Abstract
Background and aims: The liver represents one of the major sites of human glucuronidation. Many therapeutic drugs are substrates for UDP-glucuronosyltransferases (UGT) leading to the formation of usually inactive glucuronides. Hepatic glucuronidation undergoes significant changes during fetal and neonatal development requiring age adapted drug therapy. Regulation of individual UGT genes during hepatic development has not been defined.
Subjects and methods: Expression of 13 UGT genes and glucuronidation activities were analysed in 16 paediatric liver samples (aged 7–24 months), two fetal samples, and 12 adult liver samples (aged 25–75 years) using duplex reverse transcription-polymerase chain reaction, western blot, and specific catalytic UGT activity assays.
Results: No UGT transcripts were detected in fetal liver at 20 weeks' gestation. In contrast, UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A9, UGT2B4, UGT2B7, UGT2B10, and UGT2B15 transcripts were present without variation in all 28 hepatic samples after six months of age. Significantly lower expression of UGT1A9 and UGT2B4 mRNA was identified in paediatric liver. Hepatic glucuronidation activity in children aged 13–24 months was found to be lower than in adults for ibuprofen (24-fold), amitriptyline (16-fold), 4-tert-butylphenol (40-fold), estrone (15-fold), and buprenorphine (12-fold).
Conclusions: An early phase characterised by the appearance of UGT gene transcripts and a later phase characterised by upregulation of UGT expression is demonstrated during human hepatic development. The differential regulation of UGT1A9 and UGT2B4 expression extends beyond two years of age and is capable of influencing hepatic glucuronidation of common therapeutic drugs in children. The development of hepatic UGT activities is significant for paediatric drug therapy and the prevention of adverse drug effects.
Keywords: UGT1A, UGT2B, ontogenesis, drug metabolisms, drug toxicity, liver
The liver represents one of the major sites of human metabolism by glucuronidation. UDP-glucuronosyltransferases (UGT) catalyse a biochemical reaction designed to convert hydrophobic dietary constituents, environmental pollutants, and therapeutic drugs into hydrophilic β-D-glucopyranosiduronic acids (glucuronides) which are usually biologically inactive and can undergo facilitated renal and biliary elimination.1 The range of target substrates for glucuronidation is considerable and spans divergent chemical classes, among them amines, phenols, carboxylic acids, opioids, and steroids.2 Glucuronidation of hundreds of drugs and other compounds has been evaluated which has led to the realisation that glucuronidation represents one of the main detoxification pathways in humans.
To date, 15 individual UGTs have been identified in humans.2 Based on sequence homologies they are subdivided into two families: UGT1 and UGT2.3 In human liver, five UGT1A genes (UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A9)4–6 and five UGT2B genes (UGT2B4, UGT2B7, UGT2B10, UGT2B11, UGT2B15)2,7–9 are expressed and define the hepatic glucuronidation capacity. The remaining five UGT genes are expressed in extrahepatic sites.2,10,11 Expression and function of these gene products influences the metabolism of drugs administered for therapeutic purposes.
Hepatic glucuronidation has been studied mainly using adult liver samples.10,12–17 However, considerable differences in hepatic glucuronidation occur during development from fetal to adult liver. A number of developmental studies have shown that glucuronidation activity towards bilirubin and other compounds is low in fetal liver and reaches adult levels within the first six months of neonatal life.18–22 Based on western blot analyses, the number of UGTs in fetal liver was predicted to be low.23 However, data on the development of expression of individual UGT1A and UGT2B genes during the first 24 months of life are not available. The example of the toxicity of chloramphenicol and other antibiotic agents metabolised by conjugation indicates that detailed knowledge of the isoform specific development of UGT expression before adulthood is required and can impact medical pharmacotherapy in children.24,25
In this study, we analysed expression of 13 UGT genes and hepatic glucuronidation activity in fetal liver, in child liver between six and 24 months of age, as well as in adult liver. Evidence is provided for the appearance of UGT expression following the fetal period and differential upregulation of individual UGT gene expression after six months, which is not completed by 24 months of age.
MATERIALS AND METHODS
Patients
Liver tissue was obtained from 16 paediatric patients undergoing liver transplantation for extrahepatic biliary atresia, aged 6–24 months. None of these patients had serological evidence of viral hepatitis (table 1 ▶). Adult liver tissue was obtained from 12 patients (aged 25–75 years) receiving a hemihepatectomy or liver transplantation for hepatocellular carcinoma. None of the adult patients had chronic or acute viral hepatitis at the time of sampling. Genetic liver disease (haemochromatosis, Wilson's disease, α1 antitrypsin deficiency) as well as autoimmune liver diseases (autoimmune hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis) were excluded by seroimmunological and biochemical testing.26 All samples were histologically free of tumour, immediately frozen in liquid nitrogen following surgical removal of the specimen, and stored at −80°C until analysis. All samples were free of histological evidence of necrosis. The tissue procurement protocol was approved by the ethics committee of Hannover Medical School.
Table 1.
