulating effect of H pylori on pepsinogen secretion (107 cfu=1.1 (0.5)% with v 0.1 (0.4)% without calcium).
Effects of H pylori on stimulated pepsinogen secretion
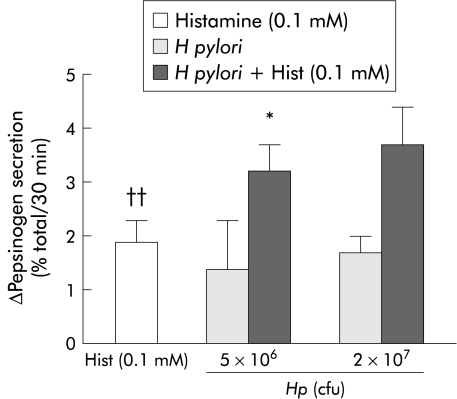
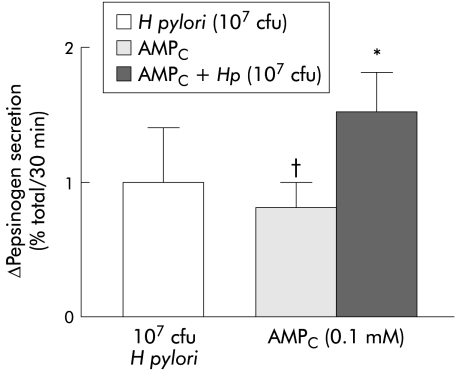
H pylori increased histamine and db-cAMP stimulated pepsinogen secretion. As previously shown,12,13 0.1 mM concentrations of histamine and db-cAMP significantly stimulated pepsinogen secretion from dispersed human peptic cells. When H pylori was combined with histamine, pepsinogen secretion was stimulated in an additive manner (fig 4 ▶). The addition of H pylori (107 cfu) further increased pepsinogen secretion in db-cAMP (0.1 mM) stimulated cells (fig 5 ▶).
Figure 4.
Helicobacter pylori at 5×106 cfu significantly increased (*p<0.05) histamine stimulated pepsinogen secretion from dispersed human peptic cells. The combination of this agent and H pylori produced an additive pepsinogen secretion response. Histamine stimulation was statistically significant (††p<0.01). Results are presented as mean (SEM) of four separate experiments. Basal secretion=5.4 (0.9)%. The H pylori strains used in these experiments correspond to strains HP25 and HP32.
Figure 5.
Helicobacter pylori increased dibutyryl-cyclic adenosine monophosphate (AMPC) stimulated pepsinogen secretion from dispersed human peptic cells. The combination of these agents potentiated pepsinogen secretion but did not have an additive effect (*p<0.05). The effect of AMPC on basal secretion was also significant (†p<0.05). Results are presented as mean (SEM) of four separate experiments. Basal secretion=5 (0.6)%. The H pylori strains used in these experiments correspond to strains HP25 and HP32.
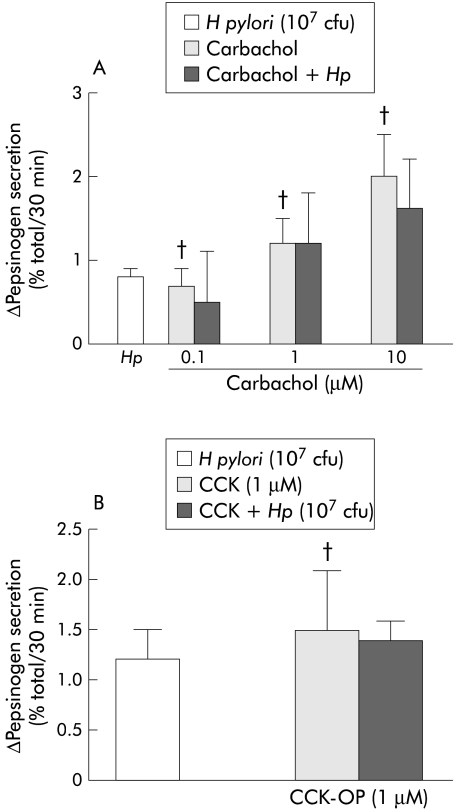
Carbachol dose dependently increased pepsinogen secretion from dispersed human peptic cells but H pylori (107 cfu) did not increase either carbachol (0.1–10 μM) or CCK (1 μM) stimulated pepsinogen secretion (fig 6 ▶).
