Abstract
Background and aims: Polypectomy in the colon has been shown to prevent colorectal cancer in both the general population and in familial colorectal cancer. Individuals with a family history of colorectal cancer have an increased risk of the disease. Over a period of 10 years, 304 subjects at risk were included in ongoing surveillance with regular colonoscopies. To compile the medical findings and experience generated during this period, a retrospective cross sectional study was performed.
Subjects: Subjects were classified into three family groups: families with hereditary non-polyposis colorectal cancer (HNPCC); families with hereditary colorectal cancer (HCC, non-Lynch syndrome); and a third group of families with only empirical risk estimates based on a family history of two close relatives (TCR) with colorectal cancer.
Methods: The risk population was studied with regard to age at onset, prevalence, number, cancer risk, size, dysplasia, and distribution of adenomas. A comparison was made within the family groups and with a reference group representing the general population.
Results: In total, 195 adenomas and six cancers were detected among 85 individuals. The relative risk of having an adenoma in the whole risk population compared with the general population was 2.6. Subjects from TCR families had most adenomas and HNPCC subjects had the least. A shift from proximal adenomas to distal carcinomas in families with HCC and TCR suggested a higher cancer risk in distal adenomas in these syndromes. HNPCC families showed a younger age at onset and adenomas with a higher degree of dysplasia. In HNPCC, there was a similar localisation of adenomas and carcinomas, suggesting a high risk of cancer in all adenomas.
Conclusions: There was clear overrepresentation of adenomas in all three family types compared with the reference population. In HNPCC, we found earlier onset of adenomas and faster progression to cancer. Families with HCC, and even more so TCR subjects, had a later onset and lower risk of cancer from proximal adenomas. Based on these results, surveillance protocols in Sweden have been revised.
Keywords: familial colorectal cancer, hereditary non-polyposis colorectal cancer, colonoscopy, surveillance, tumour progression
The lifetime risk of developing colorectal cancer is 5%, with an increased risk for individuals who have close relatives with colorectal cancer, especially if diagnosed at an early age.1 Depending on the family history and presence of an inherited mutation to colorectal cancer, the risk variability for colorectal cancer is up to 70%. Screening programmes, including colonoscopies in families with familial colorectal cancer as well as in the general population, reduce the incidence of colorectal cancer and seem to prevent mortality from colon cancer.2–4 Since 1990, families at risk have been counselled and invited to join a surveillance programme at the Karolinska Hospital with regular colonoscopy, and subjects with an increased risk of colorectal cancer of more than 10% have been offered regular colonoscopy every two years. The surveillance programme included the following types of risk syndromes: hereditary non-polyposis colorectal cancer (HNPCC); hereditary colorectal cancer (HCC); and individuals without a clear pattern of inheritance but with a family history of the disease. Patients with familial adenomatous polyposis were not included as they are usually treated with total colectomy.
HNPCC is an autosomal dominant syndrome predisposing to the development of colorectal cancer.5 It is caused by germline mutations in the DNA mismatch repair genes MSH2, MLH1, PMS1, PMS2, and MSH6.5 The syndrome is characterised by high penetrance, early onset, a more favourable prognosis than sporadic colorectal cancer, and right sided tumours.5 Tumours generally show microsatellite instability (MSI).6,7 The syndrome is also associated with a broad spectrum of extracolonic cancers, primarily in the endometrium, urinary tract, and small intestine.8 As the genes were cloned, more than 300 germline mutations in DNA mismatch repair genes were identified (ICG-HNPCC database).9
Families not fulfilling the Amsterdam criteria for HNPCC10 because of older age at onset but with a family history of three or more first degree relatives with colorectal cancer are likely to segregate unknown predisposing gene mutations, causing HCC, and they have a risk similar to families with Lynch syndrome.1,11 Apart from HNPCC and HCC, some individuals have an empiric increased risk of colorectal cancer because of a family history. Individuals with one first and one second degree or two first degree relatives (here termed two close relatives (TCR)) affected by colorectal cancer have a risk estimation of 10–20% based on empirical data.1,11 Finally, individuals with colorectal cancer at a very early age are likely to have a predisposition for the disease, and their children have an empiric increased cancer risk.1,11
A retrospective cross sectional study was performed to evaluate the surveillance programme in all subjects who had undergone regular colonoscopies for 1–10 years. An attempt was made to compare the prevalence, number, localisation, cancer risk, size, degree of dysplasia, and age at onset of adenomas between the risk groups and the general population. Sex differences were also studied.