Patients and samples
| Sample No | Age* | Age range | Sex (M:F) | Diagnosis | Operative procedure |
| 12 | 58.08 (10.44) y | 25–75 y | 11:1 | HCC | Hemihepatectomy (n=10), liver transplantation (n=2) |
| 3 | 9 (2) mo | 7–12 mo | 2:1 | Extrahepatic | Liver transplantation |
| 6 | 15.83 (2.04) mo | 13–18 mo | 2:4 | biliary atresia | (n=16) |
| 7 | 20.75 (1.58) mo | 19–24 mo | 4:3 | (n=16) |
*Mean (SD).
HCC, hepatocellular carcinoma.
RNA from two samples of human fetal liver (male, 20 weeks' gestation; female, 20 weeks' gestation) was purchased from Strategene (LaJolla, California, USA).
Isolation and purity of tissue RNA, cDNA synthesis, and microsomal protein
Tissue RNA
Tissue was pulverised under liquid nitrogen and immediately lysed in acidic phenol/guanidinium-isothiocyanate solution (Tripure, Boehringer Mannheim, Germany) for RNA isolation, as described previously.5
cDNA synthesis
Complementary DNA was synthesised as previously described in detail.5 Contamination with genomic DNA was excluded by reverse transcription-polymerase chain reaction (RT-PCR) using primers for human β-actin which span the exon 4/intron 5/exon 5 junction of the β-actin gene. PCR with cDNA leads to a 202 bp product but contamination with genomic DNA template would lead to a 312 bp PCR product.5
Isolation of microsomal protein
Approximately 200 mg of tissue were pulverised under liquid nitrogen, resuspended in 1 ml of 50 mM Tris-HCl (pH 7.4), 10 mM MgCl2, homogenised with a Potter-Elvehjam tissue grinder, and processed as described previously.4 Storage was at −80°C.
UGT enzymatic activity assay using recombinant UGT protein and tissue microsomal protein
Catalytic activity assay of human liver microsomes
The 18 substrates used to characterise liver microsomal UGT activity were solubilised in methanol. All substrates were purchased from Sigma-Aldrich (St Louis, Missouri, USA) and are listed in fig 4 ▶. Catalytic activities of 25 μg of adult or child hepatic protein were assayed in duplicate, as previously described in detail.9,11 Latency of UGT activity in microsomal proteins was controlled using 0.25–2% CHAPS and 0.25–1% alamethicin in a 30 minute incubation reaction at 37°C prior to the UGT activity assay (data not shown). After 60 minutes the UGT assay reaction protein was precipitated, the supernatants lyophilised, and resuspended in methanol prior to separation by thin layer liquid chromatography in n-butanol/acetone/acidic acid/water (35:35:10:20%). The production of 14C-labelled glucuronides was detected by autoradiography. To determine specific catalytic activities, the 14C-labelled glucuronides were quantitated using a Fujifilm BAS-1000 phosphoimager (Raytest GmbH, Straubenhardt, Germany) and TINA 2.0 software (Raytest GmbH, Straubenhardt, Germany) and expressed as pmol glucuronide formed/min/mg of microsomal or recombinant protein. As a control, autoradiography hard copies were additionally analysed with a GS-710 calibrated imaging densitometer using the Quantity One software package (BioRad Laboratories, Hercules, California, USA).
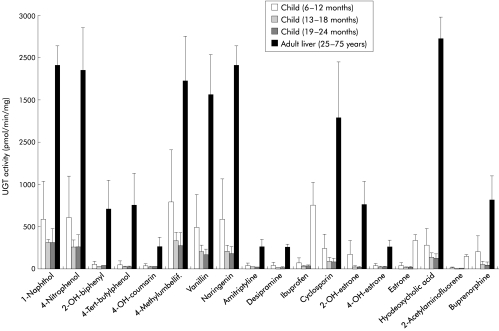
Figure 4.
Differences in catalytic activity between adult and child liver. Graphic representation of the analysis of 18 substrates tested for glucuronidation using child and adult liver. 2-OH-biphenyl, 2-hydroxybiphenyl; 4-OH-coumarin, 4-hydroxycoumarin; 2-OH-estrone, 2-hydroxyestrone; 4-OH-estrone, 4-hydroxyestrone.
Duplex reverse transcription-polymerase chain reaction for UGT1A and UGT2B transcripts
The presence of UGT1A and UGT2B transcripts in total hepatic tissue RNA was analysed by PCR amplification performed as a duplex RT-PCR (DRT-PCR) coamplification with β-actin cDNA as a control, as outlined below.
