Abstract
Background: Crohn's disease is associated with deranged intestinal permeability in vivo, suggesting dysfunction of tight junctions. The luminal contents are important for development of neoinflammation following resection. Regulation of tight junctions by luminal factors has not previously been studied in Crohn's disease.
Aims: The aim of the study was to investigate the effects of a luminal stimulus, known to affect tight junctions, on the distal ileum in patients with Crohn's disease.
Patients: Surgical specimens from the distal ileum of patients with Crohn's disease (n=12) were studied, and ileal specimens from colon cancer patients (n=13) served as controls.
Methods: Mucosal permeability to 51Cr-EDTA and electrical resistance were studied in Ussing chambers during luminal exposure to sodium caprate (a constituent of milk fat, affecting tight junctions) or to buffer only. The mechanisms involved were studied by mucosal ATP levels, and by electron and confocal microscopy.
Results: Baseline permeability was the same in non-inflamed ileum of Crohn's disease and controls. Sodium caprate induced a rapid increase in paracellular permeability—that is, increased permeation of 51Cr-EDTA and decreased electrical resistance—which was more pronounced in non-inflamed ileum of Crohn's disease, and electron microscopy showed dilatations within the tight junctions. Moreover, sodium caprate induced disassembly of perijunctional filamentous actin was more pronounced in Crohn's disease mucosa. Mucosal permeability changes were accompanied by mitochondrial swelling and a fall in epithelial ATP content, suggesting uncoupling of oxidative phosphorylation.
Conclusions: The tight junctions in the non-inflamed distal ileum of Crohn's disease were more reactive to luminal stimuli, possibly mediated via disturbed cytoskeletal contractility. This could contribute to the development of mucosal neoinflammation in Crohn's disease.
Keywords: actin, confocal microscopy, inflammatory bowel disease, tight junctions, permeability
Crohn's disease (CD) is associated with increased intestinal permeability.1, 2 Although the first report of increased permeability in relatives3 could not be confirmed,4 it has now been established that subgroups of first degree relatives of patients with CD have disturbed barrier function.5, 6 However, little is known of the passage routes and mechanisms involved and how this contributes to intestinal inflammation.7, 8 We recently reported that increased epithelial permeability to proteins precedes ileal inflammation in CD,9 suggesting barrier dysfunction as an early event in mucosal inflammation.
The tight junctions (TJs) are dynamic structures that are rate limiting for passive absorption of hydrophilic molecules in the intestine,10, 11 and should thereby determine intestinal permeability.8, 11 Small irregularities in TJ strand organisation have been found in non-inflamed ileal mucosa from CD patients12 but the pathophysiological significance of this is unclear. Although CD has been proposed as a disorder of the TJs,13 their functional properties and regulation have not previously been studied in CD patients.
The luminal contents are important for the development of intestinal inflammation in CD.14 Bowel rest or elemental diet may induce clinical remission,14 and exclusion of the faecal stream prevents ileal anastomotic recurrence after resection.15 Recently it has been shown that CD patients and their relatives have an augmented increase in intestinal permeability following ingestion of acetylsalicylic acid,16, 17 suggesting vulnerability of the intestinal mucosa to luminal stimuli. Sodium caprate (C10) is the sodium salt of capric acid which constitutes 2–3% of fatty acids in dairy products.18 Luminal C10 increases paracellular permeability in vivo in animals without damaging the intestinal mucosa,19 and C10 used in suppositories enhances rectal absorption of hydrophilic drugs in humans.20 In previous studies, we have shown that luminal exposure to C10 reversibly affects the permeability of intestinal TJs.21, 22 Hence, C10 should be a suitable model for the influence of luminal factors on intestinal TJs.
The aim of the study was to investigate the effects of luminal stimuli on TJs in the ileum of CD. Ileal mucosa from patients with and without CD were studied in Ussing chambers with regard to the effects of C10 on paracellular permeability and electrophysiology, and the mechanisms involved were studied by transmission electron microscopy (TEM) and confocal microscopy (CLSM), and by examining the energy status of the mucosa.
METHODS
Patients and ethics
The study comprised 12 patients (six men) undergoing elective surgery for ileal or ileocolic CD (eight primary resection, four re-resection), aged 37 (range 20–63) years and with a CD activity index of 240 (range 110–360). Eight patients were on maintenance treatment with corticosteroids, one with azathioprine. Thirteen patients (six men) undergoing right hemicolectomy for colon cancer, aged 71 (range 52–85) years, served as controls. The colon cancer patients had no evidence of generalised disease. No patient had received preoperative chemotherapy or radiotherapy. In the first set of experiments, distal ileal (within 50 cm of the ileocaecal junction) specimens without macroscopic disease from seven CD patients and eight colon cancer patients were investigated with regard to paracellular permeability and electrophysiology of the mucosa, and epithelial energy status. Three to seven months after surgery, all CD patients were subjected to endoscopic follow up (ileocolonoscopy) for evaluation of recurrent inflammation in the anastomosis. Endoscopic findings in the neoterminal ileum were scored according to Rutgeerts' classification.23 In the second set of experiments, to study the mechanisms involved, non-inflamed distal ileal specimens from five CD patients and five colon cancer patients were investigated with regard to the ultrastructure of the enterocyte TJs and mitochondria, as well as epithelial filamentous actin (F-actin) distribution by CLSM. The study was approved by the Ethics Committee, Faculty of Health Sciences, Linköping University, and was conducted according to the Declaration of Helsinki.