Figure 6.
(A) Helicobacter pylori (107 cfu) did not increase carbachol (0.1–10 μM) stimulated pepsinogen secretion from dispersed human peptic cells. The effects of carbachol on basal pepsinogen secretion were statistically significant with respect to control experiments (†p<0.05). Results are presented as mean (SEM) of six separate experiments. Basal secretion=6.6 (0.6)%. The H pylori strains used in these experiments correspond to strains HP33, HP34, HP42, HP43, and HP46. (B) H pylori (107 cfu) did not affect cholecystokinin octapeptide (CCK-OP 1 μM) stimulated pepsinogen secretion. The effect of CCK-OP on basal secretion was also significant (†p<0.05). Results are presented as mean (SEM) of six separate experiments. Basal secretion=5.9 (0.2)%. The H pylori strains used in these experiments correspond to strains HP50, HP52, and HP58.
Role of nitric oxide on H pylori stimulated pepsinogen secretion
Stimulating chief cells with 0.1 mM 8Br-cGMP, a cell permeable cGMP analogue, increased basal pepsinogen release (Δ secretion 2.4 (0.2)%; n=4; p<0.05). This suggests that nitric oxide is involved in the control of pepsinogen secretion in human chief cells as cGMP is the final effector of nitric oxide in target cells.
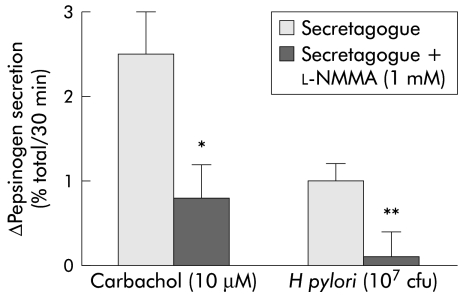
To study whether nitric oxide modulated the stimulatory effect of the bacterium on pepsinogen release, cells were preincubated alone or with 1 mM l-NMMA, an l-arginine derivative inhibitor of nitric oxide synthase, for 15 minutes and then stimulated with H pylori 107 cfu. Addition of the nitric oxide synthase inhibitor suppressed H pylori induced pepsinogen secretion (0.1 (0.3)% with v 1 (0.2)% without 1 mM l-NMMA) (fig 7 ▶). Furthermore, H pylori stimulation of peptic cells was associated with an increase in cGMP generation, which was also inhibited by cell preincubation with 1 mM l-NMMA (table 2 ▶).
Figure 7.
Cell preincubation with Nw-monomethyl-l-arginine (l-NMMA) (a nitric oxide synthase inhibitor) significatively inhibited both carbachol and Helicobacter pylori stimulated pepsinogen secretion from dispersed human peptic cells (*p<0.05; **p<0.01). Results are presented as mean (SEM) of 5–9 separate experiments. Basal secretion=6 (0.2)%. The H pylori strains used in these experiments correspond to strains HP25, HP31, HP32, HP33, and HP34.
Table 2.
Effect of Helicobacter pylori and other agents on cGMP generation by isolated chief cells
| Agent | cGMP (%) |
| Carbachol (10 μM) | 17.13% (0.3)* |
| H pylori (107 cfu) | 9.86% (0.2)* |
| Carbachol (10 μM)+l-NMMA (mM) | 4.28% (0.6) |
| H pylori (107 cfu)+l-NMMA (mM) | 1.48% (0.6) |
Results are expressed as per cent of increase over basal values. Basal concentrations of cGMP were 5.37 (0.5) fmol/106 cells (four experiments).
*p<0.05.
Carbachol, carbamylcholine;l-NMMA, Nw-monomethyl-l-arginine.
DISCUSSION
In this study, we showed that H pylori stimulated basal pepsinogen secretion from isolated human peptic cells in a concentration dependent manner. This stimulus was also observed with H pylori sonicates at the same concentration although the secretory response was lower than that achieved with the whole bacterium. The H pylori associated stimulating effect was independent of the presence of the cagA gene but dependent on the presence of extracellular calcium as cell incubation in a calcium free medium abolished increased pepsinogen secretion completely. The intracellular mechanisms involved in H pylori stimulated pepsinogen secretion were calcium and nitric oxide dependent as the H pylori increase in histamine and db-cAMP stimulated pepsinogen secretion was additive and was inhibited after preincubation with a nitric oxide inhibitor.