MATERIALS AND METHODS
Subjects
Subjects included in this study were from the Cancer Family Clinic at Karolinska Hospital from 1990 to 1999. A medical history was given by the index patient and all diagnoses in the family were confirmed by medical records, pathological reports, or in very few cases death certificates. The general screening interval was every two years. After polypectomy of at least one adenoma, a new colonoscopy was performed the following year. Data from the colonoscopies were recorded anonymously in Stat View 5.0.1. Families were classified according to family type (HNPCC, HCC, or TCR), or one close relative (OCR) using information available from clinical records (table 1 ▶). The information used for classifying families included family history and, if available, data on MSI tests in tumours and mutation screening in mismatch repair genes in affected members. All individuals were divided into risk groups as follows. Tested carriers in HNPCC families (risk group 1) were considered to have a 70% lifetime risk.12 According to the rules applied to mendelian inheritance, untested first generation members at risk in a HNPCC family have a 35% lifetime risk (risk group 2). Their children who sometimes were under surveillance have a 17% lifetime risk (risk group 3). Subjects in HNPCC families who were under surveillance before testing but tested negative for mutation at the time of the study (risk group 4) were assumed to have the same risk (5%) as the normal population. Obvious gene carriers in families with HCC (risk group 5) have empiric risk values similar to HNPCC families (70%).1,11,13 First generation (risk group 6) family members accordingly have a lifetime risk of 35% and the second generation (risk group 7) at risk in these families has an estimated lifetime risk of 17%. TCR subjects with a family history of colorectal cancer (risk group 8) have an empirical risk of 10–20%.1,11,13 OCR subjects with one relative with early age of onset (risk group 9) have a lifetime empiric risk of 20–40%.1,11,13
Table 1.
Families included in the study
| Family type | Criteria | No of families | |
| HNPCC | 1 | Germline mutation in hMSH2, hMLH1, or hMSH6 | 25 |
| 2 | Amsterdam HNPCC+MSI pos+negative mutation screen | 13 | |
| 3 | Amsterdam HNPCC+MSI nd+mutation screen nd | 4 | |
| HCC | 1 | Non-Amsterdam hereditary CRC+MSI neg+negative mutation screen | 18 |
| 2 | Amsterdam HNPCC+MSI neg+negative mutation screen | 2 | |
| 3 | Non-Amsterdam hereditary CRC+MSI nd+mutation screen nd | 5 | |
| TCR | 1 | Two close relatives+MSI neg+negative mutation screen | 5 |
| 2 | Two close relatives+MSI nd+negative mutation screen | 5 | |
| 3 | Two close relatives+MSI nd+mutation screen nd | 29 | |
| OCR | 1 | One parent with colorectal cancer before 40 y of age | 5 |
| Total no families | 111 |
HNPCC, hereditary non-polyposis colorectal cancer; HCC, hereditary colorectal cancer; TCR, two close relatives with colorectal cancer; OCR, one close relative with colorectal cancer; CRC, colorectal cancer; MSI, microsatellite instability; nd, not done.
A reference group based on three published forensic autopsy studies was used for estimates of adenoma prevalence in the normal population.14–16 In reference group 1, 185 men and 118 women underwent forensic autopsy. Colonic biopsies were examined for prevalence, size, and degree of dysplasia of identified adenomas.14 Reference group 2 consisted of 198 men and 167 women who underwent autopsy/forensic autopsy. The study included prevalence, type, and location of adenomas.15 Reference group 3 comprised autopsies in 370 women and 310 men from areas with various incidences of colorectal cancer. Prevalence of adenomas, size, degree of dysplasia, and site of the adenomas were reported.16 In a comparison of prevalence in the study group versus the reference population, only individuals less than 54 years of age were included in the analysis. The reason was an uneven distribution of subjects in different age cohorts; in the reference group 75% of the cohort were older than 54 years while only 33% were over 54 years in the study group. Also, it was considered relevant to study the prevalence of adenomas at an early age as this is typical of a predisposition to cancer. Thus in the comparison between the study group and the general population, 338 subjects from the reference group and 204 from the study group were used.