Ussing chamber experiments
Segments of the distal ileum (5 cm) were obtained at operation and mounted in modified Ussing chambers21 (Precision Instrument Design, Los Altos, California, USA) with an exposed tissue surface area of 1.78 cm2, as previously described.24 Only specimens with a normal appearance macroscopically, and as assessed by dissecting microscope (5× magnification), were included in the studies.
Electrical measurements
For determination of transepithelial potential difference (PDt), transepithelial resistance (TER), and short circuit current (Isc), a four electrode system was used.25 A PDt value of 6 mV or more at the start has previously been shown to be a sign of tissue viability,25 and was a prerequisite for inclusion of specimens.
Procedure
After mounting the mucosal specimens, reservoirs were filled with 5 ml of Krebs-Ringer buffer (KRB). Temperature was maintained at 37°C by a heating block. KRB was oxygenated with O2/CO2 (95/5%) and circulated by gas lift. After a 40 minute equilibration period to achieve steady state for the electrophysiological variables, 51Cr-EDTA was added to the mucosal side of the specimens in one of three different solutions: (1) KRB; (2) Ca2+ free KRB (vehicle); or (3) C10 in Ca2+ free KRB (C10). After 10 minutes the experiments were continued with KRB without C10 in all groups. Transmucosal permeation of 51Cr-EDTA was studied during the period of exposure to C10 or vehicle (0–15 minutes) and presented as transmucosal flux (pmol/cm2/h). Specimens exposed to Ca2+ free and Ca2+ containing buffer were equal in all studied variables; these results are pooled and termed vehicle experiments. In the second set of experiments, specimens were taken for TEM, CLSM, and analysis of energy status.
Chemicals and analyses
Krebs-Ringer bicarbonate buffer
The modified KRB, containing NaCl 110.0, CaCl2 3.0, KCl 5.5, KH2PO4 1.4, NaHCO3 29.0, Na pyruvate 5.7, Na fumarate 7.0, Na glutamate 5.7, and glucose 13.4 mM, was adjusted to pH 7.4 and equilibrated with O2/CO2 (95/5%) before use.
51Cr-EDTA
51Cr-EDTA (Du Pont, Dreieich, Germany) was used at a concentration of 0.13 μM, and its permeation determined by gamma counting (1282 Compugamma, LKB, Bromma, Sweden).
Sodium caprate (C10)
An incubation period of 10 minutes with C10 (Sigma, St Louis, Missouri, USA) (10 mM) on the mucosal side was used. Ca2+ was omitted from the mucosal side to avoid precipitation of the Ca2+ salt of C10.21 Depletion of Ca2+ on the mucosal side does not affect the integrity of epithelia as long as normal concentrations are maintained on the serosal side.21, 26 The chosen concentration induced increased paracellular permeability in our previous study of rat ileum,22 and is equivalent to the amount of capric acid in cow's milk with a 3% fat content.18
ATP, ADP, AMP analysis
Specimens obtained at operation, after equilibration, and 15 minutes after the start of the experiment were frozen in liquid nitrogen, stored at −70°C, and subsequently freeze dried. At analysis, the mucosa was dissected free of connective tissue and adenosine phosphates were extracted from 5 mg of ground freeze dried mucosa from each specimen and measured fluorometrically by enzymatic methods modified from Harris and colleagues.27 Data are presented as ATP levels, and as the energy charge potential (ECP). ECP gives the relative amounts of the adenosine phosphates in the cell, ECP=ATP+0.5ADP/ATP+ADP+AMP, which is a better estimate of the accessible energy supply.
Histology
Light microscopy
Samples taken from the margins of the resected bowel and from each specimen studied in the Ussing chamber were fixed in 4% formaldehyde, embedded in paraffin, sectioned, stained with haematoxylin-eosin, and subsequently reviewed histopathologically, with data on type of experiments and diagnosis blinded to the pathologist (LF). Assessment was made for lymphocyte infiltration, polymorphonuclear leucocyte infiltration, mucosal atrophy, and mucosal oedema, using semiquantitative scales: 0, normal appearance; 1, mild changes; 2, moderate changes; and 3, severe changes. Both macroscopic and microscopic appearance had to have no signs of inflammation for the specimens to be assigned to the non-inflamed group. In the inflamed group, all specimens had an intact mucosa—that is, no ulcers or aphthous lesions. Ten of the 12 inflamed specimens were scored 1 and 2/12 were scored 2 for inflammation.
