Abstract
Background: Interleukin 10 (IL-10) exerts anti-inflammatory actions by counteracting many biological effects of interferon γ (IFN-γ).
Aims: To investigate this in humans, we studied the effects of human recombinant IL-10 administration on IFN-γ production by patient leucocytes. Furthermore, we assessed the IFN-γ inducible molecule neopterin and nitrite/nitrate serum levels, which are indicative of endogenous nitric oxide formation.
Methods: As part of two placebo controlled double blind studies, we analysed patients with chronic active Crohn's disease (CACD) who received either subcutaneous recombinant human IL-10 (n=44) or placebo (n=10) daily for 28 days, and patients with mild to moderate Crohn's disease (MCD) treated with either subcutaneous IL-10 (n=52) or placebo (n=16) daily for 28 days. Neopterin and nitrite/nitrate concentrations were measured in serum, and ex vivo IFN-γ formation by lipopolysaccharide or phytohaemagglutinin (PHA) stimulated whole blood cells were investigated before, during, and after IL-10 therapy.
Results: In patients with CACD, the highest dose of 20 μg/kg IL-10 caused a significant increase in serum neopterin on days +15 and +29 of therapy compared with pretreatment levels. No changes were observed for nitrite/nitrate levels under either condition. In MCD, treatment with 20 μg/kg IL-10 resulted in a significant increase in PHA induced IFN-γ production.
Conclusions: High doses of IL-10 upregulate the production of IFN-γ and neopterin. This phenomenon may be responsible for the lack of efficacy of high doses of IL-10 in the treatment of CACD and MCD.
Keywords: inflammatory bowel disease, inflammation, interleukin 10, neopterin, interferon γ
Interleukin (IL)-10 is secreted by several cell populations including T helper cells (Th), monocytes/macrophages, dendritic cells, B cells, and keratinocytes.1 This cytokine suppresses inflammation by various mechanisms, including reduction of HLA class II expression, decreasing T cell secretion of IL-2, and diminishing the production of IL-1α, IL-1β, tumour necrosis factor α (TNF-α), and IL-8 by activated monocytes/macrophages.1 Gene targeted IL-10 deficient mice develop transmural inflammation of the small and large bowel, reminiscent of Crohn's disease (CD).2 This type of inflammation is aggravated by the presence of bacteria within the gut lumen, and can be prevented by administration of IL-10. Administration of IL-10 ameliorates inflammation in several other animal models not only limited to the gut.1 These data together suggested that IL-10 is a promising cytokine for treatment of inflammatory diseases. However, the clinical efficacy of recombinant human IL-10 (rHuIL-10) in the treatment of CD has been disappointing.3,4 In both animal models and endotoxin challenged volunteers, administration of recombinant IL-10 caused a significant reduction in TNF-α production.1 However, we have reported that in contrast with results obtained in various animal models, administration of rHuIL-10 in endotoxin challenged volunteers caused an increase in the production of interferon γ (IFN-γ).5
Circulating concentrations of IFN-γ and neopterin are increased in patients with active CD, and correlate with clinical disease activity, as measured by the CD activity index (CDAI).6 Neopterin, a pyrazino-pyrimidino derivate, is mainly produced by monocytes/macrophages under the control of IFN-γ, and thus is a valuable in vitro and in vivo marker for monitoring cell mediated immune function and IFN-γ activity.6,7 In addition, IFN-γ, in combination with proinflammatory cytokines such as TNF-α and IL-1β, stimulates synthesis of inducible nitric oxide synthase (iNOS), thereby increasing nitric oxide (NO) production.8
The present studies were designed to assess the impact of IL-10 on monocyte and T lymphocyte activity by (i) investigating serum levels of neopterin and nitrite/nitrate, the stable end products of NO in serum,8,9 in patients with chronic active steroid unresponsive CD (CACD) and by (ii) studying ex vivo production of TNF-α and IFN-γ in patients with mild to moderately active CD (MCD). The analysed patients were part of two large, double blind, randomised, multicentre collaborative trials studying the therapeutic effects of IL-10 in CACD and MCD.3,4
MATERIALS AND METHODS
Patients
Two multicentre, randomised, double blind, placebo controlled studies were conducted in patients with CACD and MCD (for details see Fedorak and colleagues3 and Schreiber and colleagues4). In the CACD trial, patients were included if they had active steroid resistant CD involving the colon or both the ileum and colon, with or without external fistula. Active disease was defined as a CDAI of 200–400 despite treatment with prednisone (10–40 mg/day for at least three months) given alone or in combination with 6-mercaptopurine or azathioprine prior to the study. These therapies had to be continued during the study. Patients were allowed to take aminosalicylates and/or antibiotics, providing that the dosages were kept stable during the study. Patients with CACD were randomly assigned to receive one of four doses (1, 4, 8, or 20 μ/kg body weight) of IL-10 (Schering Plough, Kenilworth, New Jersey, USA) or placebo once daily subcutaneously for 28 consecutive days. A total of 329 patients were included in the study. After the 28 day treatment period patients were followed up for four weeks.
