Abstract
Background: The histogenesis of human colorectal hyperplastic polyps and colorectal adenomas is poorly understood even now.
Method: Human colorectal adenomas, hyperplastic polyps, and normal colorectal mucosae (patients with familial adenomatous polyposis and hereditary non-polyposis colorectal carcinoma were excluded) were obtained during colonoscopy and microdissected into individual crypts. Morphology, cell proliferation characteristics, and fission indices of crypts isolated from these lesions were then studied.
Results: Crypts isolated from colorectal adenomas and colorectal hyperplastic polyps were significantly larger (p<0.001) than crypts from normal colorectal mucosae. Crypt fission was an uncommon event in normal colonic mucosae but common in crypts isolated from adenomas and hyperplastic polyps (p<0.001). Analysis of the distribution of mitoses suggested an upward expansion of the proliferation compartment in adenomas to the surface of the crypt with no reversal of proliferating cell distribution, as has previously been described.
Conclusions: Sporadic human colorectal adenomas and hyperplastic polyps grow by the process of crypt fission. Expansion of the proliferative compartment was demonstrated in crypts from adenomas, consistent with deregulation of cell cycle control.
Keywords: adenomas, hyperplastic polyps, proliferation, crypt fission, histogenesis
There is growing evidence to support the idea that accumulated genetic changes in important genes, occurring in vulnerable cells, ultimately leads to the development of neoplasia. This process is well illustrated by colorectal cancer in which there is progressive accumulation of mutations during the gradual transition from normal mucosa to carcinoma.1,2 One of the earliest mutations in colonic carcinogenesis occurs at the adenomatous polyposis coli (APC) gene.3 Hyperproliferation is believed to develop in preneoplastic epithelium, implying that an APC mutation is involved in the process. None the less, a recent study of preneoplastic intestine of human familial adenomatous polyposis (FAP) and multiple intestinal neoplasia (MIN) mice which harbour the APC mutations has shown that the major abnormality is elevated rates of crypt fission, a process that begins by basal bifurcation and is followed by longitudinal division of the crypt.4,5 In the same study, direct observations of three dimensional reconstruction of FAP microadenomas showed that both normal and atypical crypt fission events were increased in FAP and MIN adenomas and indicated that adenomas expanded mainly by the process of crypt fission.4 Crypt fission is very prominent in neonatal animals, leading to a progressive increase in crypt number in both the small and large intestine,6 and it is involved in the emergence of wholly mutated crypts from partially mutated crypts in mice after injection of carcinogens.7 The Dlb-1 locus in mice generates an intestinal binding site for the lectin Dolichos biflorus agglutinin,8 and study of the induction of mutated Dlb-1 locus in mice by N-nitroso-N-ethylurea in SWR mice showed that asymmetrical partition or segregation of the mutated progenitors is central to the process of clonal purification.9 Similarly, using FACS analysis on single cell suspensions from colonic crypts isolated from patients with longstanding ulcerative colitis, considerable areas of colonic mucosa, exceeding 9 cm in length, were colonised by the same aneuploid stem cell of the same phenotype,10,11 suggesting that mutated clones in the human colon spread by the process of crypt fission.
Crypt fission events are likely to be under the control of intestinal stem cells: the three dimensional reconstruction by Wasan et al showed that adenomatous crypts can apparently arise from normal crypts in pre-existing adenomas.4 Such a process could play a role in segregating mutated stem cell clones from normal crypt epithelium,9,12,13 and also concurs with data showing that about 75% of FAP microadenomas are polyclonal.14 Similarly, Bjerknes et al studied the replication rate of mutant crypts (aberrant crypt foci) in FAP patients and controls (non-FAP colons) and found that both FAP crypts and mutant crypts in non-FAP patients replicate at least 11 times and seven times faster than normal crypts.5 Thus the main mode of growth of colonic adenomas in FAP patients and MIN mice is by the process of crypt fission. Hereditary colonic cancers account for about 15% of all human colorectal cancers15,16 and the majority of colonic carcinomas are sporadic in nature. It is commonly believed that the colorectal adenoma is the precursor lesion of most colorectal cancer. However, the histogenesis of human sporadic colorectal adenomas remains poorly understood.
Similarly, the histogenesis of human colorectal hyperplastic polyps was under described until the study by Araki et al who examined hyperplastic polyps by scanning electron microscopy and found that branching was observed in 20% of polyps. But the accuracy of quantitative assessment in their study was limited by the fragility of the isolated crypts and the high incidence of fracture of branched crypts during the procedure.17 Thus we used a microdissection based technique to examine the crypt fission process in both sporadic colorectal adenomas and hyperplastic polyps. This method is robust, reliable, and easily reproducible.