Transmission electron microscopy (TEM)
Ileal specimens from three colon cancer patients and from non-inflamed ileum of three CD patients were studied. The mucosal specimens were fixed in 2% glutaraldehyde, postfixed in 1% osmium tetroxide, stained in 1% uranyl acetate en bloc, and embedded in Epon. Thin sections were stained with lead citrate, and examined with a JEOL 1200-EX/II transmission electron microscope at 80 kV. To evaluate changes in TJ morphology, the junctional regions of two randomly chosen villi were examined in each specimen. For morphometric assessment of mitochondria, photomicrographs were taken of every sixth epithelial cell with a Gatan BioScan CCD (Gatan, California, USA), and these were analysed with NIH Image 1.61 for Macintosh (available free of charge at http://rsb.info.nih.gov/nih-image/). In each cell the surface area of all identified mitochondria apical to the cell nucleus was measured, and the median value was calculated. Data on experiments and diagnosis were blinded to the examiners (TL and JDS).
Confocal laser scanning microscopy (CLSM)
F-actin distribution in the enterocytes was studied by CLSM in non-inflamed ileal mucosa specimens from three of the patients with Crohn's disease and from four of the colon cancer patients. Experiments were performed as above. Specimens were fixed in Ussing chambers with 4% formaldehyde in phosphate buffered saline, and subsequently labelled with rhodamine-phalloidin (1 μg/ml) to visualise F-actin. Phalloidin binds to and stabilises F-actin. The specimens were studied in a Sarastro 2000 confocal laser scanning microscope (Molecular Dynamics, Sunnyvale, California, USA) with Image Space software (Molecular Dynamics), based on a Nikon Optiphot microscope with a 60× oil immersion objective (NA 1.4). The 514 nm line (green light) from the argon laser was used for excitation of rhodamine. In each specimen 6–8 randomly chosen areas were sectioned, and in each area confocal sections were made at the apex, at the intermediate level, and at the base of the enterocytes. Data on experiments and diagnosis were blinded to the examiners (KHP and JDS).
Statistics
Data are presented as median (25–75th interquartile range). Comparisons between groups were made with the Mann-Whitney U test and Fisher's exact test, and the Wilcoxon's test was used for paired comparisons. Linear regression and Spearman's rank correlation coefficient were used to study correlations between parameters. Differences with p<0.05 were considered significant
RESULTS
Characterisation of specimens by electrophysiology and histology
All included specimens were normal macroscopically. PDt at the start of the experiments was above 6 mV in 27/30 (90%) specimens of non-inflamed mucosa (normal light microscopy assessment) from CD patients, in 8/12 (67%) specimens from inflamed (mild-moderate inflammation on light microscopy) CD mucosa, and in 41/45 (91%) of the specimens from colon cancer patients. This yielded for the first set of experiments: 15 non-inflamed specimens from five different patients with CD; eight inflamed specimens from four CD patients; and 27 specimens from eight patients with colon cancer; and for the second set of experiments: 12 non-inflamed specimens from five different patients with CD; and 14 specimens from five patients with colon cancer. Table 1 ▶ shows PDt, TER, and Isc values at the start of the experiments. There were no differences between the groups regarding baseline electrophysiology of the ileal mucosa. During the main study period, 0–15 minutes, PDt remained at 93 (91–98)% of initial values, with no differences between the study groups. At 45 minutes, the non-inflamed specimens from CD and cancer coli were the same at 90 (82–97)% whereas inflamed CD mucosa had a significantly lower PDt, 81 (79–85)% of initial values (p<0.05 v non-inflamed). During exposure to C10, PDt fell to approximately 30% of initial values with partial recovery after washout only in non-inflamed specimens.
Table 1.
Baseline electrophysiological variables of ileal mucosa in Ussing chambers
| Colon cancer (n=13) | CD non-inflamed (n=10) | CD inflamed (n=4) | |
| PDt (mV) | −9.7 (−7.8 to −10.9) | −11.1 (−8.3 to −12.2) | −10.4 (−8.8 to −13.0) |
| TER (Ωcm2) | 33.7 (29.0–35.9) | 33.5 (30.0–40.1) | 34.1 (30.0–38.8) |
| Isc (μA/cm2) | 269 (236–321) | 297 (229–400) | 273 (256–318) |
Transepithelial potential difference (PDt), transepithelial electrical resistance (TER), and short circuit current (Isc) at the start of the experiments in specimens from patients with cancer coli, non-inflamed specimens from patients with Crohn's disease (CD), and in inflamed specimens from patients with CD.
Data are median (25–75th interquartile range) after equilibration for 40 minutes in Ussing chambers.
There were no significant differences between groups with regard to baseline electrophysiological variables.
Epithelial permeability
TER
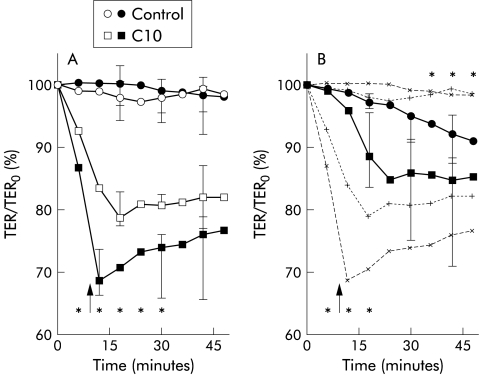
During vehicle experiments, TER in the ileal mucosa was stable in the cancer coli and non-inflamed CD (fig 1A ▶) but showed a significant fall with time in inflamed CD mucosa (fig 1B ▶).