UGT1A DRT-PCR
The UGT1A locus predicts the existence of nine proteins termed UGT1A1 and UGT1A3–1A10. UGT1A2, UGT1A11, and UGT1A12 lack an uninterrupted open reading frame and have therefore been identified as pseudogenes. DRT-PCR detection of all nine UGT1A transcripts predicted by the human UGT1A locus was performed using nine exon 1 specific sense primers and two antisense primers located within exons 2–5 or within a common portion of the 3` end of the first exons. As already reported elsewhere, exon specific primers were generated which lead to RT-PCR products of distinct molecular sizes: UGT1A1, 644 bp; UGT1A3, 483 bp; UGT1A4, 572 bp; UGT1A5, 659 bp; UGT1A6, 562 bp; UGT1A7, 754 bp; UGT1A8, 514 bp; UGT1A9, 392 bp; and UGT1A10, 478 bp. Coamplification of UGT1A first exon and β-actin sequences was performed using three cycling protocols: UGT1A1 and UGT1A6, 94°C (one minute), 59°C (one minute), 72°C (one minute); UGT1A3, UGT1A4, UGT1A5, 94°C (one minute), 56°C (one minute), 72°C (one minute); and UGT1A7, UGT1A8, UGT1A9, UGT1A10, 94°C (one minute), 64°C (one minute), 72°C (one minute). Each protocol was preceded by a three minute incubation of the reaction mixture at 94°C and followed by a seven minute elongation at 72°C. The specificity and kinetics of this assay have previously been documented in detail.5 Experiments were performed in duplicate and controls without cDNA, primers, or thermophilic polymerase included.
UGT2B DRT-PCR
Specific primer pairs were generated for the amplification of UGT2B4, UGT2B7, UGT2B10, and UGT2B15 sequences, respectively, as recently reported elsewhere.9 Cross reactivity was excluded using sequence alignments and PCGene (Oxford Molecular, Campbell, California, USA) software, as well as a computerised databank search using the Blastn software (GenBank). UGT2B cDNA was coamplified with β-actin cDNA in a starting volume of 92 μl containing 10 mM KCl, 20 mM Tris-HCl (pH 8.8), 10 mM ammonium sulphate, 2 mM magnesium sulphate, 1% Triton X-100, 0.2 mM each dNTP, and 2 μM of UGT2B primers and VENT (exo-) DNA polymerase (NEB, Beverly, Massachusetts, USA). After a hot start at 94°C for three minutes, six cycles of 94°C for 30 seconds, 57°C for 30 seconds, and 72°C for 30 seconds were run on a Perkin Elmer GeneAmp PCR 2400 system. The same β-actin primers used for UGT1A DRT-PCR were added to 0.4 μM each and cycling was resumed for a total of 32 cycles. Specificity of this assay was determined by PCR using all four primer pairs on each cloned UGT2B4, UGT2B7, UGT2B10, and UGT2B15 template cDNA to exclude cross reactivity. PCR products of the expected sizes were generated: UGT2B4, 281 bp; UGT2B7, 407 bp; UGT2B10, 388 bp; UGT2B15, 330bp. To confirm the detection of specific UGT1A and UGT2B cDNAs using this assay, the PCR products were partially sequenced to document the identity of the specific gene product.
Quantification of UGT transcript levels was performed relative to the expression of β-actin amplified in the same DRT-PCR reaction as previously outlined in detail.5 Products were quantitated using the GS-710 calibrated imaging densitometer and the Quantity One software package (BioRad Laboratories, Hercules, California, USA). Transcript levels were reported in relative arbitrary units calculated with the formula: (mean peak area for UGT/mean peak area for β-actin)×100.5 Statistical analysis was performed with the Student's t test and ANOVA (GraphPad Prism, GraphPad, San Diego, California, USA).
Western blot analysis
Microsomal protein (20 μg) from liver tissue samples was boiled for 90 seconds in loading buffer (2% sodium dodecyl sulphate, 62.5 mmol/l Tris-HCl (pH 6.8), 10% glycerol, and 0.001% bromphenol blue) with β-mercaptoethanol and resolved by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis prior to electrotransfer onto nitrocellulose membranes. As controls, a 5 μg sample of total Spodoptera frugiperda (Sf9) cell lysate expressing recombinant UGT1A1 and UGT2B7 protein as well as Sf9 cells expressing no recombinant UGT protein were included. Immunodetection was performed following published protocols.27 UGT1A1, UGT1A6, and UGT2B7 protein were detected using a monospecific rabbit antihuman UGT1A1 and rabbit antihuman UGT2B7 antibody purchased from NatuTec/Gentest (Frankfurt, Germany) at a dilution of 1:1500.
RESULTS
Differential expression of UGT1A and UGT2B transcripts in fetal, child, and adult liver
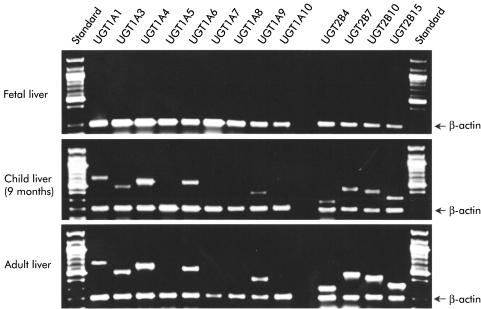
Expression of 13 individual UGT genes was analysed by specific DRT-PCR in fetal, child, and adult livers (fig 1 ▶). In fetal liver, none of the tested UGT1A or UGT2B transcripts were detected. This result is in agreement with previous findings demonstrating a reduced repertoire of UGT proteins in fetal liver.23 The data therefore demonstrate that expression of the UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A9, UGT2B4, UGT2B7, UGT2B10, and UGT2B15 genes in human liver is not regulated at 20 weeks' gestation. A comparison with child and adult liver samples indicated that expression of nine of 13 UGT genes was detectable in all of the post fetal samples. Expression of the UGT1A and UGT2B genes which represent the typical repertoire of the human liver therefore appears after 20 weeks' gestation and before six months post partum.