Maximal increase in basal pepsinogen secretion was obtained at a concentration of 107 cfu but the effect was observed at any concentration (106–2×107 cfu) using both the whole bacterium and its sonicated products. Nevertheless, the pepsinogen stimulus obtained with sonicated H pylori was 50% lower than that obtained with the whole bacterium. These data agree with those reported by Cave and Cave11 who found that H pylori sonicates increased pepsinogen secretion in isolated rabbit gastric glands and suggested that a bacterial factor was the causative agent of the stimulus. The lipopolysaccharide contained in the membrane of H pylori might be the responsible factor as Young and colleagues12 showed that purified H pylori lipopolysaccharide increased pepsinogen secretion by 50-fold while the E coli lipopolysaccharide raised this secretion by only 12-fold. However, the lower secretion obtained with H pylori sonicates in our study only suggests that bacterial membrane integrity plays a role in H pylori induced pepsinogen secretion. It is possible that in addition to lipopolysaccharide, proteins of the bacterial wall (for example, adhesins) could also be implicated, and the effect may be multifactorial. This however remains to be investigated. Another reason for the lower level of pepsinogen secretion found in our experiments may be the different experimental model used.12 In their studies, Young and colleagues12 used gastric mucosal fragments from the guinea pig which include several cell types. However, we used isolated human peptic cells, and it is possible that our data show only interspecies differences.13
It is well known that serum pepsinogen levels in patients with duodenal ulcer are higher than those observed in H pylori gastritis,8,18 and that cagA (+) strains are more prevalent in the former.19 This clinical information suggests a potential role for the cagA gene as the bacterial factor involved in the increase in pepsinogen secretion. However, we did not find differences in pepsinogen secretion using either cagA (+) H pylori or cagA (−) H pylori strains, suggesting that other factors must be involved. A recent study carried out in vitro on pig chief cell monolayer cultures20 showed that VacA and CagA proteins do not affect H pylori induced pepsinogen secretion. However, Chan and colleagues21 showed that pretreatment of human gastric adenocarcinoma cells with purified vacuolating toxin induced a concentration dependent release of pepsinogen. Differences between these studies can be explained by the use of either normal or immortalised cells whose phenotypic expression might be different. In the past it was indicated that hyperpepsinogenaemia could be determined by genetic factors.22
Another potential factor affecting pepsinogen secretion may be the characteristics of the patient whose cells were isolated to carry out the different experiments (for example, the presence of duodenal ulcer, H pylori status, etc). However, our data showed that these characteristics did not affect the interexperiment variability observed in basal secretion or pepsinogen secretion obtained after the H pylori stimulus.
With regard to the effect of the bacteria on stimulated peptic secretion, our data showed that H pylori increased histamine and db-cAMP stimulated pepsinogen secretion which supports the concept that H pylori stimulated pepsinogen secretion is mediated by calcium. When H pylori was combined with histamine, pepsinogen secretion was increased in an additive manner, indicating that the H pylori stimulus was mediated by a different intracellular mechanism. In the same way, simultaneous addition of H pylori and db-cAMP potentiated pepsinogen secretion but it did not have an additive effect. This may be because the rise in db-cAMP stimulated peptic secretion depends mainly on adenylyl cyclase activation but also on calcium dependent intracellular signals.20 Beil and colleagues20 showed that db-cAMP stimulated secretion decreased with H-8, a protein kinase A inhibitor, but also with lanthanum and W-7, a calmodulin antagonist, suggesting that pepsinogen secretion induced by db-cAMP was partially regulated by the calcium-calmodulin system.
In addition, when the effect of the bacterium on CCK-OP and carbachol stimulated pepsinogen secretion (both are agonist inducers of peptic secretion by activating the calcium intracellular system) was studied, no change in stimulated pepsinogen secretion was observed, suggesting that H pylori activates the same intracellular pathway as CCK and carbachol. On the other hand, no increase in pepsinogen secretion was found when cells and H pylori were incubated in a calcium free medium or after calcium chelation with EGTA.
