Abstract
Background: Many patients with advanced malignant dysphagia are not suitable for definitive treatment. The best option for palliation of dysphagia varies between patients. This paper looks at a simple technique for enhancing laser recanalisation.
Aim: To assess the value of adjunctive brachytherapy in prolonging palliation of malignant dysphagia by endoscopic laser therapy.
Patients: Twenty two patients with advanced malignant dysphagia due to adenocarcinoma of the oesophagus or gastric cardia, unsuitable for surgery or radical chemoradiotherapy.
Methods: Patients able to eat a soft diet after laser recanalisation were randomised to no further therapy or a single treatment with brachytherapy (10 Gy). Results were judged on the quality and duration of dysphagia palliation, need for subsequent intervention, complications, and survival.
Results: The median dysphagia score for all patients two weeks after initial treatment was 1 (some solids). The median dysphagia palliated interval from the end of initial treatment to recurrent dysphagia or death increased from five weeks (control group) to 19 weeks (brachytherapy group). Three patients had some odynophagia for up to six weeks after brachytherapy. There was no other treatment related morbidity or mortality. Further intervention was required in 10 of 11 control patients (median five further procedures) compared with 7/11 brachytherapy patients (median two further procedures). There was no difference in survival (median 20 weeks (control), 26 weeks (brachytherapy)).
Conclusions: Laser therapy followed by brachytherapy is a safe, straightforward, and effective option for palliating advanced malignant dysphagia, which is complementary to stent insertion.
Keywords: oesophageal cancer, laser therapy, brachytherapy, dysphagia, palliation
Only about half of patients with cancers of the oesophagus or gastric cardia are even considered for surgery and many are not suitable for radical radiotherapy and chemotherapy. For the majority, the cancer is too far advanced at the time of presentation for there to be any prospect of cure.1 In these circumstances, the main aim of treatment is to relieve their most important symptom, dysphagia, as rapidly, effectively, and simply as possible. For some years, the most popular option was endoscopic insertion of a silicone rubber stent to hold the oesophageal lumen open.2 More recently, expanding metal stents have been introduced. They are simpler and safer to insert as less initial dilatation is required, and they expand once in position across a tumour, but as experience with them grows, it is becoming clear that the quality of swallowing is often no better and they may have as many long term problems as the old silicone rubber stents.3
Another option is endoscopic therapy with the neodymium yttrium aluminium garnet (NdYAG) laser.4 The tumour is vaporised or coagulated under direct vision with no mechanical stress on the oesophageal wall so no stent is required. This modality is suitable for patients with exophytic tumour. Successful tumour recanalisation can be achieved in more than 90% of appropriately selected patients and following treatment return to eating solids is seen in the majority. Laser therapy does however need to be repeated every 4–6 weeks as the tumour regrows. This problem may be helped by adjuvant radiotherapy. A randomised study comparing laser recanalisation alone with laser recanalisation plus palliative external beam radiotherapy (30 Gy in 10 fractions, half the typical dose used for radical treatment) showed that adding radiotherapy increased the average interval between laser treatments from five to nine weeks.4 Others have reported that brachytherapy (intraluminal radiotherapy) in one or two fractions (total 10–15 Gy at 1 cm from the source) can more than double the interval between laser treatments with minimal morbidity.5,6 The prospective randomised study reported here compares laser therapy alone with laser therapy plus a single fraction of brachytherapy for palliation of advanced malignant dysphagia in patients who had a good initial response to laser therapy.
METHODS
Patients were recruited from individuals referred to the Middlesex Hospital, London, for palliation of advanced malignant dysphagia. Only newly diagnosed cases with biopsy proven adenocarcinoma of the oesophagus or gastric cardia who had not received previous treatment with surgery, radiotherapy, chemotherapy, or stent insertion were considered for inclusion. Patients with tumours causing predominantly extrinsic compression of the oesophagus or cardia with little or no exophytic component and those with a fistula were excluded.