In another study of similar design, patients with MCD were enrolled. Patients were eligible if CDAI was 200–350, and the disease involved the ileum and/or colon. Concurrent or recent treatment with corticosteroids (last dose 30 days) or immunosuppressives (up to 90 days) was not allowed. Systemic aminosalicylates and its topical use had to be discontinued prior to initiation of the study medication for at least 24 hours or 14 days, respectively. In this study, one of four doses (1, 5, 10, or 20 μg/kg body weight) of IL-10 or placebo was administered once daily subcutaneously for 28 consecutive days. A total of 95 patients were treated (IL-10, n=72; placebo, n=23) with a follow up of five months.
Measurement of neopterin and nitrite/nitrate in CACD patients
The analysed patients were part of the international collaborative trial described above. Serum levels of neopterin and nitrite/nitrate were analysed in 10 patients treated with placebo, 12 patients treated with 1 μg/kg, 12 patients treated with 4 μg/kg, 10 patients treated with 8 μg/kg, and 10 patients treated with 20 μg/kg body weight IL-10 subcutaneously. Serum levels of neopterin and nitrite/nitrate were determined on day −1, day +15, and day +29 of therapy and after four weeks of follow up. Neopterin levels were assessed by a specific ELISA (Brahms, Berlin, Germany). The detection limit for neopterin was 3 nmol/l and normal values are below 8 nmol/l.6 For determination of nitrite/nitrate levels, samples were deproteinised by sulphosalicylic acid and neutralised with NaOH. After enzymatic reduction of nitrate to nitrite using nitrate reductase (Sigma, Munich, Germany), total nitrite concentration was determined spectrophotometrically after addition of the Griess Ilosvay's reagent (Merck, Darmstadt, Germany).10 Sodium nitrite served as standard. The detection limit for nitrite/nitrate was 1 μmol/l.
Assessment of whole blood cell synthesis of IFN-γ and TNF-α in MCD patients
A total of 68 patients who all completed the trial were studied (placebo, n=16; 1 μg/kg, n=15; 5 μg/kg, n=12; 10 μg/kg, n=12; and 20 μg/kg, n=13). Venous blood was aseptically collected in endotoxin free heparinised tubes (final heparin concentration 10 U/ml whole blood) and immediately aliquots of 2.5 ml of blood were mixed with 25 μl phytohaemagglutinin (PHA) (final concentration 5 μg/ml; Murex Diagnostics Ltd, Dartford, UK) or with 25 μl of lipopolysaccharide (LPS) (final concentration 10 ng/ml; E coli type 055-B5). As a negative control, 2.5 ml of blood were added to 25 μl polymyxin B (1 mg/ml endotoxin free buffered saline). All samples were incubated for 24 hours at 37°C. After incubation, samples were centrifuged at 1000 g for 30 minutes at 4°C and platelet poor plasma was stored at −70° until analysis. TNF-α and IFN-γ levels were assessed by specific ELISAs (CLB, Amsterdam, the Netherlands). The detection limit for TNF-α was 1 pg/ml and for IFN-γ 7.4 pg/ml; normal values (heparinised plasma) are below 5 pg/ml and 10 pg/ml, respectively. TNF-α and IFN-γ were determined prior to administration of IL-10 on day +1, on day +8 (only TNF-α levels assessed), and 24 hours after the last dose of study medication on day +29.