Three studies have been reported which focus on the effect of H pylori on stimulated pepsinogen secretion with different agonists. In agreement with our results, Beil and colleagues20 showed that both db-cAMP and 12-O-tetradecanoylphorbol-13-acetate, a phorbol ester activator of protein kinase C, increased the stimulus of H pylori on pig chief cell monolayers. Moreover, secretion was inhibited with both lanthanum and W-7. Other authors23 have reported that H pylori sonicates significantly increased basal and histamine stimulated pepsinogen secretion from human chief glands. However, Cave and Cave11 found an additive effect with carbachol and CCK-OP. However, as noted above, it is important to note that Cave and Cave carried out their studies in rabbit glands.
Finally, our results showed that cell preincubation with l-NMMA (a nitric oxide synthase inhibitor) significantly inhibited the pepsinogen response induced by H pylori. These findings support the hypothesis that H pylori induced peptic secretion is calcium dependent and that nitric oxide acts as a second messenger in the pepsinogen secretion process induced by a Ca++ mediated agonist.17 To confirm these findings, we carried out a series of experiments where first we demonstrated that 8Br-cGMP, which is the final effector of nitric oxide, significantly increased basal pepsinogen release. In a second step, we also showed an increase in the cellular concentration of cGMP in H pylori stimulated peptic cells, which was inhibited by preincubation with l-NMMA. We tried to quantify nitric oxide generation by chief cells after carbachol and H pylori stimulation by colorimetric determination of critulline24 but unfortunately we were unable to detect this coproduct of nitric oxide which was probably due to the scarce cell mass available in our experiments which was much lower than that reported in other experiments with guinea pigs.17
In conclusion, our results showed that H pylori induced a direct stimulating effect on pepsinogen release from isolated human peptic cells by nitric oxide and calcium dependent mechanisms. From a pathogenic point of view, this effect may be of clinical relevance as in addition to other mechanisms,14,25–27 the in vitro effect of H pylori on pepsinogen secretion seen in our experiments was translated into an increase in intramucosal and/or intraluminal content of activated pepsin, which might eventually potentiate the mucosal damage associated with H pylori infection.
Acknowledgments
This study was supported by grant B162/98 from the Consejo Superior de Investigación y Desarrollo of the Aragon Government.
Abbreviations
cfu, colony forming units
db-cAMP, dibutyryl-cyclic adenosine monophosphate
carbachol, carbamylcholine
CCK-OP, cholecystokinin octapeptide
EGTA, ethylene glycol tetraacetic acid
l-NMMA
Nw-monomethyl-l-arginine
PCR, polymerase chain reaction
REFERENCES
- 1.Coghlan JG, Gilligan D, Humphries H, et al. Campylobacter pylori and recurrence of duodenal ulcers—a 12 month follow-up study. Lancet 1987;2:109–11. [DOI] [PubMed] [Google Scholar]
- 2.Rauws EA, Tytgat GN. Cure of duodenal ulcer associated with eradication of Helicobacter pylori.Lancet 1990;335:233–5. [DOI] [PubMed] [Google Scholar]
- 3.Marshall BJ, Goodwin CS, Warren JR, et al. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet 1988;2:437–42. [DOI] [PubMed] [Google Scholar]
- 4.Hentschel E, Brandstatter G, Dragosics B, et al. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. N Engl J Med 1993;328:308–12. [DOI] [PubMed] [Google Scholar]
- 5.Calam J, Gibbons A, Healey ZV, et al. How does Helicobacter pylori cause mucosal damage? Its effect on acid and gastrin physiology. Gastroenterology 1997;113(suppl 6):S43–9. [DOI] [PubMed] [Google Scholar]
- 6.Samloff IM, Liebman WM, Panitch NM. Serum group I pepsinogens by radioimmunoassay in control subjects and patients with peptic ulcer. Gastroenterology 1975;69:83–90. [PubMed] [Google Scholar]
- 7.Hunter FM, Correa P, Fontham E, et al. Serum pepsinogens as markers of response to therapy for Helicobacter pylori gastritis. Dig Dis Sci 1993;38:81–86. [DOI] [PubMed] [Google Scholar]