Statistical methods
Differences in prevalence of adenomas between the three family types and the reference population were calculated as relative risks (RR). Differences between the family types were tested by χ2 test. Logistic regression was used to test the influence of sex with respect to the risk of developing an adenoma. The number of adenomas among individual subjects with adenomas were compared using the hazard ratio. Age at first adenoma was analysed by ANOVA with two independent factors, sex and family type. The Tukey post hoc test was then used for pairwise comparisons of family types and age at onset of adenomas. Differences between the family types regarding size and degree of dysplasia were compared using the χ2 test. Differences in localisation of adenomas and carcinomas in the bowel were analysed using χ2 statistics, and corresponding 95% confidence intervals for differences in proportions were calculated.
Genetic testing
MSI tests and genetic testing were performed either as part of previous studies or as part of clinical handling and counselling after 1997, and were not part of this study. The MSI test used established methods and criteria.17,18 Methods used for mutation screening were denaturant gradient gel electrophoresis, protein truncation test, Southern blot, and direct sequencing.19
RESULTS
In total, 304 subjects underwent 765 colonoscopies (table 2 ▶). Ten colorectal cancers were found in nine individuals before recruitment into the surveillance programme. These tumours have been included in table 2 ▶ to obtain correct values for prevalence and mean age at onset of adenomas or cancer in the different family types. History of previous adenomas was unknown, and most individuals had their first colonoscopy through this programme. Four individuals had colorectal cancer detected at their first colonoscopy, and two individuals developed a metachronous colorectal cancer during surveillance.
Table 2.
Follow up of regular colonoscopies as prevention in individuals with an increased risk of colorectal cancer
| HNPCC | HCC | Family history | ||||||||
| Risk group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 (TCR) | 9 (OCR) | Total |
| Estimated risk of cancer (%) | 70 | 35 | 17 | 5 | 70 | 35 | 17 | 15 | 30 | |
| No colonscopies | 151 | 119 | 15 | 50 | 10 | 220 | 52 | 133 | 15 | 765 |
| No patients (M/F) | 45 (20/25) | 48 (27/21) | 5 (0/5) | 23 (12/11) | 4 (1/3) | 83 (33/50) | 23 (11/12) | 64 (16/48) | 9 (3/6) | 304 (123/181) |
| Mean age (y) (M/F) | 45.7 (46/45) | 42.9 (40/47) | 25 (0/25) | 40.7 (41/41) | 70.5 (72/70) | 49.5 (49/50) | 41.8 (45/39) | 50 (42/53) | 37.9 (38/38) | 46.3 (45/48) |
| Total No adenomas | 26 | 13 | 0 | 3 | 5 | 61 | 6 | 81 | 0 | 195 |
| Total No carcinomas | 15 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 16 |
| Subjects with adenomas or carcinomas | 22 | 9 | 0 | 3 | 2 | 24 | 4 | 21 | 0 | 85 |
| Prevalence (%) (M/F) | 49 (55/44) | 19 (26/10) | 0 | 13 (25/0) | 50 (100/33) | 29 (39/22) | 17 (27/8) | 33 (25/35) | 0 | 28 (34/24) |
| Mean age at first adenoma (y) (M/F) | 43.6 (41/46) | 41.4 (38/53) | 0 | 40 (40/0) | 67.5 (65/70) | 51.6 (48/56) | 45 (46/42) | 51.1 (48/52) | 0 | 48 (44/52) |
1, gene carrier; 2, first generation at risk; 3, second generation at risk; 4, family member with a negative mutation test; 5, gene carrier; 6, first generation at risk; 7, second generation at risk; 8, individuals with two close relatives (TCR) with colorectal cancer; 9, one first degree relative (OCR) with colorectal cancer <40 years of age.
Prevalence and number of adenomas
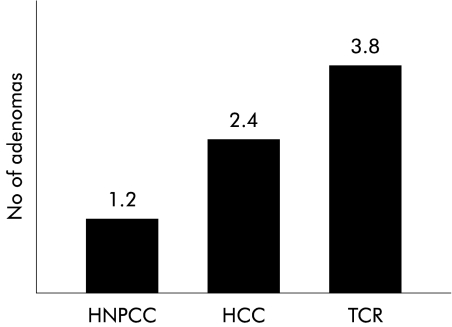
The RR value of developing an adenoma before the age of 54 years in all risk groups (except tested non-carriers) was 2.6 compared with the general population (p=0.001) (table 3 ▶). Sex had no influence on these results. RR was even higher (4.5) for tested gene carriers in HNPCC families and also statistically significantly higher for the other two family types (table 3 ▶). No difference was demonstrated (p>0.5) between the three family types for adenoma prevalence but a difference in the number of adenomas between family types was observed (fig 1 ▶). Individuals with adenomas from TCR families had more adenomas (3.8) than HCC subjects (2.4) and HNPCC families (1.2). The hazard ratio between TCR and HNPCC was 3.3 (CI 2.2–4.6) and between HCC and HNPCC 2.0 (CI 1.3–2.9). The hazard ratio between TCR and HCC was 1.6 (CI 1.1–2.18).