Figure 1.
Electrical resistance in human ileal mucosa. (A) Electrical resistance (TER) during vehicle (control) experiments and during exposure to sodium caprate (C10) in mucosa from colon cancer patients (n=8; open symbols) and non-inflamed mucosa from Crohn's disease patients (n=5; filled symbols) mounted in Ussing chambers. *p<0.05 between groups, Mann-Whitney. (B) TER in inflamed mucosa from patients with Crohn's disease (n=4; filled symbols) mounted in Ussing chambers. Broken lines indicate the curves of non-inflamed mucosa from Crohn's disease patients and colon cancer patients in (A). *p<0.05 compared with non-inflamed mucosa, Mann-Whitney. The variables are expressed as per cent of initial values (median and 25–75th interquartile range). Washout of vehicle and C10 was performed at 10 minutes (arrows).
C10 10 mM induced a rapid decrease in TER in non-inflamed mucosa and the effects were partly reversed after washout (fig 1A ▶). The fall in TER was more rapid and more pronounced in non-inflamed CD mucosa than in cancer coli (fig 1A ▶). In inflamed specimens, the C10 induced effects on TER were slower and less pronounced than in the non-inflamed groups, with a continued fall after washout (fig 1B ▶).
51Cr-EDTA flux
Ileal permeability to 51Cr-EDTA showed no differences between non-inflamed CD mucosa and cancer coli in vehicle experiments (table 2 ▶) whereas 51Cr-EDTA flux was increased in inflamed mucosa compared with cancer coli (p<0.05) (table 2 ▶).
Table 2.
Transmucosal flux of 51Cr-EDTA in ileal mucosa in Ussing chambers
| Colon cancer (n=8) | CD non-inflamed (n=5) | CD inflamed (n=4) | |
| Vehicle experiments | 3.5 (1.0–5.0) | 5.8 (2.7–7.7) | 10 (6.0–17)* |
| C10 experiments | 15 (9.8–28) | 40 (36–48)* | 9.0 (3.0–15) |
Data are given for the period of exposure to vehicle or sodium caprate (C10) as median (25–75th interquartile range) of transmucosal flux of 51Cr-EDTA (pmol/cm2/h).
*Increased permeability compared with ileal mucosa from patients with colon cancer (Mann-Whitney, p<0.05).
In parallel with changes in TER, the C10 induced increase in 51Cr-EDTA flux was augmented in non-inflamed CD mucosa compared with cancer coli (table 2 ▶). Permeation of 51Cr-EDTA during the exposure period was correlated with the fall in TER (per cent of initial TER at 12 minutes), with r=0.76 (n=24, p<0.001). In inflamed specimens, there was no increase in 51Cr-EDTA flux by C10.
Tight junctions and F-actin
TEM findings in the TJ region of ileal specimens are shown in fig 2 ▶. In C10 experiments, dilatations were observed in 37% (154/418) of the TJs compared with 5% (20/415) in vehicle experiments (p<0.001, Fisher's exact), with no differences between non-inflamed CD and colon cancer.
Figure 2.
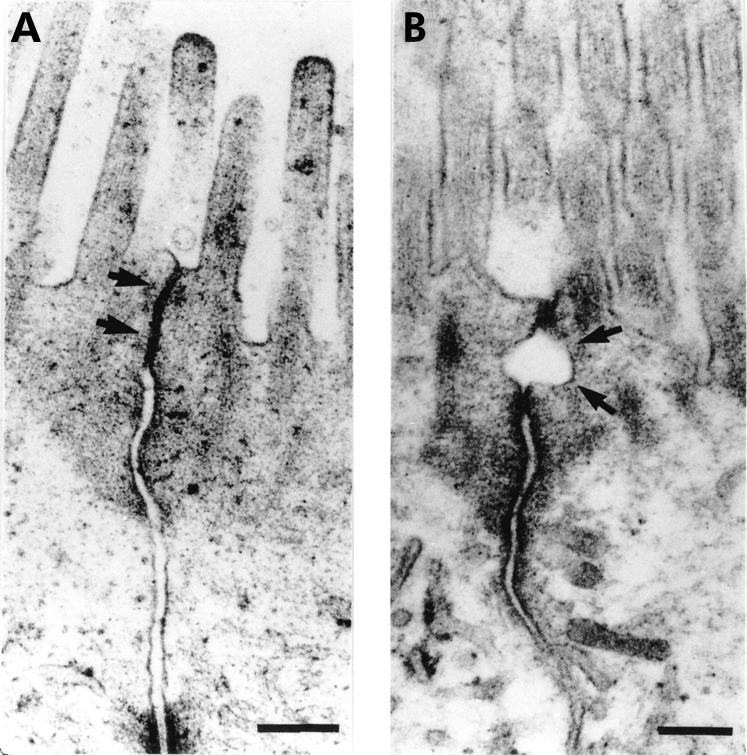
Transmission electron micrographs of the tight junction region in ileal enterocytes. Specimens were fixed after exposure to vehicle or sodium caprate (C10) in Ussing chambers. (A) Cell membranes of adjacent cells in close apposition in a tight junction exposed to vehicle (arrows). (B) Tight junction with dilatation (arrows) after exposure to C10. Bars indicate 0.2 μm. C10 induced an increased frequency of dilatations within tight junctions (37%) versus vehicle experiments (5%).