- 8.Wagner S, Haruma K, Gladziwa U, et al. Helicobacter pylori infection and serum pepsinogen A, pepsinogen C, and gastrin in gastritis and peptic ulcer: significance of inflammation and effect of bacterial eradication. Am J Gastroenterol 1994;89:211–18. [PubMed] [Google Scholar]
- 9.Kuipers EJ, Pals G, Pena AS, et al. Helicobacter pylori, pepsinogens and gastrin: relationship with age and development of atrophic gastritis. Eur J Gastroenterol Hepatol 1996;8:153–6. [DOI] [PubMed] [Google Scholar]
- 10.Chittajallu RS, Dorrian CA, Ardill JE, et al. Effect of Helicobacter pylori on serum pepsinogen I and plasma gastrin in duodenal ulcer patients. Scand J Gastroenterol 1992;27:20–4. [DOI] [PubMed] [Google Scholar]
- 11.Cave TR, Cave DR. Helicobacter pylori stimulates pepsin secretion from isolated rabbit gastric glands. Scand J Gastroenterol 1991;181:9–14. [DOI] [PubMed] [Google Scholar]
- 12.Young GO, Stemmet N, Lastovica A, et al. Helicobacter pylori lipopolysaccharide stimulates gastric mucosal pepsinogen secretion. Aliment Pharmacol Ther 1992;6:169–77. [DOI] [PubMed] [Google Scholar]
- 13.Lanas AI, Anderson JW, Uemura N, et al. Effects of cholinergic, histaminergic, and peptidergic stimulation on pepsinogen secretion by isolated human peptic cells. Scand J Gastroenterol 1994;29:678–83. [DOI] [PubMed] [Google Scholar]
- 14.Serrano MT, Lanas AI, Lorente S, et al. Cytokine effects on pepsinogen secretion from human peptic cells. Gut 1997;40:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lage AP, Godfroid E, Fauconnier A, et al. Diagnosis of Helicobacter pylori infection by PCR: comparison with other invasive techniques and detection of cagA gene in gastric biopsy specimens.J Clin Microbiol 1995;33:2.752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanas AI, Nerín J, Esteva F, et al. Non-steroidal anti-inflammatory drugs and prostaglandin effects on pepsinogen secretion by dispersed human peptic cells. Gut 1995;36:657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorucci S, Distrutti E, Chiorean M, et al. Nitric oxide modulates pepsinogen secretion induced by calcium-mediated agonist in guinea pig gastric chief cells. Gastroenterology 1995;109:214–23. [DOI] [PubMed] [Google Scholar]
- 18.Biasco G, Paganelli GM, Vaira D, et al. Serum pepsinogen I and II concentrations and IgG antibody to Helicobacter pylori in dyspeptic patients. J Clin Pathol 1993;46:826–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cover TL, Dooley CP, Blaser MJ. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun 1990;58:603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beil W, Wagner S, Piller M, et al. Stimulation of pepsinogen release from chief cells by Helicobacter pylori: evidence for a role of calcium and calmodulin. Microb Pathog 1998;25:181–7. [DOI] [PubMed] [Google Scholar]
- 21.Chan EC, Chen KT, Lin YL. Vacuolating toxin from Helicobacter pylori activates cellular signaling and pepsinogen secretion in human gastric adenocarcinoma cells. FEBS Lett 1996;399:127–30. [DOI] [PubMed] [Google Scholar]
- 22.Rotter JI, Sones JQ, Samloff IM, et al. Duodenal-ulcer disease associated with elevated serum pepsinogen I: an inherited autosomal dominant disorder. N Engl J Med 1979;300:63–6. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Hamida A, Man WK, Elgaddal AA, et al. The different effects of Helicobacter pylori strains on basal and histamine-stimulated acid and pepsin production: an in vitro study. Inflamm Res 1998;47(suppl 1):S54–5. [DOI] [PubMed] [Google Scholar]
- 24.Boyde TR, Rahmatullah M. Optimization of conditions for the colorimetric determination of citrulline, using diacetyl monoxime. Anal Biochem 1980;107:424–31. [DOI] [PubMed] [Google Scholar]
- 25.El-Omar EM, Penman ID, Ardill JE, et al. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology 1995;109:681–91. [DOI] [PubMed] [Google Scholar]
- 26.Fiorucci S, Distrutti E, Santucci L, et al. Leukotrienes stimulate pepsinogen secretion from guinea pig gastric chief cells by a nitric oxide-dependent pathway. Gastroenterology 1995;108:709–19. [DOI] [PubMed] [Google Scholar]
- 27.Fiorucci S, Lanfrancone L, Santucci L, et al. Epidermal growth factor modulates pepsinogen secretion in guinea pig gastric chief cells. Gastroenterology 1996;111:945–58. [DOI] [PubMed] [Google Scholar]