Statistics
Statistical analysis was performed using the non-parametric comparison according to Kruskal-Wallis and Friedman. ANOVA analysis and the Friedman test were used for comparison within groups for the CACD and MCD studies, respectively. The Wilcoxon signed rank test was used for comparison within groups. Correlation analysis between neopterin/cytokine values and clinical response was performed by applying the Pearson correlation test. Data are presented as mean (SEM).
RESULTS
CACD study
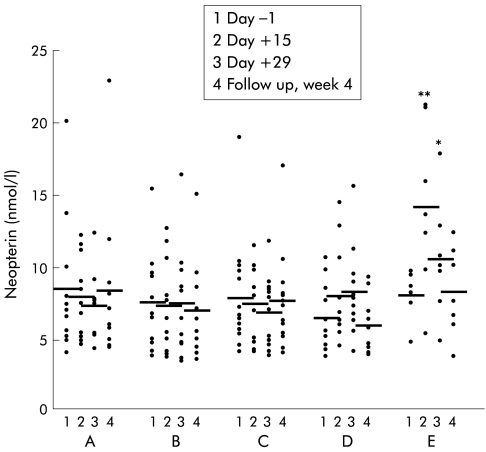
Patients who received placebo, 1 μg/kg or 4 μg/kg body weight IL-10 subcutaneously showed no change in serum neopterin values over the observation period. A dose of 8 μg/kg body weight IL-10 caused a slight but non-significant increase in neopterin levels on day +15 (8.1 (0.9) mmol/l) and day +29 (8.4 (0.9) mmol/l; p=0.07) of therapy compared with pretreatment concentrations (6.6 (0.6) mmol/l) (fig 1 ▶). This increase was more pronounced and highly significant when patients received IL-10 at the highest dose (20 μg/kg body weight). Neopterin levels then increased from 7.5 (0.5) mmol/l at baseline to 13.7 (1.7) mmol/l (p<0.003) on day +15 and to 10.4 (1.4) mmol/l (p<0.05) on day +29. During follow up (four weeks after the end of therapy) neopterin concentrations returned to baseline levels (fig 1 ▶). Pretreatment neopterin levels in all patients (n=54) were 7.9 (0.5) mmol/l (not significantly increased compared with healthy controls).6 We observed no correlation between increased neopterin synthesis and clinical response to IL-10 treatment.
Figure 1.
Serum neopterin levels in patients with chronic active Crohn's disease treated with placebo (A) (n=10), or recombinant human interleukin 10 at 1 (B) (n=12), 4 (C) (n=12), 8 (D) (n=10), or 20 (n=10) (E) μg/kg body weight subcutaneously daily over 28 days. Data are individual values/mean. *p<0.05,**p<0.005, ANOVA analysis.
There were no significant changes in serum nitrite/nitrate levels at any dose studied during the observation period in either treatment group. However, at the end of therapy (day +29) patients receiving higher doses of IL-10 (20 μg/kg body weight) presented with higher nitrite/nitrate levels than patients receiving lower IL-10 doses or placebo (table 1 ▶).
Table 1.
Serum levels of nitrite/nitrate (μmol/l) in patients with chronic active Crohn's disease treated with either placebo (n=10), or recombinant human interleukin 10 at 1 (n=12), 4 (n=12), 8 (n=10), or 20 (n=10) μg/kg body weight subcutaneously over 28 days
| Group | Day −1 | Day +15 | Day +29 | p Value* | Follow up week 4 |
| Placebo | 31.6 (3.6) | 32.4 ( 4.0) | 30.4 (3.2) | 0.79 | 36.4 (4.8) |
| 1 μg/kg | 29.6 (4.0) | 38.4 (10.6) | 22.8 (3.2) | 0.27 | 28.0 (3.6) |
| 4 μg/kg | 46.0 (9.6) | 46.8 (10.4) | 43.6 (10.8) | 0.79 | 38.0 (8.0) |
| 8 μg/kg | 38.0 (6.4) | 38.8 (5.2) | 47.6 (8.8) | 0.5 | 40.0 (5.2) |
| 20 μg/kg | 34.4 (6.0) | 39.6 (8.0) | 46.0 (11.6) | 0.29 | 32.8 (6.8) |
Data are mean (SEM).