Figure 1.
Expression of 13 UGT1A and UGT2B transcripts detected by exon specific duplex reverse transcription-polymerase chain reaction. The ethidium bromide stained gel shows representative examples of fetal liver (20 weeks' gestation, male), child liver at nine months (male), and an example of adult liver (45 years, male). De novo regulation of the UGT1A gene locus and the four UGT2B genes is apparent between the fetal and child liver samples. Between the child and adult livers the differential upregulation of UGT transcripts is visible.
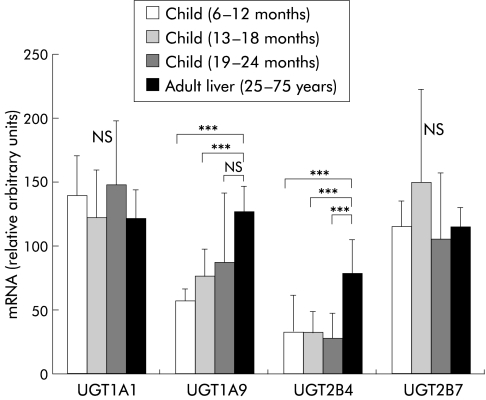
The child and adult liver RNAs were analysed for quantitative differences in UGT expression (fig 2 ▶). After six months of age differences in transcript levels were not detected for UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT2B7, UGT2B10, or UGT2B15 mRNA. However, expression of UGT1A9 mRNA increased in an age dependent fashion with significantly lower transcript levels in the age groups 6–12 months and 13–18 months (p<0.001). UGT2B4 mRNA was also expressed in an age dependent fashion with low levels between six and 24 months and significantly higher transcript levels in adults (p<0.001). These findings demonstrate an age dependent differential upregulation of the human UGT1A9 and UGT2B4 genes during the development of hepatic glucuronidation. Evidence for the de novo appearance of UGT gene expression is not detected beyond six months of age.
Figure 2.
Significant differential downregulation of UGT1A9 and UGT2B4 mRNA in child liver. Graphic representation of the quantification of mRNA levels analysed by duplex reverse transcription-polymerase chain reaction and calculated relative to the presence of β-actin.5 Statistical analysis demonstrated significant differential regulation only of UGT1A9 and UGT2B4 mRNA in children 6–18 months of age. This finding demonstrates the independent development of UGT isoform expression in human liver. ***p<0.001.
Western blot analysis of UGT1A1, UGT1A6, and UGT2B7 protein
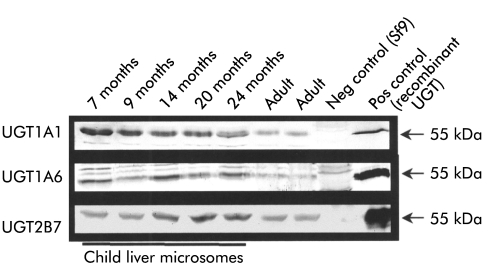
Our expression analysis indicated that there are no differences in individual UGT transcript expression, only differences in expression levels between the adult and paediatric sample population. Due to the recent availability of monospecific antisera directed against UGT1A1, UGT1A6, and UGT2B7, the specific detection of individual UGT proteins has become possible. To correlate our findings at the mRNA level with protein expression, UGT1A1, UGT1A6, and UGT2B7 protein were detected by immunoblot (fig 3 ▶). As predicted from the mRNA analysis by DRT-PCR (figs 1, 2 ▶ ▶) no differences in protein level expression were observed for UGT1A1, UGT1A6, or UGT2B7 using seven samples of child and adult liver. In the western blot shown in fig 3 ▶, which utilised the anti-UGT1A6 specific serum, two unspecific bands above the 55 kDa band were visualised which were also present using Sf9 cell extracts not expressing UGT protein. The nature of these bands is unclear but they appear to be independent of the specific band detected at 55 kDa.
Figure 3.
Expression of UGT1A1, UGT1A6, and UGT2B7 protein during hepatic development. Western blot analysis demonstrating the specific detection of UGT1A1, UGT1A6, and UGT2B7 protein in seven child and adult liver samples. As demonstrated in the duplex reverse transcription-polymerase chain reaction analysis of UGT1A1, UGT1A6, and UGT2B7 transcript expression (compare with fig 2 ▶), no significant differences were detected at the protein level, confirming the transcript analysis. All samples were found to express immunodetectable UGT protein indicating the high quality of the samples. Neg control (Sf9), Spodoptera frugiperda cells not expressing UGT protein used for negative control experiments; Pos control, positive control using the corresponding recombinant UGT for the employed antibody.
