The advent of new biological agents for the treatment of autoimmune and chronic inflammatory disorders is drastically altering the approach to management while setting higher standards for therapeutic expectations. Only a fraction of the new biological agents keeps the promise of improved efficacy and specificity but the few that do can generate impressive results as we are currently witnessing for anti-tumour necrosis factor (TNF)-α therapy in rheumatoid arthritis and Crohn's disease.1,2 Based on these results, a number of other conditions where TNF-α biological activity may play a pathogenic role, such as psoriasis, sarcoidosis, spondyloarthropathy, Behçet's syndrome, and sepsis, are being treated using TNF-α blocking antibodies with variable but generally positive results. Since the first report of the use of infliximab in human disease,1 the literature has swelled to over 200 publications on practical applications and theoretical considerations of this humanised antibody. In this enormous body of information however, disappointingly little is found on the mechanisms of action of infliximab. Almost invariably the optimism caused by the feeling of finally having discovered a magic bullet against unyielding diseases causes all interest and resources to be shifted to more clinical trials. Although this reaction is understandable, all too often it comes at the expense of investigating mechanisms of action that would ultimately lead to a safer and more reliable use of the biological agent, or even the discovery of better biologicals. Thus the study of ten Hove et al in this issue of Gut, describing induction of mucosal T cell apoptosis during infliximab treatment of Crohn's disease, is a welcome and necessary complement to our still incomplete knowledge of the effect and manipulation of TNF-α in chronic intestinal inflammation [see 206].3
The in vivo action of infliximab has been more extensively explored in rheumatoid arthritis where blocking of TNF-α alters production of interleukin (IL)-6, IL-8, monocyte chemotactic protein 1, vascular endothelial growth factor, matrix metalloproteinases 1 and 3, angiogenesis, and the recruitment of inflammatory cells.4 Targeting of these and other activities is also of obvious importance in Crohn's disease in view of the broad role of TNF-α in mucosal inflammation.5 Unfortunately, except for the demonstration of a reduction in CD4+, CD8+ T cells, and CD68+ monocytes, downregulation of cell adhesion molecules, and decrease in IL-4 and TNF-α+ cells in the gut, little else has been published on the cellular and molecular effects of infliximab in patients with Crohn's disease.6 The aetiopathogenesis of Crohn's disease is still uncertain but there is good evidence to indicate that this condition falls into the category of disease associated with defective T cell apoptosis, a fundamental mechanism of immune homeostasis indispensable to the maintenance of health.7 Without proper control of apoptosis, the complex process regulating proliferation and death of naive and memory T cells during an immune response goes awry, and an inappropriate accumulation of T cells ensues in the tissues and leads to inflammation.8 This deleterious series of events appears to occur in Crohn's disease, as indicated by studies showing that mucosal T cells are resistant to multiple apoptotic stimuli and have a reduced expression of the proapoptotic Bax protein, while an imbalance between Bax and the antiapoptotic Bcl-2 protein is present in the inflamed mucosa.9–11 Therefore, it is reasonable to assume that eliminating excessive T cells could restore the gut to its normal state of physiological inflammation or, at least, a state of controlled inflammation (fig 1 ▶). Strong evidence for this effect is provided by animal models where experimental colitis is abrogated by induction of increased T cell apoptosis with IL-12 antibodies, blockade of IL-6 trans signalling, or deletion of CD44v7+ cells.12–14
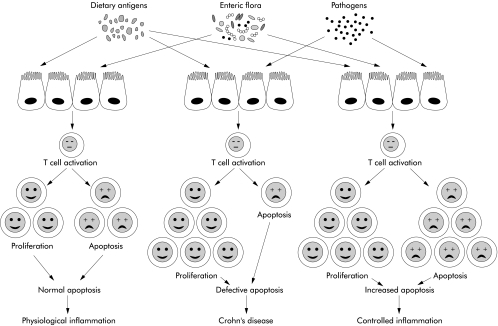
Figure 1.
Schematic representation of the various outcomes resulting from the balance between T cell proliferation and apoptosis (cell death) in the normal intestinal mucosa, or imbalance during chronic intestinal inflammation. In health, mucosal T cell proliferation induced by dietary and enteric flora antigens is counterbalanced by a baseline (normal) degree of apoptosis resulting in the low degree of physiological inflammation found in the normal gut. In Crohn's disease there is increased T cell proliferation induced by still undefined stimuli derived from bacterial antigens, food, and possibly unrecognised pathogens. At the same time, T cells die less because of their resistance to apoptosis. This defective apoptosis results in inappropriate T cell accumulation that fosters inflammation. If the degree of T cell apoptosis is increased in Crohn's disease, an effect apparently mediated by infliximab, a new balance is established between the increased rate of proliferation due to inflammation and the increased rate of apoptosis. A state of controlled inflammation is then established that is translated into clinical improvement, and later on decreased cellularity of the mucosa.