*Statistical analysis was performed using ANOVA.
MCD study
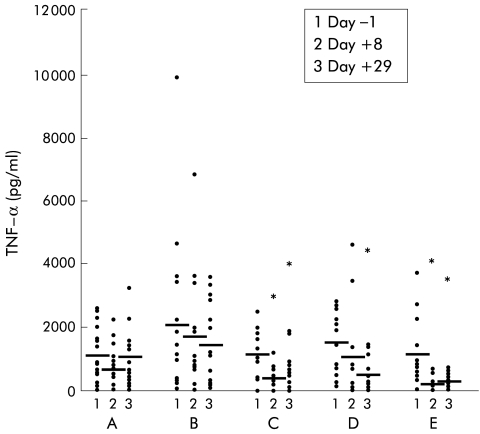
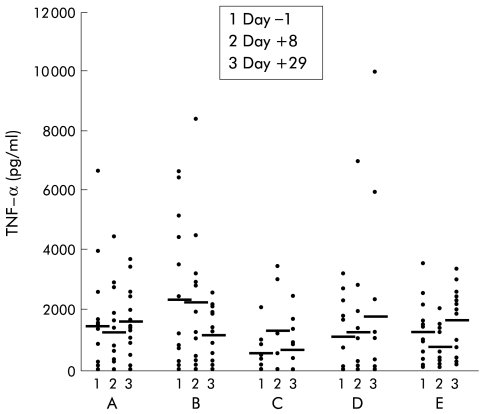
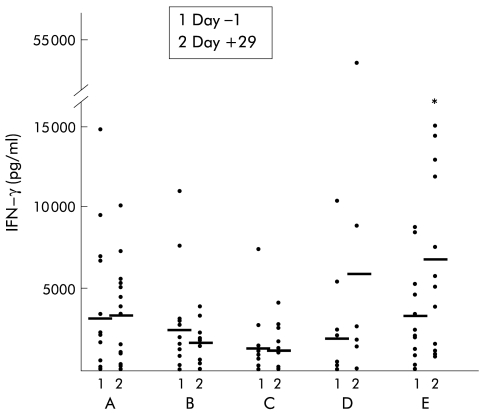
Incubation of whole blood for 24 hours with the negative control polymyxin B resulted in virtually no IFN-γ or TNF-α production (data not shown). In the placebo group, incubation with LPS caused increased TNF-α production, and no significant change over time was observed (fig 2 ▶). Treatment with IL-10 resulted in a strong dose dependent inhibition of LPS induced TNF-α release (1 μg/kg, NS; 5 μg/kg, p=0.009; 10 μg/kg, p=0.028; 20 μg/kg, p=0.008) (fig 2 ▶). In the placebo group, incubation of whole blood with PHA induced TNF-α production and again no significant change over time was observed (fig 3 ▶). Treatment with IL-10 however resulted in a slight but non-significant increase in TNF-α production in the higher dose groups (fig 3 ▶). Levels of LPS induced IFN-γ production were low on both day +1 and day +29 in all groups (data not shown). However, high IL-10 doses (20 μg/kg) significantly increased PHA induced IFN-γ production on day +29 (p=0.028) (fig 4 ▶).
Figure 2.
Whole blood cell culture plasma levels of tumour necrosis factor α (TNF-α) after lipopolysaccharide stimulation in patients with mild to moderately active Crohn's disease treated with placebo (n=16), or recombinant human interleukin 10 at 1 (B) (n=15), 5 (C) (n=12), 10 (D) (n=12), or 20 (E) (n=13) μg/kg body weight subcutaneously daily over 28 days. Data are shown as individual values/mean. *p<0.05 compared with pretreatment levels.
Figure 3.