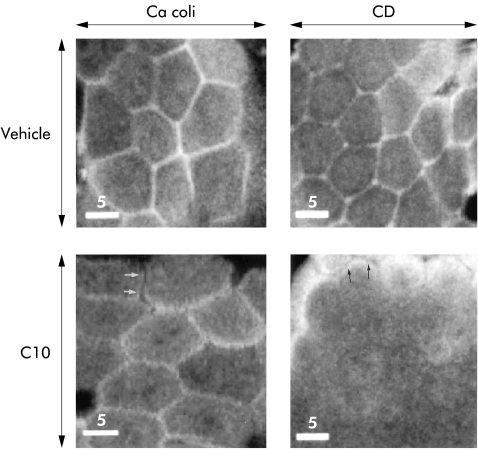
Rhodamine-phalloidin labelled F-actin was visualised by confocal microscopy as a uniformly distributed honeycomb pattern in vehicle experiments, and altered its structure and distribution in the C10 exposed specimens (fig 3 ▶). The reaction patterns in non-inflamed CD and cancer coli differed. In cancer coli specimens (n=7 specimens from four patients) exposed to C10, F-actin showed a more fragmented appearance compared with vehicle experiments (fig 3 ▶, lower left). In the non-inflamed ileal CD specimens (n=6 specimens from three patients) exposed to C10, the disassembly of F-actin at the junctional level of the cells was more pronounced (fig 3 ▶, lower right), and F-actin could only be visualised in patches in small areas of the epithelium.
Figure 3.
Perijunctional filamentous (F)-actin distribution in human ileal mucosa. Confocal en face sectioning at the apical level of enterocytes in the villus region in specimens stained with rhodamine-phalloidin. Graphs show specimens from a colon cancer patient (left) and non-inflamed mucosa from a Crohn's disease patient (right) after vehicle experiments (Vehicle; top) and after exposure to sodium caprate (C10; bottom). In the vehicle experiments, both groups showed F-actin arrayed in perijunctional rings. C10 exposed specimens demonstrated reorganisation of F-actin with marked differences in specimens from colon cancer patients and Crohn's disease patients, respectively. In cancer coli specimens (lower left), a more fragmented appearance of the perijunctional rings was seen, with occasional separation of the actin in adjacent cells (arrows). In non-inflamed ileum from Crohn's disease, F-actin staining was diminished at the junctional level (lower right), with staining seen only in patchy small areas. At the level of the microvilli, the F-actin from adjacent cells was separated after C10 exposure (arrows). Bars indicate 5 μm.
Mitochondrial structure and energy production
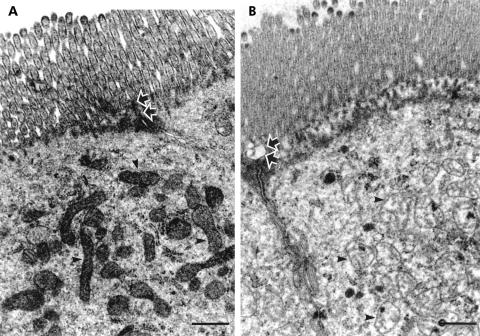
Figure 4 ▶ shows the apical region of ileal enterocytes with a large number of mitochondria. C10 exposed specimens showed an increase in mitochondrial size, with a median area of 0.11 (0.08–0.13) μm2 compared with 0.06 (0.05–0.07) μm2 in vehicle experiments (p<0.05, Mann-Whitney), with no differences between non-inflamed ileum of CD and colon cancer.
Figure 4.
Representative transmission electron micrographs of the apical part of ileal enterocytes. Specimens were fixed after exposure to vehicle or sodium caprate (C10) in Ussing chambers. (A) Enterocyte exposed to vehicle with normal mitochondria (arrowheads) and unaffected tight junction region (arrows). (B) Swollen mitochondria with derangement of the cristae (arrowheads) and a dilated tight junction (arrows) after exposure to C10. Bars indicate 0.5 μm.
Epithelial ATP concentrations and ECP during the experimental period are shown in table 3 ▶. There were no differences between the three groups in initial levels. In vehicle experiments, no changes occurred between 0 and 15 minutes in non-inflamed CD and colon cancer whereas epithelial ATP concentrations and ECP fell in the inflamed specimens (p<0.05).
Table 3.
