Abstract
Background: Thalidomide improves clinical symptoms in patients with therapy refractory Crohn's disease, as shown in two recent studies. The mechanism of this effect however is still unknown. Suppression of tumour necrosis factor α (TNF-α) by thalidomide has been suggested as a possible mechanism. However, effects on other cytokines have not been adequately investigated.
Aim: The aim of our study was to investigate the effects of thalidomide on cytokine production in patients with inflammatory bowel disease (IBD).
Methods: Ten patients with therapy refractory IBD (nine Crohn's disease, one ulcerative colitis) received thalidomide 300 mg daily in a 12 week open label study. Production of TNF-α, interleukin (IL)-1β, IL-6, and IL-12 was investigated in short term cultures of stimulated colonic lamina propria mononuclear cells (LPMC) and peripheral blood monocytes (PBMC) before and after 12 weeks of treatment. LPMC were also cultured with graded doses of thalidomide.
Results: Three patients discontinued treatment because of sedative side effects. In the other patients, disease activity decreased significantly, with four patients achieving remission. Production of TNF-α and IL-12 decreased during treatment with thalidomide: LPMC (TNF-α: 42.3 (8.3) pg/ml v 16.4 (6.3); IL-12: 9.7 (3.3) v 5.0 (2.5); p<0.04) and PBMC (TNF-α: 62.8 (14.6) v 22.5 (9.2); p<0.02). Production of IL-1β and IL-6 did not change significantly. Culturing of LPMC with thalidomide showed a dose dependent decrease in TNF-α and IL-12 production.
Conclusion: The clinical effects of thalidomide in Crohn's disease may be mediated by reduction of both TNF-α and IL-12.
Keywords: Crohn's disease, thalidomide, tumour necrosis factor α, interleukin 12
Even with the availability of various immunosuppressive therapies such as azathioprine, mercaptopurine, and methotrexate, the treatment of patients with Crohn's disease (CD) refractory to steroids still remains a clinical challenge.
The inflammatory process in inflammatory bowel disease (IBD) is characterised by increased production of proinflammatory cytokines, including tumour necrosis factor α (TNF-α), interleukin (IL)-1β, and IL-6 by intestinal lamina propria mononuclear cells (LPMC) and peripheral blood monocytes (PBMC).1–4 Production of IL-12, an immune response regulatory cytokine, has recently been demonstrated in patients with active CD.5
TNF-α is considered to be centrally involved in the inflammatory process in CD.6,7 TNF-α displays multiple effector functions, including induction of neutrophil accumulation,8 granuloma formation,9 upregulation of adhesion molecules on endothelial cells,10 procoagulant effects,11 and induction of increased intestinal permeability.12 In clinical studies, TNF-α levels in serum13 as well as in stool14 were found to be elevated in patients with active CD in comparison with normal controls.
Strong support for a central role of TNF-α comes from clinical studies with infliximab, a humanised chimeric monoclonal antibody of the IgG1 subclass. Infliximab was shown to be effective in at least two thirds of patients with steroid dependent chronic active CD.6,7
Thalidomide, another agent with TNF-α suppressive properties, was introduced into the therapy of CD by Wettstein and Meagher15 who reported remission in a case of steroid dependent CD. Thalidomide was developed in the 1950s as a sedative but was subsequently withdrawn from widespread use in the 1960s because of teratogenicity.16 After the drug was banned for more than two decades, in vitro studies demonstrating that thalidomide inhibits TNF-α production17 have led to its use in clinical conditions thought to be mediated by increased production of proinflammatory cytokines, such as refractory cutaneous lupus,18 chronic graft versus host disease,19 rheumatoid arthritis,20 and Behçet's syndrome.21
However, selective suppression of TNF-α may not be effective in CD.22,23 It would therefore be interesting to investigate the effect of thalidomide on other cytokines such as IL-12. Earlier studies showed a reduction in IL-12 by thalidomide24 but this has not been adequately investigated.
Recently, two open label trials on the treatment of refractory CD with thalidomide have reported response rates of 64% and 70% in patients after a 12 week course.25,26 To elucidate the possible mechanism of this clinical effect, we investigated production of TNF-α, IL-1β, IL-6, and IL-12 in 10 patients with IBD treated with thalidomide.