Differential catalytic glucuronidation activities in child versus adult liver microsomes
Hepatic glucuronidation activity was analysed in hepatic tissue microsomes using 18 substrates which included phenols, coumarins, flavanoids, as well as a number of therapeutic drugs such as the antidepressants amitryptyline, and desipramine, the analgesic ibuprofen, the immunosuppressant cyclosporin, the opioid buprenorphine, as well as steroid hormones such as estrone, 2-hydroxyestrone, and 4-hydroxyestrone. These substrates were selected because they represent commonly administered compounds or typical chemical structures found in medical therapeutics. The selection therefore covers opioids, analgesics, coumarins, phenols, and flavones. The number of tested substrates was limited by the available quantity of microsomal protein that permitted a complete analysis for all samples. Differences in hepatic glucuronidation activities between paediatric and adult livers were considerable and ranged from threefold to 40-fold (fig 4 ▶). At 13–24 months, UGT activities with ibuprofen, amitriptyline, 4-tert-butylphenol, estrone, and buprenorphine were 24, 16, 40, 15, and 12-fold lower than in adult liver. UGT activity analysis indicated that glucuronidation of steroid hormones, antidepressants, analgesics, opioids, flavones, and coumarins did not reach adult levels at 24 months of age.
DISCUSSION
An important process in human drug and xenobiotic catabolism is conjugation with glucuronic acid which is catalysed by members of the UGT superfamily of proteins. With the exception of a few examples including the analgesic properties of morphine-6-glucuronide,28 glucuronides are inactive water soluble conjugates committed to the elimination from the body via bile or urine.1,2 Analysis of microsomal protein from different tissue sources has demonstrated that considerable glucuronidation activity is localised in the liver.10,12,29–31 A number of the most commonly administered drugs in medicine are targeted for glucuronidation. These include paracetamol (acetaminophen),24 ibuprofen,10,32 morphine,28 amitriptyline,10,32 cyclosporin, and tacrolimus,33 as well as many steroid hormones synthesised as endobiotic substrates and administered as hormone therapy.6,7,10,11,34 Expression of UGT genes in humans is therefore an important determinant of drug efficacy with an influence on the occurrence of adverse drug reactions.24,35 Recent advances in the field of glucuronidation have led to the cloning of 15 human UGT isoforms and their presently ongoing catalytic characterisation.2 In addition, methods have been developed to specifically detect individual, highly homologous UGT transcripts in target tissues.5,6,11 Although differences in the ontogenetic development of human glucuronidation have been previously described,18–21 analysis of the contribution of individual UGT1A and UGT2B genes is not available.24 This is required to elucidate the role of individual UGTs in the glucuronidation capacity of the liver during human development.
In this study, we analysed liver tissue from children aged 6–24 months in comparison with fetal liver and adult liver. The absence of UGT1A and UGT2B gene expression in fetal liver at 20 weeks' gestation and the presence of UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A9, UGT2B4, UGT2B7, UGT2B10, and UGT2B15 in all samples of adult and child liver demonstrates that the de novo expression of UGT genes does not occur after six months of age. This is in agreement with earlier results suggesting that human glucuronidation develops up to 20 weeks' post partum.18 However, analysis of UGT transcripts by quantitative DRT-PCR demonstrated the novel finding that UGT1A9 and UGT2B4 undergo an age dependent quantitative differential regulation extending up to 24 months of age (fig 2 ▶). All of the other hepatic UGTs did not exhibit significant differences in the studied age groups. Interindividual variability in UGT expression within the sample groups was low. This finding may be a result of the absence of drug use and potential differential rates of enzyme induction prior to tissue sampling, and the fact that patients were fasting for at least 18 hours prior to surgery. A low level of interindividual UGT expression variability has been documented in other studies which analysed hepatic and other gastrointestinal tissues.4,10,36
Interestingly, there were no differences in mRNA or protein levels of the bilirubin conjugating isoform UGT1A1 (figs 2, 3 ▶ ▶) which was expressed at similar levels between six and 24 months and in adults. This is in agreement with early maturation of bilirubin glucuronidation in neonates.21 An immunoblot analysis confirmed that UGT1A1, UGT1A6, and UGT2B7, which exhibited no age dependent changes at the transcript level also showed no differences at the protein level (fig 3 ▶). However, analysis of hepatic glucuronidation activity indicated substantial differences between adults and children aged 6–24 months. Glucuronidation differences were particularly marked for a number of phenolic compounds, as exemplified by the 40-fold difference in 4-tert-butylphenol glucuronidation between adults and children aged 19–24 months (fig 4 ▶). The substrates tested and indicated in fig 4 ▶ were chosen because they represent major classes of medically relevant substrates and drugs—that is, opioids, non-steroidal analgesics, coumarins, phenolic compounds, and flavones. Of the UGT proteins expressed in human liver, UGT1A9, expression of which increased with age in this study, has been shown to exhibit the highest specific activity for phenols when catalytic analyses are compared using recombinant UGT proteins.2 UGT1A9 has been shown to glucuronidate phenols,37 anthraquinones, flavones, coumarins,38,39 and amines,9,40 which represent compounds for which considerable differences in glucuronidation activity are identified in this analysis. These data therefore suggest that the age dependent differential expression of individual UGT transcripts during human development are likely to affect levels of hepatic drug glucuronidation. This may include yet unidentified hepatic UGTs that can contribute to differential UGT activities. To expand on the clinical implication of these findings our analysis included a number of compounds and chemical classes commonly administered in medical therapy or ingested as part of our diet. This analysis included the flavanoid naringenin found in oranges, vanillin, the coumarin derivative 4-methylumbelliferone, the amine drugs amitryptyline and desipramine, the immunosuppressant cyclosporin, the analgesic ibuprofen, the opioid buprenorphine, as well as steroid hormones. All of these were glucuronidated at three to 16-fold lower levels in the child liver samples. Hence our results suggest that hepatic glucuronidation in children up to the age of two years has not matured to levels found in adults. Although the majority of UGTs are already expressed at the mRNA and protein levels indistinguishable from those found in adults, our analysis based on identification of individual UGT gene expression provides novel evidence for the differential regulation of individual UGT isoforms during human development which correlated with significantly lower hepatic glucuronidation activities.