Table 3.
Relative risk (RR) of adenoma in the study group* compared with the reference group†
| No patients without adenoma | No patients with adenoma | Total | |||
| Reference group lifetime cancer risk 5% | |||||
| Women | 110 | 12 | 122 | ||
| Men | 191 | 25 | 216 | ||
| Total | 301 | 37 | 338 | ||
| Whole study group lifetime cancer risk 10–70% | |||||
| Women | 85 | 29 | 114 | RR 2.6 | |
| Men | 60 | 30 | 90 | RR 2.8 | |
| Total | 145 | 59 | 204 | RR 2.6 | p<0.001 |
| HNPCC gene carriers lifetime risk 70% | |||||
| Women | 12 | 9 | 21 | RR 4.4 | |
| Men | 8 | 10 | 18 | RR 4.8 | |
| Total | 20 | 19 | 39 | RR 4.5 | p<0.001 |
| HCC lifetime cancer risk 35% | |||||
| Women | 23 | 6 | 29 | RR 2.1 | |
| Men | 13 | 8 | 21 | RR 3.3 | |
| Total | 36 | 14 | 50 | RR 2.6 | p<0.001 |
| TCR lifetime cancer risk 15% | |||||
| Women | 15 | 11 | 26 | RR 4.3 | |
| Men | 10 | 2 | 12 | RR 1.4 | |
| Total | 25 | 13 | 38 | RR 3.1 | p<0.001 |
*Study group was less than 54 years or age at first adenoma was below 54 years; †reference group was less than 54 years of age.
HNPCC, hereditary non-polyposis colorectal cancer; HCC, hereditary colorectal cancer; TCR, two close relatives with colorectal cancer.
Figure 1.
Mean number of adenomas among individuals presenting with adenomas in the three family types (HNPCC, hereditary non-polyposis colorectal cancer; HCC, hereditary colorectal cancer; TCR, two close relatives with colorectal cancer).
Cancer risk in adenomas
The prevalence in each risk group varied in relation to the estimated cancer risk in the various risk groups in HNPCC and HCC (table 2 ▶). The high prevalence of adenomas in the relatively low risk TCR families was unexpected. To obtain a relative value of cancer risk which could be used for comparisons between different risk groups, prevalence was related to the estimated cancer risk (table 4 ▶). The risk values (prevalence/estimated cancer risk) were highest in HNPCC, and lowest in TCR and the normal population. To obtain a relative cancer risk in each adenoma, the risk values were divided by the number of adenomas per individual (from fig 1 ▶) (table 4 ▶). Mean age of the families was similar (table 2 ▶), and the risk of cancer was the same when only subjects less than 54 years of age were compared (data not shown).
Table 4.
Comparison of cancer risks between different syndromes
| Cancer risk (risk/prevalence) | 95% CI | Cancer risk per adenoma | 95% CI | |
| HNPCC, subjects at 70% risk* | 1.43 (70/49) | 1.09–2.06 | 1.19 (1.43/1.2) | 0.91–1.72 |
| HNPCC, subjects at 35% risk* | 1.84 (35/19) | 1.16–4.30 | 1.53 (1.84/1.2) | 0.97–3.59 |
| HNPCC, subjects at 17% risk | — | — | — | — |
| HNPCC, subjects at 5% risk | 0.38 (5/13) | — | 0.32 (0.38/1.2) | — |
| HCC, subjects at 70% risk | 1.40 (70/50) | — | 0.58 (1.4/2.4) | — |
| HCC, subjects at 35% risk* | 1.21 (35/29) | 0.92–1.75 | 0.50 (1.21/2.4) | 0.38–0.73 |
| HCC, subjects at 17% risk | 1.00 (17/17) | 0.55–8.75 | 0.42 (1.00/2.4) | 0.23–3.65 |
| TCR, subjects at 15% risk* | 0.45 (15/33) | 0.39–0.80 | 0.12 (0.45/3.8) | 0.10–0.21 |
| Normal population at 5% risk | 0.45 (5/11) | — | 0.23 (0.45/2.0) | — |
*Statistically significant.
95% CI, 95% confidence interval.