METHODS
Patients
Nine patients with chronic active CD and one patient with chronic active ulcerative colitis (UC) participated in the study (table 1 ▶). Chronic active CD and UC were defined by a CD activity index (CDAI) >200, respectively, a colitis activity index (CAI) >7 despite prednisone >10 mg daily, and/or azathioprine therapy for at least three months. Diagnosis of CD and UC was established using generally accepted criteria.27,28
Table 1.
Clinical data of nine patients with Crohn's disease and one patient with ulcerative colitis
| Patient | Age (y) | Sex | Duration of disease (y) | CDAI/CAI | CDEIS | Steroid treatment (months) | Prednisone dose (mg) | Azathioprine dose (mg) | Extraintestinal manifestations | Mesalazine (g) |
| 1 | 38 | M | 7 | 342 | 16.6 | 12 | —* | 125 | Arthralgia | — |
| 2 | 22 | M | 5 | 308 | 19.8 | 8 | 10 | 100 | — | 3.0 |
| 3 | 51 | M | 2 | 202 | 12.8 | 6 | 20 | —† | — | 3.0 |
| 4 | 30 | M | 2 | 216 | 9.8 | 17 | 30 | 100 | Arthralgia | — |
| 5 | 40 | F | 13 | 218 | 10.2 | 24 | 30 | 125 | — | 4.0 |
| 6 | 33 | M | 6 | 425 | 20.4 | 15 | 20 | 100 | — | 1.5 |
| 7 | 27 | F | 4 | 325 | 14.2 | 24 | 30 | —† | — | 2.0 |
| 8 | 45 | M | 5 | 252 | 12.5 | 18 | 20 | 100 | — | 3.0 |
| 9 | 31 | M | 3 | 277 | 14.8 | 8 | 20 | 100 | — | 3.0 |
| 10‡ | 61 | M | 5 | 9 | 12 | 20 | — | — | 3.0 |
*Budenoside 9 mg; †azathioprine intolerance; ‡ulcerative colitis.
Exclusion criteria were bacterial or parasitic pathogens in the patients' stools, a positive Clostridium difficile toxin test, clinical signs of septicaemia, intestinal perforation, megacolon, signs of stenosis, and active fungal or viral infection. Because thalidomide can cause peripheral neuropathy, patients were also excluded if they had a history or clinical signs of neurological disease. Further exclusion criteria were elevated transaminases (>3 times normal), hyperbilirubinaemia (>2 times normal), signs of renal dysfunction (serum creatinine >33% elevated), or serum cholesterol concentration less than 110 mg/dl. Contraception was mandatory for female participants of childbearing potential. Informed consent was obtained from all patients. The study was granted prior approval by the local ethics review committee.
Baseline studies and follow up
A clinic visit was scheduled two weeks before the tentative start of thalidomide treatment and colonoscopy with calculation of the CD endoscopic index of severity (CDEIS)29 was performed within one week before enrollment. All patients received thalidomide (Grünenthal, Aachen, Germany) at a dose of 300 mg to be taken orally at bedtime. Patients were seen two, four, eight, and 12 weeks after the start of thalidomide treatment, and at each of these times laboratory tests and a physical examination were performed and the CDAI/CAI calculated. After two and 12 weeks, a repeat colonoscopy with calculation of CDEIS was performed. In case of clinical remission, as defined by a decrease in CDAI to below 150, (CAI below 5), prednisolone was tapered. The primary outcome measure was induction of clinical remission.
In vitro cytokine studies
Cytokine production was measured in colonic LPMC and PBMC before the start of thalidomide and after two and 12 weeks of treatment. Fetal calf sera and pokeweed mitogen (PWM) were purchased from Gibco (Grand Island, New York, USA). TNF-α , IL-1β, IL-6, and IL-12 p70 ELISA kits were obtained from R&D Systems (Minneapolis, Minnesota, USA). Sensitivity of TNF-α ELISA was 4.4 pg/ml, IL-1β 1 pg/ml, IL-6 0.7 pg/ml, and IL-12 p70 0.5 pg/ml. All other chemicals were obtained from Sigma (St Louis, Missouri, USA) unless otherwise specified. PBMC and LPMC were isolated as previously described.2,22 In brief, diluted peripheral blood was layered over a Ficoll-Hypaque gradient, and cells from the interface were harvested and incubated in petri dishes with subsequent discarding of non-adherent cells. PBMC were cultured without stimulation and in the presence of lipopolysaccharide (1% vol/vol, 24 hours). For isolation of LPMC, in brief, epithelial cells were removed from biopsies by repeated washing with EDTA, and then biopsies collagenase digested overnight. After density gradient centrifugation, LPMC were cultured without stimulation and in the presence of PWM (1% vol/vol, 48 hours). Supernatant cytokine levels were determined in duplicate by ELISA.