To date, little is known of the regulatory events underlying expression of UGT genes in humans. Experimental data suggest that expression of mRNA levels correlates with cellular UGT protein levels and modulation of microsomal UGT activity.11,36 However, experiments have also indicated that UGT activity can be significantly altered by membrane factors, including phospholipid content,41 long chain fatty acids, and acyl coenzyme A.42,43 These factors may therefore also contribute to alteration of UGT activity during hepatic development and may explain the only minor differences observed at the protein level between the adult and child liver samples (fig 3 ▶). The ability of individual regulation of gene products encoded at the human UGT1A locus and by the UGT2B genes, as evidenced in this study, has been confirmed in multiple examples.5,10,11,36
The procurement of healthy paediatric liver specimens is a difficult task. In this study, we analysed tissue from paediatric patients with extrahepatic biliary atresia, which could potentially exert an influence on the presented findings. Although this cannot be ruled out with absolute certainty, a number of observations led us to proceed with the analysis of these tissues: firstly, the pattern of UGT1A expression in all of the paediatric tissue samples reflected—without variation—a typical hepatic profile found in all liver samples analysed to date.4–6,10 Secondly, the transcript levels for all isoforms with the exception of UGT1A9 and UGT2B4 were similar in the adult and paediatric groups and exhibited comparable interindividual fluctuation (fig 2 ▶). In addition, expression of UGT1A9 transcripts was found to increase during the course of time which would not be expected if progressive liver disease influenced UGT expression. Thirdly, the level of UGT1A1, UGT1A6, and UGT2B7 protein expression did not differ between the paediatric and adult groups, indicating that microsomal protein expression is intact in both groups (fig 3 ▶). Fourthly, experimental evidence suggests that glucuronidation is not significantly altered by the presence of cirrhosis.44–46 Similar considerations apply to adult tissue. This was microscopically normal but was derived from tumour (hepatocellular carcinoma) patients. However, similar samples have been analysed previously and published in order to characterise the normal human liver.4,10,36 In the light of these considerations, we believe that the results presented in this study identify differences in UGT regulation and activity which occur during the course of human hepatic development.
In summary, we demonstrated that hepatic expression of the UGT1A and UGT2B genes is characterised by a first phase of de novo appearance of expression after 20 weeks' gestation in fetal liver, which is followed by a second phase of differential upregulation of individual isoforms extending beyond two years of age. Hepatic glucuronidation in humans has not reached adult levels at two years for steroid hormones, phenolic drugs, or opioids. These considerations are likely to impact on paediatric drug therapy and contribute to the identification and prevention of adverse drug effects.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grant Str493/3-3 (to CPS).
Abbreviations
UGT, UDP-glucuronosyltransferase
DRT-PCR, duplex reverse transcription-polymerase chain reaction
REFERENCES
- 1.Dutton GJ. Glucuronidation of drugs and other compounds. Boca Raton, Florida: CRC Boca Raton Press, 1980.