In normal colonic mucosa, proliferating cells are predominantly located in the lower two thirds of the crypts. In vitro labelling studies showed that the pattern of labelling in hyperplastic polyps is essentially similar to that of the normal mucosa.18 However, the pattern of labelling is different in adenomas, in which DNA synthesising cells are supposedly located mainly in the upper portion of the gland suggesting an upward migration of the proliferation zone.19,20 The classical ideas on the histogenesis of colonic adenomas are due to Dukes21 who proposed that tubular adenomas arise through proliferation and lateral expansion in the basal proliferative zone of the crypts while villous adenomas arise from surface proliferation. However, others had shown that tubular adenomas arise in the superficial portion of the rectal crypts.19,22 Similarly, Lipkin showed that cell proliferation was prominent at the top of the rectal crypts, not only in the small adenomas of polyposis coli but also in the intervening morphologically normal rectal crypts, supporting an origin of adenomas from the upper crypt surface.23 However, some studies refute these claims.24,25 Based on the distribution of proliferating cells by proliferating cell nucleur antigen and Ki67 immunohistochemistry, and apoptotic cells denoted by terminal deoxyuridine nick end labelling, Moss et al controversially proposed that the main direction of cell migration in adenomas is not towards the lumen but instead cells migrate towards the polyp base.26
The use of immunohistochemical staining of cell cycle related antigens has the advantage of preserving the spatial orientation of cells but the results generated are highly dependent on prior treatment of the tissue and there are two problems associated with the use of antibody techniques. The first is the difficulty in standardising immunohistochemical techniques and in setting thresholds for scoring a cell as labelled or unlabelled. The second is related to the geometrical problem of scoring the sections, as the results may be confounded by concomitant changes in crypt size. An alternate method is to count mitotic figures or arrested metaphases in microdissected crypts. This can give similar results but is far quicker and is also more robust.27 The great advantage of such techniques is that results are expressed on a per crypt basis so that one does not need to count interphase cells. Furthermore, the geometric disadvantages associated with scoring crypt sections are avoided. Microdissection of crypts enables all the mitotic figures in the entire crypt to be scored, and all the crypts in a sample can be quantified. This is often more preferable to the several in vitro methods available for the assessment of biopsies which are labour intensive, require the immediate incubation of the tissue, and may not reflect the in vivo state.28 The microdissection method has been validated by study of human intestinal biopsy samples as well as in animal studies.27,29 Biopsies need little special treatment, apart from the use of Carnoy's solution for fixation. The tissue can then be stored in 70% alcohol for years. Crypt size and area can also be determined by the use of a drawing tube, as can the position of mitoses in the crypt. Combining all of the above factors, scoring mitoses in microdissection crypts appears to be the method of choice for the study of gastrointestinal proliferation.
Thus the aims of this study were: (1) to examine the morphology and proliferation characteristics of normal human colorectal mucosae, colorectal hyperplastic polyps, and sporadic colorectal adenomas by the microdissection method; (2) to examine the zonal distribution of mitoses in the three conditions; and (3) to determine whether crypt fission is the predominant proliferation abnormality in hyperplastic polyps and sporadic colorectal adenomas.
METHODS
Specimens
Fresh colonic polyps (adenomas and hyperplastic polyps) and normal colonic mucosa were obtained by endoscopic means during colonoscopy after informed written consent. The colonoscope used was a Pentax EC-3801 or ES 3801 (Pentax, Hong Kong Ltd) with a 3.8 mm biopsy channel. Bowel preparation for colonoscopy consisted of a 45 ml phosphodosa solution followed by 2 litres of clear fluid. The majority of colonoscopies were performed by a single experienced endoscopist (BCYW). Patients with FAP, hereditary non-polyposis colorectal cancer, or any other form of hereditary colorectal cancer were excluded. The biopsy tissues were immediately fixed in Carnoy's fixative (ethanol:acetic acid:chloroform 6:1:3) for three hours and then stored in 70% alcohol before analysis. Part of the colonic polyps were fixed in formalin, sent for routine histological examination, and classified into hyperplastic polyps or adenomas. Polyp tissues without a definite pathological diagnosis were excluded. Eleven cases of adenomas (six cases of tubular adenomas (one with mild dysplasia and five with moderate dysplasia), four cases of tubulovillous adenomas (two with mild dysplasia, one with moderate dysplasia, and one with moderate to focal severe dysplasia), and one case of villous adenoma with moderate dysplasia) and five cases of hyperplastic polyps were studied. In 14 cases, non-involved colonic mucosae 10–15 cm away from the polyp lesions were also studied. Five cases of normal colonic mucosae from patients with normal colonoscopic findings were used as normal controls.