ATP and energy charge potential (ECP) levels in ileal epithelium in Ussing chambers
| Colon cancer (n=8) | CD non-inflamed (n=5) | CD inflamed (n=4) | ||||
| Time | ATP | ECP | ATP | ECP | ATP | ECP |
| 0 min | 6.3 (5.2–6.7) | 0.71 (0.68–0.76) | 6.0 (5.3–7.1) | 0.73 (0.71–0.76) | 6.1 (4.9–6.5) | 0.72 (0.69–0.74) |
| 15 min vehicle | 6.0 (4.6–6.3) | 0.70 (0.68–0.74) | 5.6 (5.1–6.1) | 0.74 (0.70–0.77) | 3.6† (3.0–4.3) | 0.64† (0.62–0.67) |
| 15 min C10 | 2.1* (1.7–2.6) | 0.53* (0.51–0.57) | 2.4* (2.2–2.7) | 0.56* (0.50–0.59) | 2.3* (1.8–2.4) | 0.54* (0.47–0.56) |
Data are given as median (25–75th percentiles) at the start of the experiment—that is, after 40 minutes of equilibration in Ussing chambers (0 minutes), and after 15 minutes exposure to vehicle or sodium caprate (C10).
ATP concentrations are given as μg/mg dry weight.
*Decreased ATP concentrations compared with vehicle experiments (p<0.05); †decrease compared with baseline values in the same group and compared with vehicle experiments in the two non-inflamed groups (p<0.05).
C10 exposure induced a significant decrease in ATP concentrations and ECP in all three groups (p<0.05).
Endoscopic follow up
Ileocolonoscopy 3–7 months postoperatively revealed pre-anastomotic ileal inflammation in all five CD patients in the non-inflamed group despite no residual macroscopic or microscopic inflammation after resection. Patients showed neoterminal ileitis, with aphthous lesions in one patient, and ulcers of 3–20 mm in size in the remaining four. The endoscopy score was 1 in one patient, 2 in two patients, and 4 in two patients.
DISCUSSION
Although several in vivo permeability studies suggest a disturbance of TJ function in CD, and the luminal contents are important for determining permeability28 and development of inflammation,15 TJ regulation by luminal stimuli has not been studied previously in CD. The present study showed that non-inflamed ileal mucosa from CD patients was more reactive than control ileum to luminal exposure to C10, known to affect TJs. Electron microscopy demonstrated dilatations of ileal TJs, and the augmented increase in paracellular permeability in non-inflamed CD specimens was paralleled by a more pronounced disassembly of perijunctional F-actin. This suggests that TJs in CD patients are more vulnerable to noxious factors in the lumen, possibly mediated via altered cytoskeletal regulation.
We studied specimens from the part of the distal ileum that was anastomosed to the colon—that is, the neoterminal ileum. The neoterminal ileum is highly prone to recurrent inflammation in CD patients29, 30 and in this study pre-anastomotic recurrence was demonstrated by endoscopy within seven months postoperatively, despite no residual microscopic inflammation after resection. The faecal stream14, 15 and the proximity to the colon30 are important factors in this process, starting within one week after ileal exposure to faecal fluid.31 Taken together, our data suggest an epithelial vulnerability to luminal factors in CD which precedes ileal inflammation. This could contribute to the rapid induction of recurrent inflammation.
Our “control group” of colon cancer patients was substantially older than the CD patients but intestinal permeability does not seem to be affected by aging.8, 32 Increased intestinal permeability has been found in advanced malignancy33 or related to chemotherapy.8 However, the cancer patients included in our study had no signs of generalised disease, did not receive chemotherapy, and were in a good nutritional condition. Most CD patients were receiving treatment with corticosteroids and mesalazine. In experimental intestinal inflammation these drugs seem to tighten the barrier34, 35 and are not likely to explain the differences between the patient groups in our study. All specimens studied in the Ussing chamber as well as the resection margins from all patients were scrutinised by histopathology. The specimens grouped as “non-inflamed” should thus be unaffected from a clinical perspective. Nevertheless, there is a possibility that the observed TJ vulnerability in non-inflamed CD mucosa could be secondary to changes caused by the disease. For example, scanning electron microscopy studies have shown abnormalities in villus architecture and goblet cells in microscopically normal mucosa.36 Moreover, the non-inflamed specimens were taken in proximity to inflamed mucosa, and effects on the TJs by neighbouring inflammation have been shown in animal models.37, 38 Further studies addressing these issues are in progress at our laboratory.