In patients with exophytic tumour causing dysphagia who are not candidates for potentially curative surgery, it is our policy to debulk endoscopically accessible tumour with NdYAG laser therapy under direct vision as the initial treatment. Prominent tumour nodules are vaporised and less prominent areas coagulated with debulking achieved by sloughing of dead tissue over the subsequent few days. Full treatment usually takes 2–3 sessions, at intervals of a few days. Patients invited to participate in this study were those who were able to swallow a soft diet or better after laser therapy and who were not considered suitable for radical chemotherapy or radiotherapy, and hence the main aim of treatment was to maintain their improvement in swallowing for as long as possible. Swallowing was graded on a scale of 0–4 (0, normal diet; 1, some solids; 2, soft diet/semisolids; 3, liquids only; 4, dysphagia to fluids). Once swallowing had improved to a semisolid diet (grade 2) or better, patients who agreed to participate in the study were asked to give written informed consent and were then randomised by sealed envelope to receive either no further treatment until dysphagia worsened or a single dose of brachytherapy. For brachytherapy, the tumour limits were identified on a contrast swallow and by radio-opaque markers positioned with endoscopic guidance. A nasogastric tube was passed and an iridium 192 wire was inserted using the Selectron afterloading technique (Nucletron, UK). The total dose delivered was 10 Gy at 1 cm from the source. This usually took 15–30 minutes, after which the nasogastric tube was removed. The whole procedure was normally done as a day case.
The last treatment in the initial course of therapy (laser or brachytherapy) was designated as the index treatment. Laser therapy causes local oedema, which may make swallowing worse immediately after treatment and can take several days to settle, and hence the best dysphagia grade is seen 1–2 weeks after the last laser treatment. Thus for this study, the dysphagia grade was documented at presentation, before the index treatment, and two, four, six, and 10 weeks after the index treatment. Patients were followed by telephone interview and asked to return if their dysphagia deteriorated by one grade or more. The period between the index treatment and the need for treatment for recurrent dysphagia was designated the dysphagia palliated interval (DPI). Subsequent treatments were applied as clinically indicated. Recurrence of exophytic nodules was treated with further laser therapy and fibrous strictures were dilated. Failure to respond to these measures with continued difficulty in swallowing a semisolid diet was considered an indication for stent insertion. Although some expanding metal stents were available at the time this study commenced, we were unhappy with the complications associated with their insertion, particularly pain. In contrast, we had achieved excellent results with Celestin tubes over a period of years with very few complications and so we decided to continue to use these tubes as the stent of first choice in this study.
Whenever feasible, treatments were carried out as day cases, with due consideration for the patients' disability, frailty, and fitness to travel to and from hospital, but some individuals required inpatient care. Any patient who had a stent inserted was kept in hospital for at least one night and a contrast swallow performed the following morning to ensure that there was no perforation and that the stent had not slipped and was functioning correctly. The study was approved by the ethics committee of the University College London Hospitals.
The primary end points of the study were duration of DPI and total survival time from the date of the first treatment. Non-parametric tests were used for statistical analysis. Kaplan-Meier plots were constructed and analysis was carried out using the log rank test. Comparison of the number of treatments required in the two groups was evaluated using the Mann-Whitney test. Quality of life was assessed prospectively using a linear analogue self assessment (LASA) scale.7 This questionnaire comprised five visual analogue scales measuring 10 cm in length. Each line addressed the symptoms associated with the disease process, including odynophagia, lethargy, appetite, nausea/vomiting, and general well being. Each end of every line was labelled with a word denoting the worst (0 cm) and best (10 cm) extremes. After appropriate instruction, the patient was encouraged to place an X on the line corresponding to their perception of the symptom, giving a total score of 0–50. When originally used (for patients with breast cancer), this questionnaire had as many as 20 lines addressing all aspects of symptomatology but we encountered difficulties with patient compliance in previous studies on carcinoma of the oesophagus. This was because any deterioration became increasingly obvious to the patient and served to remind them of their impending demise.8 Consequently, we limited the questionnaire to five lines and only asked patients to complete it before treatment, before the index treatment, and two, four, six, and 10 weeks after the index treatment. Differences in paired data were analysed using the Wilcoxon signed rank test.