Statistics
Results are expressed as mean (SEM). The Wilcoxon signed rank test was used with paired data. A p value <0.05 was considered significant.
Results
Effect on clinical activity
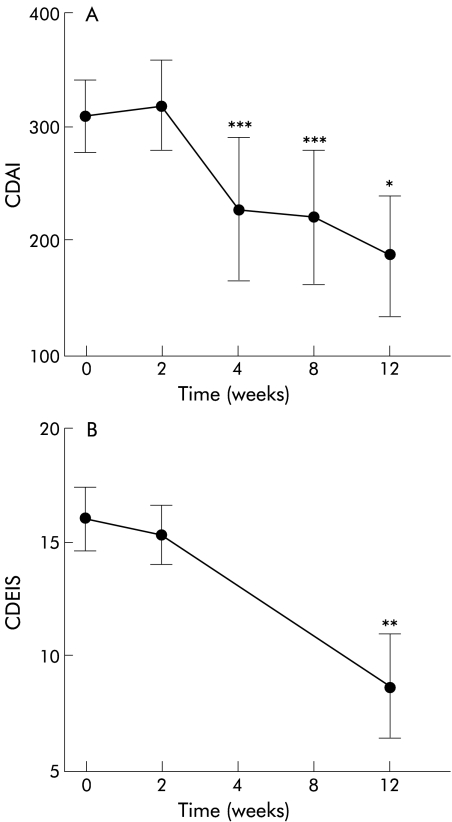
Three patients discontinued treatment with thalidomide within two weeks because of sedative side effects. The CDAI of the remaining six patients with CD decreased from 309 (31) before treatment and 319 (40) after two weeks to 227 (63) after four weeks (p<0.05), 216 (56) after eight weeks (p<0.05), and 187 (53) after 12 weeks of treatment (p<0.02) (fig 1A ▶). Four of these patients achieved remission (two patients at week 4 and two patients at week 12). The CAI of the patient with UC was 9 before thalidomide and 7 after 12 weeks of treatment. CDEIS scores changed from 16.1 (1.4) before treatment to 15.4 (1.3) at week 2 and 8.7 (2.3) at week 12 (p<0.04) (fig 1B ▶). C reactive protein level decreased from 4.9 (1.1) mg/dl before treatment to 4.5 (1.0) after two weeks, 2.6 (0.7) after four weeks (p<0.02), 2.3 (0.7) after eight weeks (p<0.04), and 2.1 (0.9) after 12 weeks (p<0.04). All patients were receiving steroid therapy at study entry. Three patients were able to discontinue steroid treatment; in one patient prednisolone was reduced from 20 mg to 10 mg daily. In the other patients, steroid doses were unchanged.
Figure 1.
Crohn's disease activity index (CDAI) and Crohn's disease endoscopic index of severity (CDEIS) after treatment with thalidomide. CDAI scores (A) decreased from 309 (31) before treatment to 227 (63) after four weeks, 216 (56) after eight weeks, and 187 (53) after 12 weeks of treatment. CDEIS (B) also significantly decreased after treatment with thalidomide. *p<0.02, **p<0.04, ***p<0.05.