- 2.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 2000;40:581–616. [DOI] [PubMed] [Google Scholar]
- 3.Burchell B, Nebert DW, Nelson DR, et al. The UDP glucuronosyltransferase gene superfamily: suggested nomenclature based on evolutionary divergence. DNA Cell Biol 1991;10:487–94. [DOI] [PubMed] [Google Scholar]
- 4.Strassburg CP, Manns MP, Tukey RH. Differential down-regulation of the UDP-glucuronosyltransferase 1A locus is an early event in human liver and biliary cancer. Cancer Res 1997;57:2979–85. [PubMed] [Google Scholar]
- 5.Strassburg CP, Oldhafer K, Manns MP, et al. Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissue. Mol Pharmacol 1997;52:212–20. [DOI] [PubMed] [Google Scholar]
- 6.Strassburg CP, Manns MP, Tukey RH. Expression of the UDP-glucuronosyltransferase 1A locus in human colon. Identification and characterization of the novel extrahepatic UGT1A8. J Biol Chem 1998;273:8719–26. [DOI] [PubMed] [Google Scholar]
- 7.Belanger A, Hum DW, Beaulieu M, et al. Characterization and regulation of UDP-glucuronosyltransferases in steroid target tissues. J Steroid Biochem Mol Biol 1998;65:301–10. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu M, Levesque E, Tchernof A, et al. Chromosomal localization, structure, and regulation of the UGT2B17 gene, encoding a C19 steroid metabolizing enzyme. DNA Cell Biol 1997;16:1143–54. [DOI] [PubMed] [Google Scholar]
- 9.Strassburg CP, Strassburg A, Nguyen N, et al. Regulation and function of family 1 and family 2 UDP-glucuronosyltransferase genes (UGT1A, UGT2B) in human oesophagus. Biochem J 1999;338:489–98. [PMC free article] [PubMed] [Google Scholar]
- 10.Strassburg CP, Nguyen N, Manns MP, et al. UDP-glucuronosyltransferase activity in human liver and colon. Gastroenterology 1999;116:149–60. [DOI] [PubMed] [Google Scholar]
- 11.Strassburg CP, Kneip S, Topp J, et al. Polymorphic gene expression and interindividual variation of UDP-glucuronosyltransferase activity in human small intestine. J Biol Chem 2000;275:36164–71. [DOI] [PubMed] [Google Scholar]
- 12.Bock KW, Lilienblum W, von Bahr C. Studies of UDP-glucuronyltransferase activities in human liver microsomes. Drug Metab Dispos 1984;12:93–7. [PubMed] [Google Scholar]
- 13.Burchell B, Coughtrie MW, Jansen PL. Function and regulation of UDP-glucuronosyltransferase genes in health and liver disease: report of the Seventh International Workshop on Glucuronidation, September 1993, Pitlochry, Scotland. Hepatology 1994;20:1622–30. [DOI] [PubMed] [Google Scholar]
- 14.Matern H, Lappas N, Matern S. Isolation and characterization of hyodeoxycholic-acid: UDP-glucuronosyltransferase from human liver. Eur J Biochem 1991;200:393–400. [DOI] [PubMed] [Google Scholar]
- 15.Matern H, Matern S, Gerok W. Formation of bile acid glucosides by a sugar nucleotide-independent glucosyltransferase isolated from human liver microsomes. Proc Natl Acad Sci USA 1984;81:7036–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pacifici GM, Back DJ. Sulphation and glucuronidation of ethinyloestradiol in human liver in vitro. J Steroid Biochem 1988;31:345–9. [DOI] [PubMed] [Google Scholar]
- 17.Peters WH, Allebes WA, Jansen PL, et al. Characterization and tissue specificity of a monoclonal antibody against human uridine 5`-diphosphate-glucuronosyltransferase. Gastroenterology 1987;93:162–9. [DOI] [PubMed] [Google Scholar]
- 18.Burchell B, Coughtrie M, Jackson M, et al. Development of human liver UDP-glucuronosyltransferases. Dev Pharmacol Ther 1989;13:70–7. [DOI] [PubMed] [Google Scholar]
- 19.Kawade N, Onishi S. The prenatal and postnatal development of UDP-glucuronyltransferase activity towards bilirubin and the effect of premature birth on this activity in the human liver. Biochem J 1981;196:257–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leakey JE, Hume R, Burchell B. Development of multiple activities of UDP-glucuronyltransferase in human liver. Biochem J 1987;243:859–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onishi S, Kawade N, Itoh S, et al. Postnatal development of uridine diphosphate glucuronyltransferase activity towards bilirubin and 2-aminophenol in human liver. Biochem J 1979;184:705–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacifici GM, Franchi M, Giuliani L, et al. Development of the glucuronyltransferase and sulphotransferase towards 2-naphthol in human fetus. Dev Pharmacol Ther 1989;14:108–14. [PubMed] [Google Scholar]
- 23.Coughtrie MW, Burchell B, Leakey JE, et al. The inadequacy of perinatal glucuronidation: immunoblot analysis of the developmental expression of individual UDP-glucuronosyltransferase isoenzymes in rat and human liver microsomes. Mol Pharmacol 1988;34:729–35. [PubMed] [Google Scholar]