RESULTS
Twenty two patients with advanced adenocarcinoma of the oesophagus or gastric cardia were recruited into this study between July 1993 and August 1996 (12 men, 10 women; aged 45–88 years, median 83). The main reasons for purely palliative therapy were advanced local disease as detected on computed tomography scanning or endoscopic ultrasound (seven cases) or general frailty and other comorbidity (15 cases). All randomised patients complied with the study requirements and there were no withdrawals. In both groups, median length of the tumour was 7 cm. In no patient was the top of the tumour less than 23 cm from the incisors, and the median distance from the incisors to the top of the tumour was 33 cm in the laser group and 30 cm in the combination group.
The median number of procedures undertaken up to and including the index treatment was four in each group over a median period of two weeks for the laser group and three weeks for the combination group. The longer period in the latter group reflected the logistics of arranging brachytherapy after laser treatment. The dysphagia grades are given in table 1 ▶. By definition, all patients achieved grade 2 or better by the time of the index treatment. As expected, the best results were seen two weeks after the index treatment when the median dysphagia grade for all patients in the study was 1.
Table 1.
Dysphagia grades for all patients. Follow up data were complete for all patients except one
| Time interval | Median | Range |
| Pretreatment | 3 | 1–4 |
| Pre-index treatment | 2 | 0–2 |
| 2 weeks post index | 1 | 0–3 |
| 4 weeks post index | 1 | 0–3 |
| 6 weeks post index | 1 | 0–4 |
| 10 weeks post index | 1 | 0–3 |
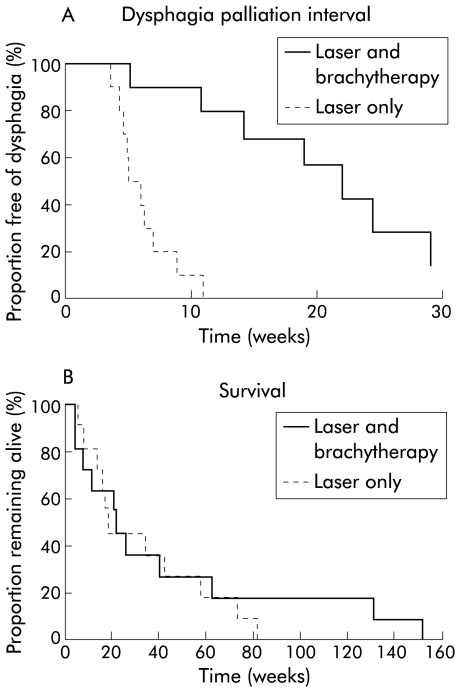
Details of duration of dysphagia palliation after the index treatment are given in table 2 ▶ and shown in fig 1A ▶. The striking finding is that there was a statistically significant difference in the median time to recurrence of dysphagia in the laser group (five weeks) compared with the combination group (19 weeks) (χ2=17, p<0.001). There was no significant difference in median survival times in the two groups (20 weeks in the laser group, 26 weeks in the combination group) (fig 1B ▶). Initial therapy palliated dysphagia until death in one patient in the laser group (two weeks after the index treatment) and in four patients in the combination group (four, 13, 21, and 152 weeks after the index treatment). Details of procedures required after recurrence of dysphagia in the other patients are shown in table 3 ▶. One patient in the laser group had recurrent dysphagia after 11 weeks but declined further treatment, preferring to continue to only swallow fluids. Oesophageal stents (Celestin tube in 8/9 cases) were inserted if there was no endoscopic target for further laser treatment or if dysphagia recurred less than one month after laser treatment or dilatation. One stent needed replacement after six months due to rotting, perhaps related to previous laser treatment of stent overgrowth by the tumour.
Table 2.
Treatments applied and duration of dysphagia relief
| Laser | Laser+brachytherapy | |
| No of patients | 11 | 11 |
| No of procedures during initial course of therapy | 4 (3–5) | 4 (2–8) |
| Duration of dysphagia palliated interval (weeks) | 5 (2–11) | 19 (4–152) |
| No of further procedures after index treatment | 5 (0–11) | 1 (0–23) |
| Survival from first treatment (weeks) | 20 (5–83) | 26 (11–152) |
Values are median (range).
Figure 1.
(A) Kaplan-Meier plot of the dysphagia palliation interval after the index treatment. The interval was significantly longer in the combination group (laser plus brachytherapy) (p<0.001). (B) Kaplan-Meier plot of survival from the date of the first treatment. There was no significant difference between the two groups.