HNPCC, hereditary non-polyposis colorectal cancer; HCC, hereditary colorectal cancer; TCR, two close relatives with colorectal cancer.
Localisation of adenomas and carcinomas
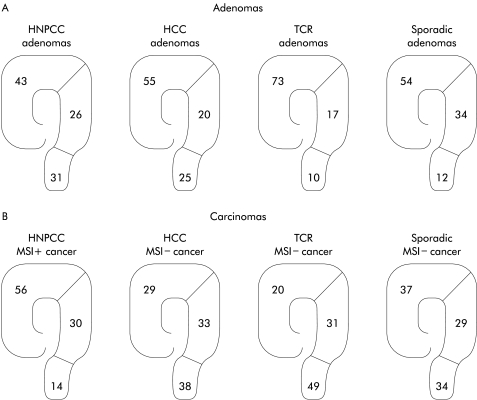
Adenomas were located throughout the colon and rectum, as depicted in fig 2 ▶. While adenomas were evenly distributed in HNPCC, HCC, and in the general population, TCR adenomas seemed to be mostly proximal (fig 2 ▶).
Figure 2.
Percentage distribution of adenomas in the three familial types (HNPCC, hereditary non-polyposis colorectal cancer; HCC, hereditary colorectal cancer; TCR, two close relatives with colorectal cancer) and in the reference population (A). Percentage distribution of carcinomas in the same family types and in the general population (B).
To determine if there was a difference in cancer risk depending on location, a comparison was made between adenoma location and carcinoma location in the different family types (fig 2 ▶). Localisation of cancers among the total number of family relatives was obtained from investigation of all 111 families in the study. In total, there were 81 HNPCC cancers, 52 HCC cancers, and 51 TCR cancers. To determine the localisation of sporadic cancer in the general population, three published reports20–22 were used (fig 2 ▶). There was a clear difference in percentage of distal outcome between adenomas and carcinomas in families with TCR (p<0.001) but also in HCC (p<0.05) and the normal population (p<0.001) (fig 2 ▶).
Mean age at first observed adenoma
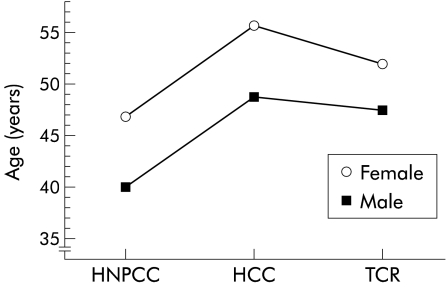
Mean age at identification of the first adenoma was 43 years in HNPCC, 50 years in HCC, and 52 years in TCR (fig 3 ▶). There was a statistically significant difference between age at first adenoma in HNPCC and TCR (p=0.006). The difference between HNPCC and HCC was as expected (p=0.02) as this was defined by the inclusion criteria.
Figure 3.
Mean age at onset of adenoma or carcinoma in men and women in the three family types (HNPCC, hereditary non-polyposis colorectal cancer; HCC, hereditary colorectal cancer; TCR, two close relatives with colorectal cancer).
Sex and age
Overall, there was a difference (p<0.05) between the prevalence of adenomas in men (34%) and women (24%) (tables 2, 3 ▶ ▶). Men and women showed a systematic difference in mean age at first adenoma (fig 3 ▶). There was no interaction effects; the sex difference was assumed to be constant over family type and the difference between family types was assumed to be constant over sex.
Histopathology
In total, 152 tubular adenomas, 18 tubulovillous adenomas, two villous adenomas, 18 serrated adenomas, and five unclassified adenomas were removed. The vast majority (88%) were less than 5 mm. In four of the adenomas estimation of size was not done. Size and degree of dysplasia in adenomas from the second or later colonoscopies were compared. Thirteen adenomas were >5 mm in size but only three showed a high degree of dysplasia (table 5 ▶). In total, eight subjects had 11 adenomas with high dysplasia, and six of those eight subjects were from HNPCC families (table 6 ▶). There was a statistically significant difference between the number of adenomas with high degree dysplasia in the HNPCC compared with the other two family types (χ2=6.7, p<0.01).
Table 5.