Previously we have shown that human ileal mucosa maintains integrity and metabolism in vitro in Ussing chambers for 90 minutes if PDt (dependent on both TER and Isc) is above 6 mV at the start of experiments,25 and this was a prerequisite for inclusion of specimens in this study. Moreover, PDt values of the included specimens were at the same level as previous in vivo recordings of human small intestine.39 In vehicle experiments, no differences were seen between non-inflamed ileum from CD and colon cancer patients regarding baseline PDt, development of PDt over time, or energy levels, and there was no difference in the proportion of specimens excluded due to low PDt. This indicates equal viability and integrity of the non-inflamed specimens from the two patient groups. In contrast, inflamed CD mucosa showed a spontaneous drop over time in ATP and ECP, PDt, and TER, and had increased baseline permeability, suggesting impaired viability in vitro. The observations of slow and reduced effects by C10 in inflamed mucosa could be due to poor viability but could also be a technical artefact. By alternate current impedance analysis, differentiating epithelial and submucosal resistance, Schmitz et al showed that the barrier defect in intestinal inflammation is underestimated with standard resistance measurements.40
C10 affected mitochondrial function as shown both by ultrastructural changes and by a reduced ATP content. Similar changes have been described after induction of uncoupling of oxidative phosphorylation in the intestine.41, 42 Being a lipid soluble acid, C10 has the chemical attributes needed,42 and fatty acids similar to C10 are known to induce uncoupling.43 Changes in ATP levels and mitochondrial size were similar in CD and colon cancer, which suggests equal intracellular access of C10, and make variations in mucus layer and cell membrane composition less likely as causes of intergroup differences in mucosal permeability. On the other hand, the difference in F-actin response to C10 may be instrumental. Structural and functional links between perijunctional actin and the TJs are well established,11, 41 and contraction of the actomyosin ring enhances TJ permeability. The change in the perijunctional actin pattern was more pronounced in CD than in cancer coli patients. In non-inflamed CD specimens, staining of apical F-actin was diminished and only found in occasional patchy areas. This F-actin pattern has previously been associated with contraction of the perijunctional actomyosin ring and increased TJ permeability in intestinal epithelia by various stimuli—for example, inhibition of the rho GTP binding proteins,44 interferon γ treatment,45 and exposure to copper salts.46 Previous studies in animals and cell monolayers have shown a C10 induced calmodulin dependent rise in intracellular Ca2+ in parallel with increased permeability.47–49 Phosphorylation of the myosin light chain has been shown to be an important regulatory step in Ca2+ induced cytoskeletal contraction,50 as well as in physiological TJ regulation,51 and the effects of C10 in Caco-2 monolayers can be diminished by inhibition of myosin light chain kinase.49 Thus it could be speculated that the observed differences in TJ reactivity to C10 between CD and cancer coli could be caused by alterations in cytoskeletal regulation.
In vivo studies have shown an exaggerated increase in intestinal permeability in response to acetylsalicylic acid in 30–40% of CD relatives, suggesting a hereditary disturbance of the mucosal defence system.16, 17 Interestingly, acetylsalicylic acid has been found to increase TJ permeability via an effect on mitochondrial oxidative phosphorylation.42, 52 These data corroborate our present findings and lend further support to the notion of a primary vulnerability to luminal factors in the epithelial TJs in CD. In recent studies, we9 and others53 have found increased transcytosis of proteins in the ileum of CD. A similar combination of enhanced antigen transcytosis and increased paracellular permeability has also been recognised in animal models of stress54 and food hypersensitivity.55 Whether this implicates involvement of stress and hypersensitivity reactions in CD, or a combination of transcellular and paracellular barrier defects in small bowel inflammation in general, is not known at present. Our findings suggest interplay between an impaired epithelial barrier and luminal factors in the initiation of intestinal inflammation and corroborate the hypothesis that CD patients develop an abnormal response to “normal” antigens in the intestinal lumen.56 Further studies clarifying the cross talk between luminal stimuli and the epithelium could yield important clues to the pathogenesis of CD.
Acknowledgments
The authors wish to thank Mrs Lisbeth Hedman and Mrs Ylva Braaf for excellent laboratory work; Tapio Nikkilä and Bengt-Arne Fredriksson for technical support in the electron microscopy studies; and Johan Gråsjö for technical support with the electrical measurement devices. This study was supported by grants from the County Council of Östergötland, “Förenade liv” Mutual Group Life Insurance Company, Stockholm, King Gustav Vth 80 year Foundation, the Swedish Society for Medical Research, the Swedish Society of Medicine, and the Swedish Medical Research Council (projects 12618, 05983, and 06251).
Abbreviations
CD, Crohn's disease
C10, sodium caprate
CLSM, confocal laser scanning microscopy
ECP, energy charge potential
F-actin, filamentous actin
Isc, short circuit current
KRB, Krebs-Ringer buffer
PDt, transepithelial potential difference
TEM, transmission electron microscopy
TER, transepithelial electrical resistance
TJ, tight junction
REFERENCES