Table 3.
Further treatments applied after recurrence of dysphagia. These values apply only to the 10 patients in the laser group and seven patients in the laser plus brachytherapy group who received further endoscopic procedures after the index treatment
| Laser | Laser+brachytherapy | |
| No of further procedures | 5 (1–11) | 2 (1–23) |
| Average interval between further procedures (weeks) | 5 (2–10) | 5 (1–9) |
| No of patients treated by (alone or in combination) | ||
| Laser | 9 | 3 |
| Dilatation | 5 | 6 |
| Stent | 7 | 2 |
Values are median (range) or number.
One patient in the combination group was reluctant to have a stent despite multiple dilatations over a period of more than two years. When he did agree, there was so much fibrosis that it was felt hazardous to dilate to 18 mm to insert a Celestin tube, and therefore an uncovered metal stent was used but this never expanded satisfactorily and he died a few weeks later. Only one other patient in the combination group required a stent; this was inserted 25 weeks after the index treatment and she survived a further 13 weeks. Overall, patients treated with brachytherapy required less than half the number of treatments per month alive than those treated with laser alone (p=0.04).
There was no laser related morbidity or mortality despite the application of endoscopic laser therapy on 121 occasions. The 30 day mortality from first treatment was zero in both groups. From the index treatment, there was one death within 30 days from disease progression in each group. Brachytherapy did not lead to deterioration in swallowing and did not cause any systemic side effects, although three patients did have some pain on swallowing. This started a week or so after brachytherapy, was relieved by antacids, and resolved spontaneously within six weeks. The LASA quality of life scores are shown in table 4 ▶.
Table 4.
Linear analogue self assessment (LASA) scales for laser and laser plus brachytherapy groups
| Time interval | No | Median | Mean | Range | 95% CI |
| Pretreatment | |||||
| Laser only | 9 | 29 | 26 | 13–39 | 19–34 |
| Brachytherapy | 9 | 27 | 22 | 5–38 | 14–30 |
| Pre-index | |||||
| Laser only | 9 | 29 | 31 | 13–41 | 25–37 |
| Brachytherapy | 9 | 35 | 34 | 21–43 | 29–39 |
| 2 weeks post index | |||||
| Laser only | 9 | 35 | 34 | 25–41 | 29–40 |
| Brachytherapy | 9 | 35 | 32 | 20–39 | 27–38 |
| 4 weeks post index | |||||
| Laser only | 9 | 33 | 31 | 15–33 | 26–37 |
| Brachytherapy | 9 | 36 | 34 | 13–43 | 27–41 |
| 6 weeks post index | |||||
| Laser only | 8 | 26 | 27 | 15–39 | 20–33 |
| Brachytherapy | 9 | 29 | 30 | 21–39 | 25–35 |
| 10 weeks post index | |||||
| Laser only | 7 | 27 | 26 | 7–39 | 16–35 |
| Brachytherapy | 7 | 31 | 32 | 26–39 | 28–36 |
The quality of life improved significantly as a result of the initial laser recanalisation in both the entire study group (p<0.001) and in the brachytherapy group (p<0.01) although it just failed to reach statistical significance in the laser only group (p<0.07). There was no significant difference, and in particular no deterioration, in LASA scores as a result of brachytherapy compared with laser treatment alone from the time of the index treatment until the end of the quality of life follow up 10 weeks later.
DISCUSSION
This study has shown that the duration of laser palliation of malignant dysphagia can be increased by a factor of nearly four (five weeks to 19 weeks) by adding a single day case brachytherapy treatment after successful laser recanalisation. In the laser only group, DPI from the time of the index treatment until death or recurrent dysphagia covered a median of 23% (range 8–100%) of the patient's remaining lifetime whereas in the combination group the value was 84% (range 22–100%). The LASA data showed that the initial laser treatment significantly improved the quality of life of these patients and that there was no deterioration in quality of life associated with brachytherapy.