Effects of thalidomide on cytokine production
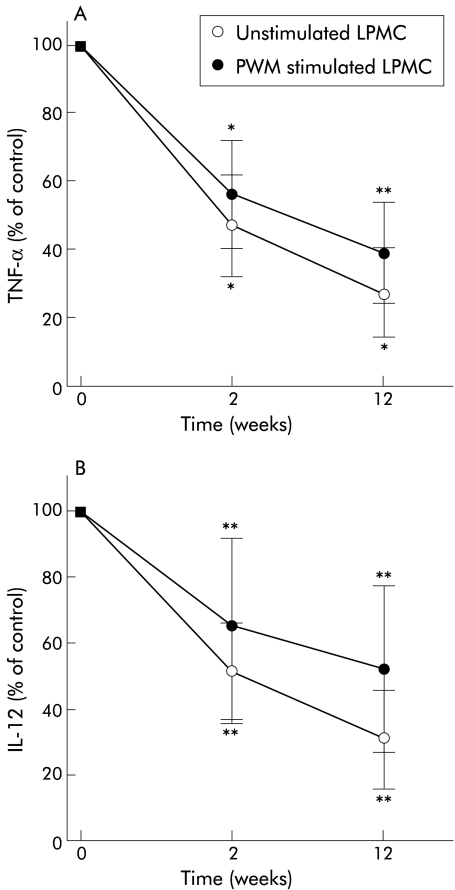
TNF-α production by stimulated and unstimulated PBMC and LPMC was studied in all seven patients (six CD, one UC) who completed the study. TNF-α production by stimulated LPMC decreased from 42.3 (8.3) pg/ml before treatment with thalidomide to 24.0 (6.8) at week 2 (p<0.02) and 16.4 (6.3) (p<0.04) at week 12 (fig 2A ▶). TNF-α in unstimulated cells decreased from 17.0 (3.9) pg/ml to 8.0 (2.5) (week 2; p<0.02) and 4.6 (2.2) (week 12; p<0.02). Similarly, production of TNF-α by PBMC decreased from 62.8 (14.6) pg/ml (unstimulated 28.8 (7.2) pg/ml) before thalidomide to 37.3 (9.9) (unstimulated 17.7 (5.6)) at week 2 (p<0.01, p<0.02), and 22.5 (9.2) (unstimulated 8.8 (4.6)) at week 12 (p<0.02). Production of IL-12 by LPMC decreased from 9.7 (3.2) pg/ml (unstimulated 7.3 (1.9)) before thalidomide to 6.3 (2.7) (unstimulated 3.7 (1.1)) after week 2 (p<0.04) and 5.0 (2.5) (unstimulated 2.3 (1.1)) after 12 weeks of thalidomide (p<0.04) (fig 2B ▶). Production of IL-1β and IL-6 did not change significantly during treatment with thalidomide. Production of IL-1β (stimulated) by LPMC was 465 (74) pg/ml before treatment versus 417 (49) after 12 weeks. Production of IL-6 (stimulated) by LPMC was 2498 (286) versus 2702 (376) after 12 weeks.
Figure 2.
Production of tumour necrosis factor α (TNF-α) (A) and interleukin 12 (IL-12) (B) by pokeweed mitogen (PWM) stimulated and unstimulated lamina propria mononuclear cells (LPMC) after treatment with thalidomide (n=7). TNF-α and IL-12 production by LPMC decreased within two weeks of treatment with thalidomide. Data show cytokine production as a percentage of levels before treatment with thalidomide: TNF-α: 42.3 (8.3) pg/ml (stimulated), 17.0 (3.9) pg/ml (unstimulated); IL-12: 9.7 (3.2) pg/ml (stimulated), 7.3 (1.9) pg/ml (unstimulated). *p<0.02, **p<0.04.
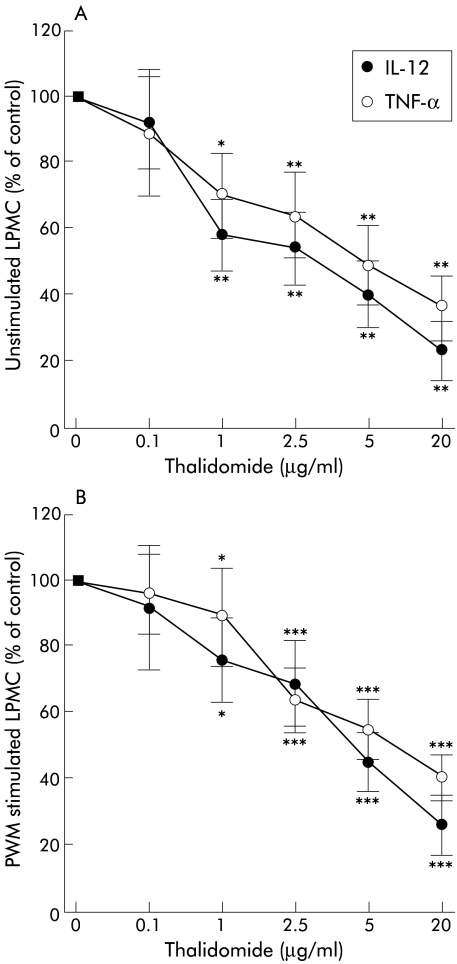
Colonic LPMC of eight patients with CD were incubated with increasing doses of thalidomide. Doses of 0.1, 1, 2.5, 5, and 20 μg were added to unstimulated and PWM stimulated 106 LPMC. Production of TNF-α and IL-12 was detectable in all supernatants without thalidomide. With increasing doses of thalidomide, levels of both TNF-α and IL-12 by unstimulated as well as PWM stimulated LPMC significantly decreased, beginning at a concentration of 1 μg/ml (fig 3 ▶). At higher doses, TNF-α and IL-12 were both strongly suppressed by thalidomide; 50% suppression of IL-12 was observed at lower doses of thalidomide than suppression of TNF-α (IC50 for IL-12 ∼ 3–4 μg/ml; IC50 for TNF-α 5–10 μg/ml). Production of IL-1β and IL-6 did not change significantly in the presence of thalidomide.