Whole blood cell culture plasma levels of tumour necrosis factor α (TNF-α) after phytohaemagglutinin stimulation in patients with mild to moderately active Crohn's disease treated with placebo (n=16), or recombinant human interleukin 10 at 1 (B) (n=15), 5 (C) (n=12), 10 (D) (n=12), or 20 (E) (n=13) μg/kg body weight subcutaneously daily over 28 days. Data are shown as individual values/mean.
Figure 4.
Whole blood cell culture plasma levels of interferon γ (IFN-γ) after phytohaemagglutinin stimulation in patients with mild to moderately active Crohn's disease treated with placebo (n=16), or recombinant human interleukin 10 at 1 (B) (n=15), 5 (C) (n=12), 10 (D) (n=12), or 20 (E) (n=13) μg/kg body weight subcutaneously daily over 28 days. Data are shown as individual values/mean. *p<0.05 compared with pretreatment levels.
When correlating PHA induced IFN-γ production and clinical response, we found that IFN-γ production was lower in responding patients (data not shown) but the effect was not statistically significant. There was however no correlation between LPS induced TNF-α values on day +8 and day +29 and clinical response.
DISCUSSION
IL-10 is an important negative regulator of cell mediated immunity.1 In many animal models, IL-10 has been shown to inhibit IFN-γ secretion from activated Th1 cells and natural killer cells and antagonise the effects of IFN-γ towards target cells such as macrophages.1,11–14 In mice, the immunoregulatory roles of IL-10 however appear to be complex. Murray et al have shown that IL-10 transgenic mice are unable to clear mycobacterial infection.15 In this study, excess administration of IL-10 did not inhibit T cell responses to mycobacteria and IFN-γ production in these mice.15 This study suggested that IL-10 enhances IFN-γ production by antigen specific Th1 cells and/or non-specifically by natural killer cells under chronic inflammatory conditions. Furthermore, several studies indicate that IL-10 may act as an immunostimulatory agent.16–18 In mice with graft versus host disease (GVHD), IL-10 administration dose dependently decreased survival.16 Peritt et al demonstrated that proliferation of IL-2 activated natural killer cells is enhanced by IL-10, and the production of IFN-γ and TNF-α by IL-2 activated natural killer cells is significantly stimulated by IL-10.17 More importantly, Shibita et al showed that IL-10 may enhance IFN-γ production by natural killer cells.18
In humans, IL-10 can also have a dual role. We have previously reported that in low dose endotoxaemia in human volunteers, a high dose of IL-10 (25 μg/kg) increases serum levels of IFN-γ.5 IL-10 treatment also enhanced the release of the IFN-γ dependent chemokines IFN-γ inducible protein 10 and monokine induced by IFN-γ in vivo. In addition, increased levels of soluble granzymes were measured after IL-10 treatment, reflecting activation of cytotoxic T lymphocytes and natural killer cells.5
Whether the stimulatory or inhibitory effect of IL-10 on IFN-γ production may be predominant in a chronic inflammatory disease such as CD however is not yet known. In the current study, we decided to assess cytokine production by lymphocytes and monocytes separately, using specific stimuli (that is, PHA which directly stimulates lymphocytes and LPS which stimulates monocytes). Our data showed increased formation of neopterin and IFN-γ in response to high doses of IL-10. This indicates that in CACD and MCD, IL-10 may stimulate IFN-γ production thereby limiting its anti-inflammatory activities. This effect appears to be dose dependent as low doses of IL-10 had no influence on neopterin/IFN-γ values. Interestingly, our data from the MCD study indicate that IL-10 differently affects the two proinflammatory cytokines TNF-α and IFN-γ, as high doses of IL-10 decrease LPS induced TNF-α synthesis whereas they enhance PHA stimulated IFN-γ synthesis. This discrepancy between TNF-α and IFN-γ after IL-10 treatment is probably a consequence of different effects of IL-10 on the source cells for both cytokines. IFN-γ is exclusively produced by lymphocytes and natural killer cells whereas TNF-α is produced by both monocytes and lymphocytes.