Microdissected techniques for isolating crypts
Individual crypts were microdissected using the method described by Goodlad and colleagues.27 The tissue was taken from its storage in 70% ethanol, hydrated (in 50% ethanol for 10 minutes followed by 25% ethanol for 10 minutes), and hydrolysed in 1 M HCl at 60°C for 10 minutes. It was then stained by Feulgen reaction for at least 45 minutes. The tissue was transferred onto a slide with 45% acetic acid and gently teased apart under a dissecting microscope (at ×25 magnification). A coverslip was then placed over the wet tissue and tapped until the crypts began to separate. Microdissected crypts were examined under the compound microscope, and the outline of the crypts was traced using a Leitz drawing tube (Leica UK, Milton Keynes, UK). The number of mitoses in each gland or crypt was counted. The criteria used to define mitoses were strict and only distinct late prophases, metaphases, anaphases, and telophases were scored. Crypt size (area) was obtained by scanning the tracings with a flatbed scanner connected to an Apple Macintosh computer and the area was measured using the program Image (NIH public domain).
Measurement of crypt fission index, cell proliferation, and distribution of mitoses
Microdissected Feulgen stained crypts were counted under ×40 magnification. Mitoses were counted in at least 20 microdissected crypts per biopsy using a compound microscope at 160 magnification. By focusing up and down through the width of the crypt, a total mitotic count for each crypt was obtained. Crypts were then divided into five zones (zones 1–5, from base to top) by projecting a grid into the microscope field or view, and the distribution of mitoses per zone was recorded. Crypts with complex branching were orientated correctly before calculation of zonal mitotic count. The mean crypt area of all crypts isolated was plotted against the crypt fission index to determine the relationship between the two variables.
Statistical analysis
All results are presented as group means (SEM). The statistics used included the two sided t test using the SPSS 7.5 (Inc., 1989) package. The association between crypt fission index and crypt area was measured by calculating the Pearson correlation coefficient. A p value ≤0.05 was considered to be statistically significant.
RESULTS
Morphology of colonic crypts from uninvolved mucosa and normal controls
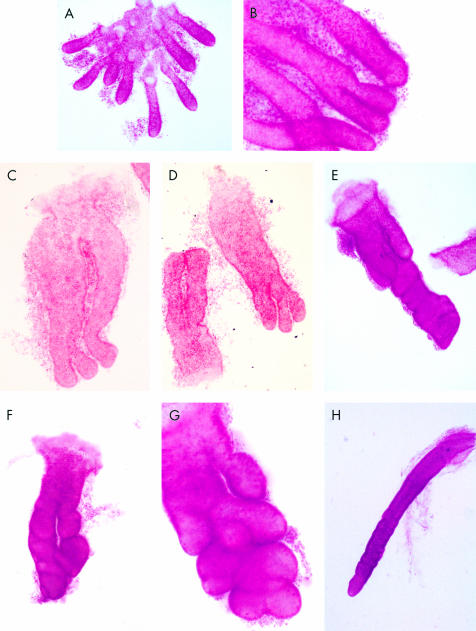
Normal colonic crypts were cylindrical in shape and mitotic figures were mainly concentrated at the base of the crypt—that is, zone 1 (fig 1A, B ▶). Crypt fission was rare and began with basal bifurcation at the base of the gland (fig 1B ▶). No asymmetrical or atypical types of branching were observed.
Figure 1.
Microdissected crypts from normal colonic mucosae, hyperplastic polyps, and adenomas. (A) Low power view of crypts isolated from normal colonic mucosae. (B) Symmetrical fission of normal colonic crypts. (C) Crypts isolated from hyperplastic polyps. Note that these crypts are larger in size and with frequent crypt branching. (D) Crypts from hyperplastic polyps with multiple branching. (E) A crypt isolated from an adenoma. Crypt fission was very frequent with atypical and asymmetrical branching. (F) Another crypt from an adenoma with bizarre shape and asymmetrical branching. (G) Multiple budding of a crypt isolated from an adenomas. (H) Markedly elongated crypt isolated from the margins of the adenoma.