Figure 3.
Titration of lamina propria mononuclear cells (LPMC) with increasing doses of thalidomide. Inhibition by thalidomide of production of tumour necrosis factor α (TNF-α) and interleukin 12 (IL-12) was observed in both unstimulated (A) and pokeweed mitogen (PWM) stimulated LPMC (B) (n=8). Data show cytokine production as a percentage of control without thalidomide: TNF-α: 20.8 (5.0) pg/ml (unstimulated), 49.8 (6.9) pg/ml (stimulated); IL-12: 12.0 (2.0) pg/ml (unstimulated), 17.2 (3.3) pg/ml (stimulated). *p<0.04, **p<0.01, ***p<0.001 compared with controls.
Side effects
All patients reported transient fatigue. In three patients sedation was so severe that they discontinued treatment within the first two weeks of the study. Peripheral neuropathy was seen in one patient after six weeks and was completely reversible after dose reduction to 200 mg. In one of two patients, in which thalidomide therapy was continued after the 12 week study period, peripheral neuropathy developed after 36 weeks and disappeared after discontinuation of therapy. No pregnancies occurred during the study.
DISCUSSION
Recently, two open label trials on the treatment of refractory CD with thalidomide reported clinical efficacy with response rates of 64% and 70% in patients after a 12 week course.25,26 Our study showed similar results. As TNF-α is considered to be centrally involved in the inflammatory process in IBD and thalidomide has been shown to suppress TNF-α production,17 this mechanism could be responsible for its clinical efficacy. In fact, we found a strong effect of thalidomide on TNF-α production in LPMC as well as in peripheral monocytes. However, exclusive suppression of TNF-α may not be sufficient to explain the clinical improvement observed, as a recent study with pentoxifylline, another TNF-α suppressor,23 failed to demonstrate clinical improvement in refractory CD.22 Also, the effect of anti-TNF-α antibody (infliximab) is attributed not only to its direct effect on TNF-α but rather to the combination with other immunmodulating effects.25,30
To shed more light on the mechanisms by which thalidomide might work, we also investigated the potency of thalidomide in reducing other proinflammatory cytokines. We found a significant reduction in the production of IL-12 by LPMC after treatment with thalidomide.
IL-12, an immunoregulatory cytokine considered to be centrally involved in the induction of cellular immune responses, has been shown to be released by LPMC in patients with CD.5 Expression of the IL-12 receptor β2 subunit was also found to be upregulated in CD.31 Recently, an experimental animal model of colitis has been developed in which anti-IL-12 antibody treatment prevented colitis, suggesting an important role for IL-12 in the pathogenesis of intestinal inflammation.32
The effects of thalidomide on TNF-α and IL-12 seem to be rather specific as no change in IL-1β and IL-6 production was seen in our study. A decrease in these two cytokines could have been expected in accordance with reduced disease activity. In addition, suppression of TNF-α and IL-12 preceded clinical improvement; this was already present two weeks after treatment with thalidomide whereas a decrease in disease activity parameters was first noted after four weeks. In vitro incubation of LPMC with thalidomide showed a dose dependent decrease in TNF-α and IL-12 production, supporting this interpretation. It is interesting that thalidomide reduces production of two cytokines which are centrally involved in the inflammatory cascade in IBD. This combination may explain why thalidomide is more effective in these patients than other selective inhibitors of single pathways.22,23
Compared with the other open label trials recently presented,24,25 we used a relatively high dose of 300 mg of thalidomide daily, which probably accounts for the high rate of dropouts (3/10 patients) in our study due to sedative side effects. In the study of Ehrenpreis and colleagues,25 who mainly used 200 mg thalidomide daily, eight of 22 (37%) patients withdrew because of side effects or lack of perceived improvement. Vasiliauskas and colleagues26 used low dose thalidomide (50–100 mg) observing less toxicity, allowing all 12 patients to complete the 12 week study phase. Interestingly, despite the different dosage used, both groups had similar response rates (64% and 70%, respectively). Although dose dependent suppression of TNF-α by thalidomide has been demonstrated in vitro,17 only limited in vivo data are presently available. A study in microsporidiosis patients found a non-significant decrease in faecal TNF-α levels with 100 mg thalidomide daily33 whereas reduction of TNF-α in whole blood cultures was found in patients with rheumatoid arthritis treated with 100 mg thalidomide daily in combination with pentoxifylline.34 Further studies are necessary to find the optimal dose in IBD.