Elevated levels of neopterin have been demonstrated in CD6 but in our patient population baseline neopterin concentrations were similar to healthy controls. This may be due to concomitant anti-inflammatory treatment with steroids as all of our patients in the CACD trial were treated with steroids and these agents can suppress neopterin synthesis.6 Neopterin is a pteridine derivate which is produced by human monocytes/macrophages in response to IFN-γ.6,7 This effect is due to direct stimulation of the key enzyme of the pteridine pathway, GTP cyclohydrolase I, by IFN-γ.7 Thus it is well established that elevated levels of neopterin reflect enhanced endogenous IFN-γ activity.6 This has been demonstrated in many different disease states including bone marrow and solid organ transplantation, immune mediated disorders, tumours, and infectious diseases.6,19–22
Nitrite/nitrate levels were not significantly changed over the observation period in the CACD study. In contrast with neopterin, which is produced after stimulation of macrophages by a single cytokine such as IFN-γ,6,7 iNOS expression in macrophages or hepatocytes requires combined treatment with IFN-γ/TNF-α, IL-1, and/or LPS to be fully induced.8,9,23,24 Thus increased serum concentrations of a single protein, such as IFN-γ, as might occur after therapeutic administration of high doses of IL-10, stimulate neopterin formation but are not sufficient enough to induce NO formation to an extent to alter serum nitrite/nitrate levels. This notion is also supported by the observation that a “cytokine storm”, as occurs during GVHD or allograft rejection, can increase endogenous NO formation as well as neopterin production while viral complications in this setting increase only neopterin but not NO levels.25 Thus neopterin is a more reliable parameter to detect endogenous IFN-γ activity than nitrite/nitrate. Nevertheless, it would also appear reasonable that the lack of differences in nitrite/nitrate levels over the observation period may be a consequence of the fact that all patients investigated in this study received corticosteroids, as corticosteroids can downregulate NO formation by blocking iNOS expression.26
van Deventer et al demonstrated that IL-10 administered as a daily intravenous bolus injection over one week was safe and well tolerated and this small study suggested clinical efficacy.27 In both the MCD and CACD studies, IL-10 was also safe and showed some clinical efficacy.3,4 Interestingly, in the MCD study, the major effective dose was 5 μg/kg body weight, a dose which did not induce IFN-γ in our study.3 Whereas a tendency towards clinical improvement but not remission was observed in the 8 μg/kg dose group in the CACD study, a dose of 20 μg/kg IL-10 was not associated with any clinical benefit in both the CACD and MCD studies. Higher doses of IL-10 are associated with systemic side effects such as fever, headache, and malaise; this may be caused by upregulation of IFN-γ and neopterin production in vivo, probably reflecting endogenous formation of IFN-γ by activated lymphocytes or natural killer cells.
In conclusion, out data strongly indicate that IL-10 at a high dose (20 μg/kg) increases production of IFN-γ by peripheral blood lymphocytes. At this dose, no clinical efficacy was observed in patients with active CD, and side effects including fever and headache were observed. This suggests that the use of systemically administered rHuIL-10 is limited by its proinflammatory effects. This problem may be circumvented by approaches that result in effective mucosal delivery without causing an increase in systemic IL-10 concentrations.
Acknowledgments
We thank Margot Haun for technical assistance. The authors thank the following collaborators for their input: Dr B Duclos, Department of Gastroenterology, University of Strasbourg, France and Dr RA van Hogezand, Department of Gastroenterology, University of Leiden, the Netherlands
This work was supported by grant P14641 (HT) and P12186 (GW) from the Austrian Science Foundation.