Morphology of adenomas and hyperplastic polyps
The mean area of colonic crypts isolated from adenomas was significantly larger compared with uninvolved mucosae and normal controls (p<0.001) (fig 2B ▶), and significantly larger than hyperplastic polyps (p=0.001). The shapes of these crypts were irregular and asymmetrical. Multiple branching and budding were observed in a single crypt, suggesting an accelerated rate of crypt fission (fig 1E–G ▶). Fission was mostly asymmetrical as opposed to fission in normal colonic mucosae (fig 1B ▶). Superficial budding was commonly seen in contrast with normal controls in which all budding was basal. Crypts isolated from hyperplastic polyps were significantly larger than crypts isolated from non-involved mucosae (p<0.001) (fig 2B ▶). Mean crypt area of the hyperplastic polyps was also larger than that of normal controls but this difference was not statistically significant. Crypts from hyperplastic polyps appeared less irregular in shape but fission events were still very common. Crypt fission was more symmetrical in these crypts compared with adenomatous crypts and usually started from the crypt base (basal budding). Multiple fission events were observed (fig 1C, D ▶) in some of the crypts isolated. Interestingly, we also observed the presence of some very elongated crypts adjacent to adenomas (fig 1H ▶).
Figure 2.
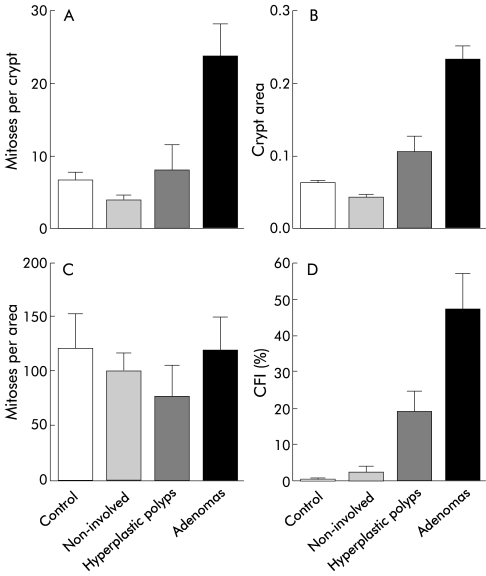
Mitoses per crypt (A), mean crypt area (B), mitoses per area (C), and crypt fission index (CFI, proportion of crypts in fission) (D) for adenomas, hyperplastic polyps, non-involved mucosae, and normal controls.
Proliferation characteristics of adenomas and hyperplastic polyps
The mean crypts counted for mitotic scores were 26.5 (1.6). Crypt mitotic scores were significantly greater in adenomas (p<0.001) and hyperplastic polyps (p=0.03) compared with non-involved mucosae (fig 2A ▶). Mitoses per crypt for adenomas were also significantly greater than for normal controls (p=0.02) (fig 2A ▶). Mean mitoses per crypt for hyperplastic polyps was greater than that for normal controls but this was not statistically significant. When the effect of size was taken into account—that is, mitoses per area—there were no differences between adenomas, hyperplastic polyps, and normal controls (fig 2C ▶).
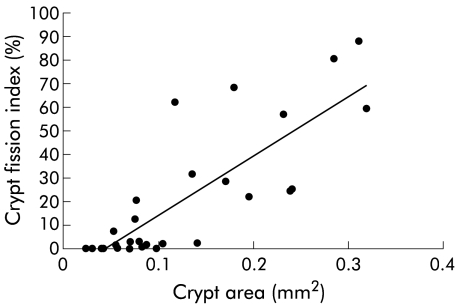
Mean crypts counted for crypt fission index per control and per adenoma were 292 (SEM 39; range 100–828) and 91 (SEM 17; range 21–202), respectively. The crypt fission index (proportion of crypts in fission) for adenomas was significantly greater than that for hyperplastic polyps (p=0.049), non-involved mucosae (p<0.001), and normal controls (p=0.003) (fig 2D ▶). The crypt fission index for hyperplastic polyps was significantly greater than that for non-involved mucosae (p<0.001) and normal controls (p=0.01) (fig 2D ▶). The correlation between crypt fission index and crypt area was 0.83 (p<0.001) (fig 3 ▶). Crypt mitotic scores were higher in adenomas with moderate dysplasia compared with adenomas with mild dysplasia (30.6 v 11.1; p=0.03) but mean crypt area, mitoses per area, and crypt fission index were similar.