Based on the above reasoning and experimental evidence, ten Hove et al hypothesised that infliximab, in addition to neutralising soluble TNF-α, could improve Crohn's disease by inducing apoptosis of mucosal T cells.3 To test this hypothesis, the authors measured markers of activation and cell death in peripheral and mucosal T cells of patients with clinically active Crohn's disease receiving a therapeutic infusion of infliximab. In patients with a clinical response they found only minor changes in the properties and apoptosis of circulating T cells while the number of apoptotic cells, primarily CD3+ T cells, significantly increased in mucosal biopsies taken 24 hours after the start of treatment. They complemented these observations by demonstrating that infliximab could induce in vitro apoptosis of activated but not resting Jurkat T cells. As mucosal T cells in active Crohn's disease are in an enhanced state of activation, the authors concluded that the beneficial effects of infliximab may be mediated by killing of activated mucosal T cells (fig 1 ▶). This conclusion is warranted even though in vitro studies on infliximab mediated apoptosis of resting and activated peripheral blood and lamina propria T cells were not performed. The results could have reinforced the conclusion reached by the authors, and shed some light on whether defective apoptosis in Crohn's disease is an intrinsic systemic defect or one that is only detectable on exposure of T cells to the immunological challenges of the mucosa.15
A number of interesting issues, questions, and speculations are raised by this work. For starters, as ten Hove et al point out, the exact mechanism of infliximab mediated killing of mucosal T cells remains to be explored, especially knowing that apoptosis is not induced by direct in vitro exposure of these cells to TNF-α.10 Is induction of mucosal T cell apoptosis the only mechanism responsible for the beneficial effects of infliximab? Most likely not in view of the multiplicity of biological activities of TNF-α and this antibody.4,5 Whether induction of apoptosis is the dominant mechanism of action should be ascertained in the near future once studies similar to the one reported in this issue of Gut are repeated in other diseases that also benefit from TNF-α blockade. Finally, if indeed killing of activated T cells is the modus operandi of infliximab, this could have broad therapeutic implications. In fact, any condition characterised by increased numbers of activated T cells may profit from killing of these cells in the affected organs. There is preliminary evidence that infliximab provides clinical benefit for some patients with steroid refractory ulcerative colitis,16 which is also characterised by high numbers of activated T cells in the mucosa. Expansion of the ten Hove study to ulcerative colitis and other chronic inflammatory conditions should provide rather interesting answers to the questions and speculation raised in this commentary.
REFERENCES
- 1.Elliott MJ, Maini RN, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet 1994;344:1105–10. [DOI] [PubMed] [Google Scholar]
- 2.Targan SR, Hanauer SB, van Deventer SJH, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn's disease. N Engl J Med 1997;337:1029–35. [DOI] [PubMed] [Google Scholar]
- 3.ten Hove T, van Montfrans C, Peppelenbosch MP, et al. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn's disease. Gut 2002;50:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldmann M, Maini RN. Anti-TNFα therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 2001;19:163–96. [DOI] [PubMed] [Google Scholar]
- 5.Papadakis KA, Targan SR. Tumor necrosis factor: biology and therapeutic inhibitors. Gastroenterology 2000;119:1148–57. [DOI] [PubMed] [Google Scholar]
- 6.Baert FJ, D'Haens GR, Peeters M, et al. Tumor necrosis factor α antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology 1999;116:22–8. [DOI] [PubMed] [Google Scholar]
- 7.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995;267:1456–62. [DOI] [PubMed] [Google Scholar]
- 8.Sprent J, Tough DF. T cell death and memory. Science 2001;293:245–58. [DOI] [PubMed] [Google Scholar]
- 9.Boirivant M, Marini M, Di Felice G, et al. Lamina propria T cells in Crohn's disease and other gastrointestinal inflammation show defective CD2 pathway-induced apoptosis. Gastroenterology 1999;116:557–65. [DOI] [PubMed] [Google Scholar]
- 10.Ina K, Itoh J, Fukushima K, et al. Resistance of Crohn's disease T-cells to multiple apoptotic stimuli is associated with a Bcl-2/Bax mucosal imbalance. J Immunol 1999;163:1081–90. [PubMed] [Google Scholar]
- 11.Itoh J, delaMotte C, Strong SA, et al. Decreased Bax expression by mucosal T-cells favours resistance to apoptosis in Crohn's disease (CD). Gut 2001;49:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuss IJ, Marth T, Neurath MF, et al. Anti-interleukin-12 treatment regulates apoptosis of Th1 T cells in experimental colitis in mice. Gastroenterology 1999;117:1078–88. [DOI] [PubMed] [Google Scholar]
- 13.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med 2000;6:583–8. [DOI] [PubMed] [Google Scholar]
- 14.Wittig BM, Johansson B, Zoller M, et al. Abrogation of experimental colitis correlates with increased apoptosis in mice deficient for CD44 variant exon 7 (CD44v7). J Exp Med 2000;12:2053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornillie F, Shealy D, D'Haens G, et al. Infliximab induces potent anti-inflammatory and local immunomodulatory activity but no systemic immune suppression in patients with Crohn's disease. Aliment Pharmacol Ther 2001;15:463–73. [DOI] [PubMed] [Google Scholar]
- 16.Sands BE, Tremaine WJ, Sandborm WJ, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis 2001;7:83–8. [DOI] [PubMed] [Google Scholar]