Adenomas greater than 5 mm from the second and subsequent colonoscopies
| Family | Patient | Family type | Age (y) | Screen | Size (mm) | Histology | Dysplasia | Location |
| 69 | 77 | HNPCC | 35 | 3rd | 10 | TA | Low | A |
| 5 | 141 | HNPCC | 34 | 6th | 9 | TA | High | D |
| 87 | 248 | HCC | 68 | 4th | 10 | TA | Low | DC |
| 87 | 248 | HCC | 69 | 5th | 10 | TA | Low | T |
| 87 | 247 | HCC | 44 | 3rd | 7 | VA | Low | A |
| 134 | 90 | HCC | 46 | 2nd | 6 | TA | Low | R |
| 24 | 179 | HCC | 70 | 2nd | 15 | TA | High | D |
| 26 | 204 | HCC | 75 | 3rd | 10 | TVA | Low | R |
| 26 | 204 | HCC | 76 | 4th | 10 | TVA | Low | R |
| 26 | 208 | HCC | 42 | 3rd | 7 | TA | High | R |
| 23 | 30 | TCR | 40 | 4th | 6 | TA | Low | A |
| 23 | 30 | TCR | 41 | 5th | 6 | TA | Low | A |
| 123 | 331 | TCR | 59 | 2nd | 10 | TA | Low | T |
HNPCC, hereditary non-polyposis colorectal cancer; HCC, hereditary colorectal cancer; TCR, two close relatives with colorectal cancer; TA, tubular adenoma; TVA, tubulovillous adenoma; VA, villous adenoma; A, ascending colon; T, transverse colon; D, descending colon; R, rectum.
Table 6.
Adenomas with moderate and high dysplasia from second and subsequent colonoscopies
| Family | Patient | Family type | Age (y) | Screen | Size (mm) | Histology | Dysplasia | Location |
| 3 | 135 | HNPCC | 53 | 3rd | 5 | TVA | High | D |
| 3 | 138 | HNPCC | 58 | 5th | Unknown | TVA | High | A |
| 3 | 138 | HNPCC | 58 | 6th | 24 | Carcinoma Dukes' A | A | |
| 5 | 139 | HNPCC | 57 | 2nd | Unknown | Adenoma unclassified | High | D |
| 5 | 141 | HNPCC | 34 | 6th | 9 | TA | High | D |
| 28 | 299 | HNPCC | 40 | 2nd | Unknown | Carcinoma Dukes' A | R | |
| 183 | 326 | HNPCC | 36 | 3rd | Unknown | TA | High | R |
| 24 | 179 | HCC | 70 | 2nd | 15 | TA | High | D |
| 24 | 179 | HCC | 71 | 3rd | Unknown | TA | High | A |
| 100 | 265 | TCR | 69 | 3rd | 3 | TVA | High | A |
| 100 | 265 | TCR | 69 | 3rd | 1 | TVA | High | D |
HNPCC, hereditary non-polyposis colorectal cancer; HCC, hereditary colorectal cancer; TCR, two close relatives with colorectal cancer; TA, tubular adenoma; TVA, tubulovillous adenoma; VA, villous adenoma; A, ascending colon; T, transverse colon; D, descending colon; R, rectum.
DISCUSSION
In the following discussion of the results, we will use the RR of adenomas as an estimate of tumour initiation rate in individuals. When the actual cancer risk in an individual is higher than the increase in initiation rate, this is considered to depend on an increased tumour progression rate in adenomas.
A higher initiation rate in all risk groups
There is much discussion as to whether the prevalence and frequency of adenomas in individuals with an increased risk of colorectal cancer are higher than in the general population.23–26 In this study, gene carriers in HNPCC had an RR of 4.5 compared with the general population of presenting with an adenoma before the age of 54 years. All risk groups combined had an RR of 2.6. The increased risk of adenomas in all groups compared with the general population indicates that in all families there is an increased initiation rate that explains, at least in part, the increased risk of colorectal cancer. The different numbers of adenomas in the family types suggests that the initiation rate is increased most in TCR families and increased least in HNPCC.
Autopsy studies to assess the number of adenomas in the general population are not optimal. However, the autopsy studies chosen for this study were specifically designed to give adenoma values representing the normal population. Furthermore, some authors found autopsies to be more reliable than colonoscopies in detecting adenomas <10 mm.27 Other studies have shown a missrate of 15–27% for detecting adenomas <5 mm using colonoscopies.28,29 However, as most of the study objects were included in a surveillance programme, a minute adenoma would show up at the next screening if missed at the previous one, thus giving correct values for prevalence in the study group.