Abbreviations
CACD, chronic active Crohn's disease
CD, Crohn's disease
CDAI, Crohn's disease activity index
GVHD, graft versus host disease
IFN-γ, interferon γ
IL, interleukin
rHuIL-10, recombinant human IL-10
iNOS, inducible nitric oxide synthase
LPS, lipopolysaccharide
MCD, mild to moderately active CD
NO, nitric oxide
PHA, phytohaemagglutinin
Th, T helper cells
TNF-α, tumour necrosis factor α
REFERENCES
- 1.Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993;75:263–74. [DOI] [PubMed] [Google Scholar]
- 3.Fedorak RN, Gangl A, Elson CO, et al. Recombinant human interleukin-10 in the treatment of patients with mild to moderately active Crohn's disease. Gastroenterology 2000;119:1473–82. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber S, Fedorak RN, Nielsen OH, et al. Safety and efficacy of recombinant human interleukin-10 in chronic active Crohn's disease. Gastroenterology 2000;119:1461–72. [DOI] [PubMed] [Google Scholar]
- 5.Lauw FN, Pajkrt D, Hack CE, et al. Proinflammatory effects of interleukin-10 during human endotoxemia. J Immunol 2000;165:2783–9. [DOI] [PubMed] [Google Scholar]
- 6.Wachter H, Fuchs D, Hausen A, et al. Neopterin. Biochemistry-methods-clinical application. Berlin: De Gruyter, 1992.
- 7.Huber C, Batchelor JR, Fuchs D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med 1984;160:310–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993;329:2002–12. [DOI] [PubMed] [Google Scholar]
- 9.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem 1994;269:13725–8. [PubMed] [Google Scholar]
- 10.Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1982;126:131–8. [DOI] [PubMed] [Google Scholar]
- 11.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 1991;174:915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiorentino DF, Zlotnik A, Mosmann TR, et al. IL-10 inhibits cytokine production by activated macrophages. J Immunol 1991;147:3815–22. [PubMed] [Google Scholar]
- 13.D'Andrea A, Aste Amezaga M, Valiante NM, et al. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med 1993;178:1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med 1991;174:1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray PJ, Wang L, Onufryk C, et al. T cell-derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol 1997;158:315–21. [PubMed] [Google Scholar]
- 16.Blazar BR, Taylor PA, Smith S, et al. Interleukin-10 administration decreases survival in murine recipients of major histocompatibility complex disparate donor bone marrow grafts. Blood 1995;85:842–51. [PubMed] [Google Scholar]
- 17.Peritt D, Aste Amezaga M, Gerosa F, et al. Interleukin-10 induction by IL-12: a possible modulatory mechanism? Ann N Y Acad Sci 1996;795:387–9. [DOI] [PubMed] [Google Scholar]
- 18.Shibata Y, Foster LA, Kurimoto M, et al. Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFN-gamma-inducing factors but enhances NK cell production of IFN-gamma. J Immunol 1998;161:4283–8. [PubMed] [Google Scholar]
- 19.Tilg H, Vogel W, Aulitzky WE, et al. Neopterin excretion after liver transplantation and its value in differential diagnosis of complications. Transplantation 1989;48:594–9. [PubMed] [Google Scholar]
- 20.Niederwieser D, Herold M, Woloszczuk W, et al. Endogenous IFN-gamma during human bone marrow transplantation. Analysis of serum levels of interferon and interferon-dependent secondary messages. Transplantation 1990;50:620–5. [DOI] [PubMed] [Google Scholar]
- 21.Troppmair J, Nachbaur K, Herold M, et al. In-vitro and in-vivo studies on the induction of neopterin biosynthesis by cytokines, alloantigens and lipopolysaccharide (LPS). Clin Exp Immunol 1988;74:392–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss G, Kronberger P, Conrad F, et al. Neopterin and prognosis in patients with adenocarcinoma of the colon. Cancer Res 1993;53:260–5. [PubMed] [Google Scholar]
- 23.Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 1992;256:225–8. [DOI] [PubMed] [Google Scholar]
- 24.Geller DA, Lowenstein CJ, Shapiro RA, et al. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci USA 1993;90:3491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss G, Schwaighofer H, Herold M, et al. Nitric oxide formation as predictive parameter for acute graft-versus-host disease after human allogeneic bone marrow transplantation. Transplantation 1995;60:1239–44. [PubMed] [Google Scholar]
- 26.Radomski MW, Palmer RM, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci USA 1990;87:10043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Deventer SJH, Elson CO, Fedorak RN for the Crohn's Disease Study Group. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn's disease. Gastroenterology 1997;113:383–9. [DOI] [PubMed] [Google Scholar]