Dysphagia recurred in 10 patients in the laser group compared with seven in the combination group but when dysphagia did recur, the average interval between subsequent treatments was the same in each group (five weeks (range 2–10) for the laser only group; five weeks (range 1–9) weeks for the combination group). In contrast, in our previous study using palliative external beam irradiation, the initial DPI was only increased to nine weeks rather than 19 weeks in this study, but the longer average interval between interventions was maintained for the remainder of the patients' lives.4 It is likely that the external beam irradiation slowed regrowth in most of the bulk of the primary tumour whereas brachytherapy only slowed regrowth of tumour close to the oesophageal lumen so when tumour grew back from areas unaffected by the brachytherapy it grew faster. These results suggest that some combination of external and intraluminal irradiation may be more beneficial than either alone. Despite this, brachytherapy required only one treatment session and resulted in a reduction in endoscopic interventions by 60% per month the patient remained alive. The morbidity of both irradiation regimens was low although the single treatment required for brachytherapy is simpler and cheaper than the 10 fractions required for external beam therapy. There was no improvement in median survival in those treated with external or internal irradiation compared with those treated with laser alone although two patients in the brachytherapy group survived more than two years.
Laser therapy and oesophageal stent insertion are complementary as palliative techniques. In theory, insertion of a self expanding metal stent is a one stage procedure and undoubtedly these stents are easy and safe to insert, but as more detailed follow up studies are published, so more problems emerge.3,9 Early problems with bleeding through the mesh of uncovered stents have been eased by using covered stents but this increases the risk of slippage. More intransigent problems include incorrect positioning (which in contrast with silicon rubber Celestin stents is difficult to correct), poor function due to incomplete expansion, and chest pain (which can be severe and lifelong). In routine practice, swallowing has been reported as no better than with silicone rubber stents and significant stent related morbidity was seen in more than 20% of cases.10
No palliative treatment is ideal for all patients with advanced malignant dysphagia. If a fistula is present, a stent is essential, but for patients without a fistula who have a major exophytic component to their tumour, this study has shown that laser therapy followed by brachytherapy is a safe, straightforward, and effective option. This report adds to the mounting body of evidence that these unfortunate individuals are best treated in specialist referral centres that have a broad range of therapeutic options available so the optimum combination of treatments can be chosen for each individual.
Abbreviations
NdYAG, neodymium yttrium aluminium garnet
DPI, dysphagia palliated interval
LASA scale, linear analogue self assessment scale
REFERENCES
- 1.Muller JM, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg 1990;77:845–57. [DOI] [PubMed] [Google Scholar]
- 2.Ogilvie AL, Dronfield MW, Ferguson R, et al. Palliative intubation of oesophagogastric neoplasms at fibreoptic endoscopy. Gut 1982;23:1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gevers AM, Macken E, Hiele M, et al. A comparison of laser therapy, plastic stents, and expandable metal stents for palliation of malignant dysphagia in patients without a fistula. Gastrointest Endosc 1998;48:383–8. [DOI] [PubMed] [Google Scholar]
- 4.Sargeant IR, Tobias JS, Blackman G, et al. Radiotherapy enhances laser palliation of malignant dysphagia: A randomised study. Gut 1997;40:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shmueli E, Srivastava E, Dawes PJ, et al. Combination of laser treatment and intraluminal radiotherapy for malignant dysphagia. Gut 1996;38:803–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer GM, Thorpe SM, Sargeant IR, et al. Laser and brachytherapy in the palliation of adenocarcinoma of the oesophagus and cardia. Gut 1996;39:726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Priestman TJ, Baum M. Evaluation of quality of life in patients receiving treatment for advanced breast cancer. Lancet 1976;1:899–901 [DOI] [PubMed] [Google Scholar]
- 8.Loizou LA, Rampton D, Atkinson M, et al. A prospective assessment of quality of life after endoscopic intubation and laser therapy for malignant dysphagia. Cancer 1992;70:386–91. [DOI] [PubMed] [Google Scholar]
- 9.Cwikiel W, Tranberg KG, Cwikiel M, et al. Malignant dysphagia: palliation with esophageal stents—long-term results in 100 patients. Radiology 1998;207:513–18. [DOI] [PubMed] [Google Scholar]
- 10.Lovat LB, Thorpe SM, Gertner D, et al. Relief of dysphagia with self expanding metal stents is far from perfect. Gut 2000;46(suppl II):A38. [Google Scholar]