Figure 3.
Relationship between crypt area and crypt fission index (r=0.83, p<0.001).
Zonal distribution of mitoses (fig 4 ▶)
Figure 4.
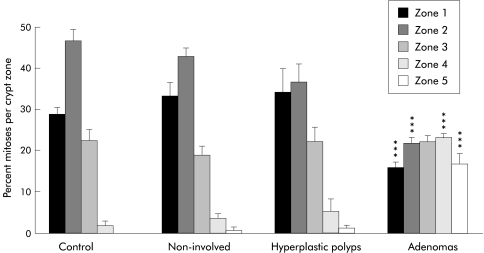
Percentage mitoses per crypt zone for adenomas, hyperplastic polyps, non-involved mucosae, and normal controls. ***p<0.001 compared with controls and non-involved mucosae.
The zonal distribution of mitoses in adenomas, hyperplastic polyps, non-involved mucosae, and normal controls is shown in fig 4 ▶. In normal colonic mucosae, the mitotic figures were mainly located in zones 1 and 2—that is, the base of the crypts. In hyperplastic polyps, mitotic figures were mainly concentrated in zones 1–3, but in adenomas mitoses were almost evenly distributed between the five zones. The mean percentage zonal mitoses of zones 1 and 2—that is, the base of the crypts—were significantly smaller in adenomas (zone 1=16%; zone 2=22%) compared with non-involved mucosae (zone 1=34%, zone 2=43%; p<0.001 for both zones) and normal controls (zone 1=29%, zone 2=47%; p<0.001 for both zones). On the other hand, mean percentage zonal mitoses of zones 4 and 5—that is, the top of the crypts—were significantly greater in adenomas (zone 4=23%, zone 5=17%) compared with non-involved mucosae (zone 4=4%, zone 5=1%; p<0.001 for both zones) and normal controls (zone 4=2%, zone 5=0.1%; p<0.001 for both zones). There was no difference in mean percentage zonal mitoses between hyperplastic polyps and normal controls for all zones (NS) but the percentage zonal mitoses of zone 4 were greater in hyperplastic polyps compared with non-involved mucosae (10% v 4%; p=0.05).
DISCUSSION
It is widely believed that colonic polyps are the precursor lesions of sporadic colonic cancer. Colonic adenomas enlarge slowly and the development of carcinoma may take decades. We have reported previously that in rats given carcinogens and in FAP colons, there was an increased rate of crypt fission. Crypt fission is also the process that leads to the evolution of monoclonal crypts after administration of carcinogens, with segregation of mutated progenitor cells, which is central to the process of clonal purification.7,9 In the flat mucosa of FAP colons, crypt fission was atypical and asymmetrical. Crypt fission is thus likely to be the main process that leads to enlargement of microadenomas. Here we have reported a microdissection study in which colonic hyperplastic polyps and adenomas grew by the process of crypt fission and was the major proliferation abnormality.
Colonic crypts isolated from both hyperplastic and adenomas were significantly larger than those from normal colonic crypts (fig 2B ▶). The crypt fission index was also significantly larger in both hyperplastic polyps and adenomas (fig 2D ▶). Our findings support the mouse studies of Totafurno and colleagues30 in which dividing crypts were generally larger (fig 3 ▶) and crypt size was therefore believed to be an important factor in initiating crypt fission. It has been proposed by Löeffler et al that crypts will divide if the number of stem cells exceeds a certain threshold, called the Sf.31 Thus it is reasonable to assume that a larger crypt will contain a greater number of stem cells and hence a greater rate of crypt fission compared with normal sized crypts. Furthermore, we also found that the proliferation characteristics of non-involved mucosae adjacent to adenomas were similar to those of normal controls. Thus hyperproliferation appears to be localised on colonic adenomas rather than a generalised and diffuse hyperproliferation of the whole colon, as previously suggested.32 Jass et al showed that there was no difference in the Ki67 labelling index between patients with hereditary non-polyposis colorectal cancer and those with some positive family history of colorectal cancer,33 and proposed that hyperproliferation is not a general phenomenon in the early stages of colonic carcinogenesis. The crypt mitotic score correlated with the degree of dysplasia but the number of case studies was small in our study. A large scale study is warranted to determine the relationship between crypt mitotic score and the severity of dysplasia.