A higher rate of progression in the high risk syndromes
The excess in cancer risk in adenomas in HNPCC and HCC compared with TCR and the general population in this study suggested that apart from increased initiation there is also an increased rate in tumour progression. As the genes for HCC are still unknown, we estimated the penetrance as equal to that of HNPCC. If this is an overestimation, the cancer risk in each adenoma in HCC is also overestimated and could be even lower. In particular, HNPCC subjects have an up to eightfold greater cancer risk in each adenoma compared with TCR subjects and the normal population (table 4 ▶). This fits well with an increased mutation rate in tumours because of deficient mismatch repair in this syndrome. The shift from proximal adenomas to distal carcinomas among the latter two groups and also in the general population suggests that there is a difference in cancer risk in adenomas depending on the location in the colorectum (fig 2 ▶). Thus distal adenomas seem to be associated with a much higher risk of cancer in all patients except HNPCC where the risk of cancer seems equally high in proximal and distal adenomas.
Differences in mean age between syndromes
Mean age at first observed adenoma was 44 years in HNPCC which is in accordance with previous studies (fig 1 ▶). The higher penetrance for men in HNPCC found in this study (table 3 ▶) confirmed previous reports.30 A higher penetrance for men was also suggested in HNPCC and HCC in this study, while TCR subjects were not informative in this respect depending on different mean ages among men and women.
In summary, there was a clear overrepresentation of adenomas in all three family types. This seems to justify regular colonoscopy surveillance for prevention in these patients. The data support a proposed model (table 7 ▶) with increased rates of both initiation and tumour progression in HNPCC. In HNPCC it appears that there is a rapid transformation from adenoma to carcinoma as there was a higher grade of dysplasia in HNPCC adenomas unrelated to size. HNPCC also displayed the highest risk of cancer in each adenoma, regardless of location (table 4 ▶). In HCC families, an increased initiation rate as well as an increased progression rate were also found although the cancer risk in each adenoma was lower in proximal adenomas and the progression from adenoma to carcinoma is likely to be slower than in HNPCC. TCR families seem to have the highest increase in initiation rate as they had the highest number of adenomas. In TCR there seems to be a low cancer risk in proximal adenomas and a relatively higher cancer risk in distal adenomas. The differences between the three family types (HNPCC, HCC, and TCR) with regard to age at onset, prevalence, location, size and degree of dysplasia, and cancer risk of adenomas have been used to revise surveillance protocols in Sweden. The guidelines from the Swedish National Oncogenetic Counsil now recommend the following: in HNPCC, regular colonoscopy every 1–2 years; in HCC, regular colonoscopy every 3–5 years; and in TCR, colonoscopy (or alternating colonoscopy/sigmoidoscopy) every 3–5 years.
Table 7.
Modelling of tumour initiation rate and tumour progression rate in the three family types (HNPCC, HCC, TCR) in this study compared with sporadic colorectal cancer where no increased initiation and progression are known, and a fourth syndrome, familial adenomatosis polyposis, where an increased initiation rate is well documented for adenomas
| Family type | Tumour initiation | Tumor progression |
| Sporadic colorectal cancer | 0 | 0 |
| HNPCC | + | +++++ |
| HCC | ++ | +++ |
| TCR | +++ | +? (distal adenomas) |
| FAP | +++++ | 0 |
HNPCC, hereditary non-polyposis colorectal cancer; HCC, hereditary colorectal cancer; TCR, two close relatives with colorectal cancer; FAP, familial adenomatosis polyposis.
0, no increased rate; + (1–5), various degrees of increased rates. This estimation is approximate and only chosen to present the idea of different degrees of increased tumour initiation and tumour promotion rates characterising different families with an increased risk of colorectal cancer because of an inherited predisposition.
Acknowledgments
We are indebted to Marianne Törnblom for excellent management of the patient registry and Jan Kowalski for statistical analysis. The Swedish Cancer Society, the Stockholm County Council, and the Cancer Foundation in Stockholm supported the study.