Taken together, all three clinical studies on thalidomide in IBD demonstrated that it is less effective than other new therapies—for example, infliximab. However, analogues of thalidomide with enhanced anti-TNF-α activity and supposed less toxic/teratogenic effects have recently been developed35,36 which may offer therapeutic options in the future. In the light of our data, it may be important to study not only effects on TNF-α but also on IL-12 for the development of such analogues.
In conclusion, our study indicates that the effects of thalidomide on TNF-α and IL-12 may explain its clinical efficacy. The cytokine suppressive properties and therapeutic potential of thalidomide and its analogues35,36 in CD should be further investigated.
Abbreviations
CAI, colitis activity index
CD, Crohn's disease
CDAI, Crohn's disease activity index
CDEIS, Crohn's disease endoscopic index of severity
IBD, inflammatory bowel disease
IL, interleukin
LPMC, lamina propria mononuclear cells
PWM, pokeweed mitogen
PBMC, peripheral blood monocytes
TNF-α, tumour necrosis factor α
REFERENCES
- 1.Mahida YR, Wu K, Jewell DP. Enhanced production of interleukin 1-β by mononuclear cells isolated from mucosa with active ulcerative colitis or Crohn's disease. Gut 1989;30:835–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinecker HC, Steffen M, Witthoet T, et al. Enhanced secretion of tumor necrosis factor-alpha, IL-6 and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol 1993;94:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDonald TT, Hutchings P, Choy MY, et al. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol 1990;81:301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isaacs KL, Sartor RB, Haeskil JS. Cytokine messenger RNA profiles in inflammatory bowel disease detected by polymerase chain reaction amplification. Gastroenterology 1992;103:1587–95. [DOI] [PubMed] [Google Scholar]
- 5.Monteleone G, Biancone L, Marasco R, et al. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology 1997;112:1169–78. [DOI] [PubMed] [Google Scholar]
- 6.Van Dullemen HM, Van Deventer SJH, Hommes DW, et al. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric antibody (cA2). Gastroenterology 1995;109:129–35. [DOI] [PubMed] [Google Scholar]
- 7.Targan SR, Hanauer SB, Van Deventer SJH, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn's disease. N Engl J Med 1997;337:1029–35. [DOI] [PubMed] [Google Scholar]
- 8.Rampart M, De Smet W, Fiers W, et al. Inflammatory properties of recombinant tumor necrosis in rabbit skin in vivo. J Exp Med 1989;169:2227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kindler V, Sappino AP, Gran GE, et al. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 1989;56:731–40. [DOI] [PubMed] [Google Scholar]
- 10.Clauss M, Ryan J, Stern D. Modulation of endothelial cell hemostatic properties by TNF: Insights into the role of endothelium in the host response to inflammatory stimuli. In: Beutler B, ed. Tumor necrosis factors: The molecules and their emerging role in medicine. New York: Raven Press, 1992:49–63.