Our findings support those of Araki et al who performed a morphological study of hyperplastic polyps in which the length of the crypts isolated from hyperplastic polyps were significantly greater than normal colonic crypts and branching of crypts was observed in 22% of isolated crypts.17 They suggested that hyperplastic polyps grow by the process of crypt fission. We found that mitoses were significantly greater in adenomas and hyperplastic polyps compared with non-involved mucosa (fig 2A ▶). Interestingly, when the effect of size was taken into account, mitoses per area were actually similar between the three types of crypts isolated (fig 2C ▶). Thus the main proliferation abnormality in both hyperplastic polyps and adenomas appears to be an increase in the rate of crypt fission.
Analysis of the distribution of mitoses (fig 4 ▶) showed that the distribution of mitoses in crypts isolated from hyperplastic polyps followed the same pattern of normal colonic crypts and was similar to previous reports.34–36 But in adenomas, mitoses were almost evenly distributed between the five zones. Thus there appears to be an upward expansion of the proliferation compartment towards the surface of the crypt in adenomas.32,37 Weibecke et al tackled the same issue by studying the mitotic distribution curves of normal mucosa, hyperplastic polyps, and adenomas.38 There was a progressive upward increase in the extent of the proliferative compartment, most marked in the tubular and villous adenomas, whereas hyperplastic polyps merely showed a minor expansion in the size of the proliferative compartment. Serial reconstruction studies showed that branching was concentrated in the upper parts of the neoplastic crypts (zones 4–5). These findings were essentially confirmed by Maskens20 who noted that branched neoplastic glands grew selectively in the upper part of the mucosa and were associated with increased cell proliferation near the apex of the bud. Multiple budding and branching near the surface of the crypt would then lead to a horizontal pressure with a fan shaped spreading, giving a typically fungiform appearance. Thus we propose that the markedly increased atypical and asymmetrical crypt fission near the surface of the crypt and the associated elevated proliferation activity probably account for the observed upward migration of the proliferation compartment. Furthermore, earlier micro-reconstruction studies of adenomas may have included admixed normal crypts and hence may have given a false impression of low proliferative activity in lower crypt compartments. From the analysis of the zonal distribution of mitoses, proliferating cells were evenly distributed among the five zones in contrast with the reverse proliferating cell distribution previously described by Moss and colleagues.26 A cell kinetics study may still be warranted to study the direction of cell migration and thus refute the hypothesis of Moss et al.
We also observed the presence of very elongated crypts at the margins of adenomatous polyps with features resembling those of the transitional mucosa described previously.39–41 Bjerknes and Cheng found greatly elongated crypts at the margins of adenomas from MIN mice and these crypts had normal APC function.42 Our findings correlate well with the mouse data of Bjerknes and Cheng. It is reasonable to assume that these elongated crypts isolated in our study also have preserved APC function. However, the exact mechanism for the marked enlargement of these crypts is still uncertain.
In conclusion, sporadic human colorectal adenomas and hyperplastic polyps grow by the process of crypt fission. Expansion of the proliferative compartment was demonstrated in crypts from adenomas, consistent with deregulation of cell cycle control.
Note added in proof
A recent article by Shih et al (Proc Natl Acad Sci USA 2001;98:2640–5) has suggested that adenomas grow by a “top-down morphogenesis,” taking origin from mutant stem cells on the surface of the colonic mucosa, and the adenomatous epithelium grows downwards to occupy adjacent crypts. This concept diverges markedly from the present study, which indicates that adenomas grow, in the main, by crypt fission. The debate also extends into how clonal patches of dysplasia spread in the colon in ulcerative colitis—“top down” by lateral migration or “bottom up” by crypt fission, or both? Which mode of morphogenesis prevails will have considerable implications for stem cell biology in the gut.
Acknowledgments
We thank Nurse Specialist M Chong, endoscopy nurses KW Wong, VSY Tang, MY Lee, KK Chang, YC Fan, and HS Lee for their nursing assistance, and Fiona Fung, Stanley Yeung, and Samuel Law for technical assistance.