Abbreviations
HNPCC, hereditary non-polyposis colorectal cancer
HCC, hereditary colorectal cancer
TCR, two close relatives with colorectal cancer
OCR, one close relative with colorectal cancer
MSI, microsatellite instability
RR, relative risk
REFERENCES
- 1.Fuchs CS, Giovanni EL, Colditz GA, et al. Family history increases the risk of colorectal cancer. Gastroenterology 1995;109:1015–19. [DOI] [PubMed] [Google Scholar]
- 2.Järvinen HJ, Mecklin J-P, Sistonen P. Screening reduces colorectal cancer rate in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 1995;108:1405–11. [DOI] [PubMed] [Google Scholar]
- 3.Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in hereditary nonpolyposis colorectal cancer. Gastroenterolgy 2000;118:829–34. [DOI] [PubMed] [Google Scholar]
- 4.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977–81. [DOI] [PubMed] [Google Scholar]
- 5.Lynch HT, de la Chapelle A. Genetic susceptibility to non-polyposis colorectal cancer. J Med Genet 1999;36:801–18. [PMC free article] [PubMed] [Google Scholar]
- 6.Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993;260:812–16. [DOI] [PubMed] [Google Scholar]
- 7.Tannergård P, Liu T, Weger A, et al. Tumorigenesis in colorectal tumors from patients with hereditary non-polyposis colorectal cancer. Hum Genet 1997; 101:56–60. [DOI] [PubMed] [Google Scholar]
- 8.Watson P, Lynch HT. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer 1993;71:677–85. [DOI] [PubMed] [Google Scholar]
- 9.ICG-HNPCC database: http://www.nfdht.nl/database/mdbchoice.htm.
- 10.Vasen HFA, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999;116:1453–6. [DOI] [PubMed] [Google Scholar]
- 11.Lovett E. Family studies in cancer of the colon and rectum. Br J Surg 1976;63:13–18. [DOI] [PubMed] [Google Scholar]
- 12.Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 1999;81:214–18. [DOI] [PubMed] [Google Scholar]
- 13.St John DJB, McDermott T, Hopper JL, et al. Cancer risk in relatives of patients with common colorectal cancer. Ann Intern Med 1993;118:785–90. [DOI] [PubMed] [Google Scholar]
- 14.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut 1992;33:1508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams AR, Balasooriya BAW, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut 1982;23:835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark JC, Collan Y, Eide TJ, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer 1985;36:179–86. [DOI] [PubMed] [Google Scholar]
- 17.Liu T, Wahlberg S, Burek E, et al. Microsatellite instability as a predictor of a mutation in a DNA mismatch repair gene in familial colorectal cancer. Genes Chrom Cancer 2000;1:17–25. [DOI] [PubMed] [Google Scholar]
- 18.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for Cancer Detection and Familial Predisposition: Development of International Criteria for the Determination of Microsatellite Instability in Colorectal Cancer: Cancer Res 1998;58:5248–57. [PubMed] [Google Scholar]
- 19.Wahlberg S, Liu T, Lindblom P, et al. Different mutations screening techniques in the DNA mismatch repair genes hMSH2 and hMLH1. Genet Test 1999;3:259–64. [DOI] [PubMed] [Google Scholar]
- 20.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science 1993;260:816–19. [DOI] [PubMed] [Google Scholar]
- 21.Breivik J, Lothe RA, Meiling GI, et al. Different genetic pathways to proximal and distal colorectal cancer influenced by sex-related factors. Int J Cancer 1997;74:664–9. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994;145:148–56. [PMC free article] [PubMed] [Google Scholar]
- 23.Lanspa JS, Lynch HT, Smyrk TC, et al. Colorectal adenomas in the Lynch syndromes. Results of a colonoscopy screening program. Gastroenterology 1990:98:1117–22. [DOI] [PubMed] [Google Scholar]
- 24.Jass JR, Stewart SM. Evolution of hereditary non-polyposis colorectal cancer. Gut 1992;33:783–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponz de Leon M, Della Casa G, et al. Frequency and type of colorectal tumors in asymptomatic high-risk individuals in families with hereditary nonpolyposis colorectal cancer. Cancer Epidemiol Biom Prev 1998;7:639–41. [PubMed] [Google Scholar]
- 26.Jass JR, Stewart SM, Stewart J, et al. Hereditary non-polyposis colorectal cancer—morphologies, genes and mutations. Mutat Res 1994;310:125–33. [DOI] [PubMed] [Google Scholar]
- 27.Byrd RL, Boggs HW Jr, Slagle GW, et al. Reliability of colonoscopy. Dis Colon Rectum 1989;32:1023–5. [DOI] [PubMed] [Google Scholar]
- 28.Hixon LJ, Fennerty MB, Sampliner RE, et al. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. J Natl Cancer Inst 1990;82:1769–72. [DOI] [PubMed] [Google Scholar]
- 29.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determinded by back-to-back colonoscopies. Gastroenterology 1997;112:24–8. [DOI] [PubMed] [Google Scholar]
- 30.Dunlop MG, Farrington SM, Carothers AD, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet 1997;6:105–10. [DOI] [PubMed] [Google Scholar]