- 11.Sun XL, Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J Clin Invest 1988;81:1328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullin JM, Snock KV. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res 1990;50:2172–6. [PubMed] [Google Scholar]
- 13.Murch SH, Lamkin VA, Savage MO, et al. Serum concentrations of release tumor necrosis factor-alpha in childhood chronic inflammatory bowel disease. Gut 1991;32:913–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braegger CP, Nicholls SW, Murch SH, et al. Tumor necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet 1992;339:89–91. [DOI] [PubMed] [Google Scholar]
- 15.Wettstein AR, Meagher AP. Thalidomide in Crohn's disease. Lancet 1997;350:1445–6. [DOI] [PubMed] [Google Scholar]
- 16.McBride WG. Thalidome and congenital abnormalities. Lancet 1961;ii:1958–60. [Google Scholar]
- 17.Sampaio EP, Sarno EN, Galilly R, et al. Thalidomide selectively inhibits tumor necosis factor alpha production by stimulated human monocytes. J Exp Med 1991;173:699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holm AL, Bowers KE, McMeekin TO, et al. Chronic cutaneous lupus erythematosus treated with thalidomide. Arch Dermatol 1993;129:1548–50. [PubMed] [Google Scholar]
- 19.Vogelsang GB, Farmer ER, Hess AD, et al. Thalidomide for the treatment of chronic graft-versus-host disease. N Engl J Med 1992;326:1055–8. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez-Rodriguez O, Starusta-Bacal P, Gutierrez-Montes O. Treatment of refractory rheumatoid arthritis—the thalidomide experience. J Rheumatol 1989;16:158–63. [PubMed] [Google Scholar]
- 21.Hamuryudan V, Mat C, Saip S, et al. Thalidomide in the treatment of the mucocutaneous lesions of the Behcet syndrome. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1998;228:443–50. [DOI] [PubMed] [Google Scholar]
- 22.Bauditz J, Haemling J, Ortner M, et al. Treatment with tumor necrosis factor inhibitor oxpentifylline does not improve steroid dependent chronic active Crohn's disease in a pilot study. Gut 1997;40:470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimund JM, Dumont S, Muller CD, et al. In vitro effects of oxpentifylline on inflammatory cytokine release in patients with inflammatory bowel disease. Gut 1997;40:475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moller DR, Wysocka M, Greenlee BM, et al. Inhibition of IL-12 production by thalidomide. J Immunol 1997;159:5157–61. [PubMed] [Google Scholar]
- 25.Ehrenpreis ED, Kane SV, Cohen LB, et al. Thalidomide therapy for patients with refractory Crohn's disease: An open-label trial. Gastroenterology 1999;117:1271–7. [DOI] [PubMed] [Google Scholar]
- 26.Vasiliauskas EA, Kam LY, Abreu-Martin MT, et al. An open-label pilot study of low-dose thalidomide in chronically-active, steroid-dependent Crohn's disease. Gastroenterology 1999;117:1278–87. [DOI] [PubMed] [Google Scholar]
- 27.Malchow H, Ewe K, Brandes JW, et al. European cooperative Crohn's disease study (ECCDS): Results of drug treatment. Gastroenterology 1984;86:249–66. [PubMed] [Google Scholar]
- 28.Rachmilewitz D. Coated mesalazine (5-aminosalicyc acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 1989;298:82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.GETAID represented by Mary JY and Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: A prospective, multicentre study. Gut 1989,30:983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scallon BJ, Moore MA, Trinh DM, et al. Chimeric anti-TNF-α monoclonal antibody cA2 binds recombinant transmembrane TNF-α and activates immune effector functions. Cytokine 1995;7:251–9. [DOI] [PubMed] [Google Scholar]
- 31.Parrello T, Monteleone G, Cucchiara S, et al. Upregulation of the IL-12 receptor β2 beta unit chain in Crohn's disease. J Immunol 2000;165:7234–9. [DOI] [PubMed] [Google Scholar]
- 32.Neurath MF, Fuss I, Kelsall BL, et al. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 1995;182:1281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharpstone D, Rowbottom A, Francis N, et al. Thalidomide: A novel therapy for microsporidiosis. Gastroenterology 1997;112:1823–9. [DOI] [PubMed] [Google Scholar]
- 34.Huizinga TWJ, Dijkmans BAC, Van der Velde EA, et al. An open study of pentoxifylline and thalidomide as adjuvant therapy in the treatment of rheumatoid arthritis. Ann Rheum Dis 1996;55:833–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marriott JB, Westby M, Cookson S, et al. CC 3052: a water-soluble analog of thalidomide and potent inhibitor of activation-induced TNF-alpha production. J Immunol 1998;161:4236–43. [PubMed] [Google Scholar]
- 36.Oliver SJ, Freeman SL, Corral LG, et al. Thalidomide analogue CC 1069 inhibits development of rat adjuvant arthritis. Clin Exp Immunol 1999;118:315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]