This work was supported by the ICRF and Croucher Foundation Fellowship to WMW and CNPq to SBG
Abbreviations
APC gene, adenomatous polyposis coli gene
FAP, familial adenomatous polyposis
MIN, multiple intestinal neoplasia
REFERENCES
- 1.Morson BC. Genesis of colorectal cancer. In: Sherlock P, Zamchek N, eds. Cancer of the gastrointestinal tract. Clinics in gastroenterology, vol 5. Eastbourne: Saunders, 1976:505–25. [PubMed]
- 2.Fearon ER, Vogelstein B. A genetic model of colorectal tumorigenesis. Cell 1990;61:759–67. [DOI] [PubMed] [Google Scholar]
- 3.Powell SM, Zilz N, Beazer BY, et al. APC mutations occur early during colorectal tumorigenesis. Nature 1992;359:235–7. [DOI] [PubMed] [Google Scholar]
- 4.Wasan HS, Park HS, Liu KC, et al. APC in the regulation of intestinal crypt fission. J Pathol 1998;185:246–55. [DOI] [PubMed] [Google Scholar]
- 5.Bjerknes M, Cheng H, Hay K, et al. APC mutation and the crypt cycle in murine and human intestine. Am J Pathol 1997;150:833–9. [PMC free article] [PubMed] [Google Scholar]
- 6.St Clair WH, Osborne JW. Crypt fission and crypt number in the small and large bowel of postnatal rats. Cell Tissue Kinet 1985;18:255–62. [DOI] [PubMed] [Google Scholar]
- 7.Park HS, Goodlad RA, Wright NA. Crypt fission in the small intestine and colon: A mechanism for the emergence of G6PD locus-mutated crypts after treatment with mutagens. Am J Pathol 1995;147:1416–27. [PMC free article] [PubMed] [Google Scholar]
- 8.Winton DJ, Blount MA, Ponder BAJ. A clonal marker induced by mutation in mouse intestinal epithelium. Nature 988;333:463–6. [DOI] [PubMed]
- 9.Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 1999;116:7–14. [DOI] [PubMed] [Google Scholar]
- 10.Levin DS, Rabinovitch PS, Haggit RC, et al. Distribution of aneuploid cell populations in ulcerative colitis with dysplasia or cancer. Gastroenterology 1991;101:1198–210. [DOI] [PubMed] [Google Scholar]
- 11.Burmer GC, Rabinovitch PS, Haggit RC, et al. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology 1992;103:1602–10. [DOI] [PubMed] [Google Scholar]
- 12.Ponder BA, Wilkinson MM. Direct examination of the clonality of carcinogen-induced colonic epithelial dysplasia in chimeric mice. J Natl Cancer Inst 1986;77:967–76. [PubMed] [Google Scholar]
- 13.Chang WW. Morphological basis of multistep process in experimental colonic carcinogenesis. Virchows Arch B (Cell Pathol Incl Mol Pathol) 1982;41:17–37. [DOI] [PubMed] [Google Scholar]
- 14.Novelli MR, Williamson JA, Tomlinson IP, et al. Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science 1996;272:1187–90. [DOI] [PubMed] [Google Scholar]
- 15.Cannon-Albright LA, Skolnick MH, Bishop DT, et al. Common inheritance of susceptibility to colonic adenomatous polyps and associated colorectal cancers. N Engl J Med 1988;319:533–7. [DOI] [PubMed] [Google Scholar]
- 16.Houlston RS, Collins A, Slack J, et al. Dominant genes for colorectal cancer are not rare. Ann Hum Genet 1992;56:99–103. [DOI] [PubMed] [Google Scholar]
- 17.Araki K, Ogata T, Kobayashi M, et al. A morphological study on the histogenesis of human colorectal polyps. Gastroenterology 1995;109:1468–74. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Yatari R, Apostol J, et al. Pathogenesis of hyperplastic polyps of the colon; a hypothesis based on ultrastructure and in-vitro kinetics. Gastroenterology 1974;66:347–56. [PubMed] [Google Scholar]
- 19.Cole JW, McKalen A. Studies on the morphogenesis of adenomatous polyp in the human colon. Cancer 1963;16:998–1002. [DOI] [PubMed] [Google Scholar]
- 20.Maskens AP. Histogenesis of adenomatous polyps in the human large intestine. Gastroenterology 1979;77:1245–51. [PubMed] [Google Scholar]
- 21.Dukes CT. Explanation of differences between papilloma and adenoma of the rectum. Proc R Soc Med 1947;40:829–30. [PMC free article] [PubMed] [Google Scholar]
- 22.Valdes-Dapena A, Beckfield WJ. Adenomatous polyps of the large intestine: pathology and histogenesis. Gastroenterology 1957;32:452–61. [PubMed] [Google Scholar]