- 24.de Wildt SN, Kearns GL, Leeder JS, et al. Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin Pharmacokinet 1999;36:439–52. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes KH, Henry NK. Antibiotic therapy for severe infections in infants and children. Mayo Clin Proc 1992;67:59–68. [DOI] [PubMed] [Google Scholar]
- 26.Strassburg CP, Obermayer-Straub P, Manns MP. Autoimmunity in liver diseases. Clin Rev Allergy Immunol 2000;18:127–39. [DOI] [PubMed] [Google Scholar]
- 27.Strassburg CP, Obermayer-Straub P, Alex B, et al. Autoantibodies against glucuronosyltransferases differ between viral hepatitis and autoimmune hepatitis. Gastroenterology 1996;111:1576–86. [DOI] [PubMed] [Google Scholar]
- 28.Coffman BL, Rios GR, King CD, et al. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab Dispos 1997;25:1–4. [PubMed] [Google Scholar]
- 29.Matern S, Matern H, Farthmann EH, et al. Hepatic and extrahepatic glucuronidation of bile acids in man. Characterization of bile acid uridine 5'-diphosphate-glucuronosyltransferase in hepatic, renal, and intestinal microsomes. J Clin Invest 1984;74:402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matern H, Matern S, Schelzig C, et al. Bile acid UDP-glucoronyltransferase from human liver. Properties and studies on aglycone substrate specificity. FEBS Lett 1980;118:251–4. [DOI] [PubMed] [Google Scholar]
- 31.Pacifici GM, Franchi M, Bencini C, et al. Tissue distribution of drug-metabolizing enzymes in humans. Xenobiotica 1988;18:849–56. [DOI] [PubMed] [Google Scholar]
- 32.Green MD, King CD, Mojarrabi B, et al. Glucuronidation of amines and other xenobiotics catalyzed by expressed human UDP-glucuronosyltransferase 1A3. Drug Metab Dispos 1998;26:507–12. [PubMed] [Google Scholar]
- 33.Strassburg CP, Barut A, Obermayer-Straub P, et al. Identification of cyclosporine A and tacrolimus glucuronidation in human liver and the gastrointestinal tract by a differentially expressed UDP-glucuronosyltransferase: UGT2B7. J Hepatol 2001;34:865–72. [DOI] [PubMed] [Google Scholar]
- 34.Hum DW, Belanger A, Levesque E, et al. Characterization of UDP-glucuronosyltransferases active on steroid hormones. J Steroid Biochem Mol Biol 1999;69:413–23. [DOI] [PubMed] [Google Scholar]
- 35.Sharp S, Mak LY, Smith DJ, et al. Inhibition of human and rabbit liver steroid and xenobiotic UDP-glucuronosyltransferases by tertiary amine drugs–implications for adverse drug reactions. Xenobiotica 1992;22:13–25. [DOI] [PubMed] [Google Scholar]
- 36.Strassburg CP, Nguyen N, Manns MP, et al. Polymorphic expression of the UDP-glucuronosyltransferase UGT1A gene locus in human gastric epithelium. Mol Pharmacol 1998;54:647–54. [PubMed] [Google Scholar]
- 37.Wooster R, Sutherland L, Ebner T, et al. Cloning and stable expression of a new member of the human liver phenol/bilirubin: UDP-glucuronosyltransferase cDNA family. Biochem J 1991;278:465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wooster R, Ebner T, Sutherland L, et al. Drug and xenobiotic glucuronidation catalysed by cloned human liver UDP-glucuronosyltransferases stably expressed in tissue culture cell lines. Toxicology 1993;82:119–29. [DOI] [PubMed] [Google Scholar]
- 39.Ebner T, Burchell B. Substrate specificities of two stably expressed human liver UDP-glucuronosyltransferases of the UGT1 gene family. Drug Metab Dispos 1993;21:50–5. [PubMed] [Google Scholar]
- 40.Nowell SA, Massengill JS, Williams S, et al. Glucuronidation of 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human microsomal UDP-glucuronosyltransferases: identification of specific UGT1A family isoforms involved. Carcinogenesis 1999;20:1107–14. [DOI] [PubMed] [Google Scholar]
- 41.Tukey RH, Billings RE, Autor AP, et al. Phospholipid-dependence of oestrone UDP-glucuronyltransferase and p-nitrophenol UDP-glucuronyltransferase. Biochem J 1979;179:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita A, Watanabe M, Tonegawa T, et al. Acyl-CoA binding and acylation of UDP-glucuronosyltransferase isoforms of rat liver: their effect on enzyme activity. Biochem J 1995;312:301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashita A, Nagatsuka T, Watanabe M, et al. Inhibition of UDP-glucuronosyltransferase activity by fatty acyl-CoA. Kinetic studies and structure-activity relationship. Biochem Pharmacol 1997;53:561–70. [DOI] [PubMed] [Google Scholar]
- 44.Furlan V, Demirdjian S, Bourdon O, et al. Glucuronidation of drugs by hepatic microsomes derived from healthy and cirrhotic human livers. J Pharmacol Exp Ther 1999;289:1169–75. [PubMed] [Google Scholar]
- 45.Debinski HS, Lee CS, Danks JA, et al. Localization of uridine 5'-diphosphate-glucuronosyltransferase in human liver injury. Gastroenterology 1995;108:1464–9. [DOI] [PubMed] [Google Scholar]
- 46.Debinski HS, Mackenzie PI, Lee CS, et al. UDP glucuronosyltransferase in the cirrhotic rat liver. J Gastroenterol Hepatol 1996;11:373–9. [DOI] [PubMed] [Google Scholar]