- 23.Lipkin M. Phase I and phase 2 proliferative lesions of colonic epithelial cells in premalignant diseases leading to colonic cancer. Cancer 1974;34:878–88. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S, Kino I, Baba S. Cell kinetics analysis of background colonic mucosa of patients with intestinal neoplasm by ex vivo autoradiography. Gut 1988;29:4280–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura S, Kino I, Baba S. Nuclear DNA content of isolated crypts of background colonic mucosa from patients with familial adenomatous polyposis and sporadic colorectal cancer. Gut 1993;34:1240–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss SF, Liu TC, Petrotos A, et al. Inward growth of colonic adenomatous polyps. Gastroenterology 1996;111:1425–32. [DOI] [PubMed] [Google Scholar]
- 27.Goodlad RA, Levi S, Lee CY, et al. Morphometry and cell proliferation in endoscopic biopsies: evaluation of a technique. Gastroenterology 1991;101:1235–41. [DOI] [PubMed] [Google Scholar]
- 28.Wanders SL, ten Kate J, van der Linden E, et al. Does ex vivo labelling of proliferating cells in colonic and vaginal mucosa reflect the S-phase fraction in vivo? Histochemistry 1992;98:267–70. [DOI] [PubMed] [Google Scholar]
- 29.Millis SJ, Mathers JC, Chapman PD, et al. Colonic crypt cell proliferation state assessed by whole crypt microdissection in sporadic neoplasia and familial adenomatous polyposis. Gut 2001;48:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Totafurno J, Bjerknes M, Cheng H. The crypt cycle: crypt and villus production adult intestinal epithelium. Biophys J 1987;52:279–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Löeffler M, Grossman B. A stochastic branching model with formation of subunits applied to the growth of intestinal crypts. J Theor Biol 1991;150:175–91. [DOI] [PubMed] [Google Scholar]
- 32.Ponz de Leon M, Roncucci L, Di Donato P, et al. Pattern of epithelial cell proliferation in colorectal mucosa of normal subjects and of patients with adenomatous polyps or cancer of the large bowel. Cancer Res 1988;48:4121–6. [PubMed] [Google Scholar]
- 33.Jass JR, Ajioka Y, Radojkovic M, et al. Failure to detect colonic mucosal hyperproliferation in mutation positive members of a family with hereditary non-polyposis colorectal cancer. Histopathology 1997;30:201–7. [DOI] [PubMed] [Google Scholar]
- 34.Maskens AP. Distribution du compartiment prolifératif dans la muqueuse recto-collique normale, prénéoplasique et néoplasique. Acta Gastroenterol Belg 1978;41:226–40. [PubMed] [Google Scholar]
- 35.Lane N, Kaplan H, Pascal RR. Minute adenomatous and hyperplastic polyps of the colon: divergent patterns of epithelial growth with specific associated mesenchymal changes. Gastroenterology 1971;60:537–51. [PubMed] [Google Scholar]
- 36.Hayashi T, Yatani R, Apostol J, et al. Pathogenesis of hyperplastic polyps of the colon: a hypothesis based on ultrastructure and in vitro cell kinetics. Gastroenterology 1974;66:347–56. [PubMed] [Google Scholar]
- 37.Shpitz B, Bomstein Y, Mekori Y, et al. Proliferating cell nuclear antigen as a marker of cell kinetics in aberrant crypt foci, hyperplastic polyps, adenomas, and adenocarcinomas of the human colon. Am J Surg 1997;174:425–30. [DOI] [PubMed] [Google Scholar]
- 38.Wiebecke B, Brandts A, Eder M. Epithelial proliferation and morphogenesis of hyperplastic, adenomatous and villus polyps of the human colon. Virchows Arch Path A 1974;364:35–49. [DOI] [PubMed] [Google Scholar]
- 39.Dawson PA, Filipe MI. An ultrastructural and histochemical study of the mucous membrane adjacent to and remote from carcinoma of the colon. Cancer 1976;37:2388–98. [DOI] [PubMed] [Google Scholar]
- 40.Riddell RH, Levin B. Ultrastructure of the “transitional” mucosa adjacent to large bowel carcinoma. Cancer 1977;40(suppl 5):2509–22. [DOI] [PubMed] [Google Scholar]
- 41.Listinsky CM, Riddell RH. Patterns of mucin secretion in neoplastic and non-neoplastic diseases of the colon. Hum Pathol 1981;12:923–9. [DOI] [PubMed] [Google Scholar]
- 42.Bjerknes M, Cheng H. Colossal crypts bordering colon adenomas in APCmin mice express full-length APC. Am J Pathol 1999;154:1831–4. [DOI] [PMC free article] [PubMed] [Google Scholar]