- 1.Olaison G, Sjödahl R, Tagesson C. Abnormal intestinal permeability in Crohn's disease. A possible pathogenic factor. Scand J Gastroenterol 1990;25:321–8. [DOI] [PubMed] [Google Scholar]
- 2.Meddings JB. Review article: Intestinal permeability in Crohn's disease. Aliment Pharmacol Ther 1997;11(suppl 3):47–53. [DOI] [PubMed] [Google Scholar]
- 3.Hollander D, Vadheim CM, Brettholz E, et al. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med 1986;105:883–5. [DOI] [PubMed] [Google Scholar]
- 4.Lindberg E, Söderholm JD, Olaison G, et al. Intestinal permeability to polyethylene glycols in monozygotic twins with Crohn's disease. Scand J Gastroenterol 1995;30:780–3. [DOI] [PubMed] [Google Scholar]
- 5.May G, Sutherland L, Meddings JB. Is small intestinal permeability really increased in relatives of patients with Crohn's disease? Gastroenterology 1993;104:1627–32. [DOI] [PubMed] [Google Scholar]
- 6.Peeters M, Geypens B, Claus D, et al. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology 1997;113:802–7. [DOI] [PubMed] [Google Scholar]
- 7.Travis S, Menzies I. Intestinal permeability: Functional assessment and significance. Clin Sci 1992;82:471–88. [DOI] [PubMed] [Google Scholar]
- 8.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology 1995;108:1566–81. [DOI] [PubMed] [Google Scholar]
- 9.Söderholm JD, Holmgren Peterson K, Olaison G, et al. Epithelial permeability to proteins in the non-inflamed ileum of Crohn's disease? Gastroenterology 1999;117:65–72. [DOI] [PubMed] [Google Scholar]
- 10.Balda MS, Matter K. Tight junctions. J Cell Sci 1998;111:541–7. [DOI] [PubMed] [Google Scholar]
- 11.Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol 1998;60:143–59. [DOI] [PubMed] [Google Scholar]
- 12.Marin ML, Greenstein AJ, Geller SA, et al. A freeze fracture study of Crohn's disease of the terminal ileum: changes in epithelial tight junction organization. Am J Gastroenterol 1983;78:537–47. [PubMed] [Google Scholar]
- 13.Hollander D. Crohn's disease—a permeability disorder of the tight junction? Gut 1988;29:1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janowitz HD, Croen EC, Sachar DB. The role of the fecal stream in Crohn's disease: an historical and analytical review. Inflamm Bowel Dis 1998;4:29–39. [DOI] [PubMed] [Google Scholar]
- 15.Rutgeerts P, Geboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet 1991;338:771–4. [DOI] [PubMed] [Google Scholar]
- 16.Hilsden RJ, Meddings JB, Sutherland LR. Intestinal permeability changes in response to acetyl-salicylic acid in relatives of patients with Crohn's disease. Gastroenterology 1996;110:1395–403 [DOI] [PubMed] [Google Scholar]
- 17.Söderholm JD, Olaison G, Lindberg E, et al. Different intestinal permeability patterns in relatives and spouses of patients with Crohn's disease—an inherited defect in mucosal defence? Gut 1999;44:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padley FB, Gunstone FD, Harwood JL. Occurrence and characteristics of oils and fats. Animal fats: milk and depot fats. In: Gunstone FD, Harwood JL, Padley FB, eds. The lipid handbook. London: Chapman & Hall, 1994:147–66.
- 19.Chao AC, Nguyen JV, Broughall M, et al. In vitro and in vivo evaluation of effects of sodium caprate on enteral peptide absorption and on mucosal morphology. Int J Pharm 1999;191:15–24. [DOI] [PubMed] [Google Scholar]
- 20.Lindmark T, Söderholm JD, Olaison G, et al. Mechanism of absorption enhancement in humans after rectal administration of ampicillin in suppositories containing sodium caprate. Pharm Res 1997;14:930–5. [DOI] [PubMed] [Google Scholar]
- 21.Anderberg EK, Lindmark T, Artursson P. Sodium caprate elicits dilatations in human intestinal tight junctions and enhances drug absorption by the paracellular route. Pharm Res 1993;10:957. [DOI] [PubMed] [Google Scholar]
- 22.Söderholm JD, Öman H, Blomquist L, et al. Reversible increase in tight junction permeability to macromolecules in rat ileal mucosa in vitro by sodium caprate, a constituent of milk fat. Dig Dis Sci 1998;43:1547–52. [DOI] [PubMed] [Google Scholar]
- 23.Rutgeerts P, Geboes K, Vantrappen K, et al. Predictability of the postoperative course of Crohn's disease. Gastroenterology 1990;99:956–63. [DOI] [PubMed] [Google Scholar]
- 24.Grass G M, Sweetana SA. In vitro measurement of gastrointestinal tissue permeability using a new diffusion cell. Pharm Res 1988;5:372–6. [DOI] [PubMed] [Google Scholar]
- 25.Söderholm JD, Hedman L, Artursson P, et al. Integrity and metabolism of human ileal mucosa in vitro in the Ussing chamber. Acta Physiol Scand 1998;162:47–56. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson M. Integrity of the occluding barrier in high-resistant thyroid follicular epithelium in culture. I. Dependence of extracellular Ca2+ is polarized. Eur J Cell Biol 1991;56:295–307. [PubMed] [Google Scholar]
- 27.Harris RC, Hultman E, Nordesjö L-O. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 1974;33:109–20. [PubMed] [Google Scholar]
- 28.Madsen KL, Malfair D, Gray D, et al. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis 1999;5:262–70. [DOI] [PubMed] [Google Scholar]
- 29.Rutgeerts P, Geboes K, Vantrappen G, et al. Natural history of recurrent Crohn's disease at the ileocolonic anastomosis after curative surgery. Gut 1984;25:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olaison G, Smedh K, Sjödahl R. Natural course of Crohn's disease after ileocolic resection—Early endosopic ileal ulcers preceding symptoms. Gut 1992;33:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998;114:262–7. [DOI] [PubMed] [Google Scholar]
- 32.Salzman JR, Kowdley KV, Perrone G, et al. Changes in small-intestine permeability with aging. Am Geriatr Soc 1995;43:160–4. [DOI] [PubMed] [Google Scholar]
- 33.Ryan CM, Atkins MB, Mier JW, et al. Effects of malignancy and interleukin-2 infusion on gut macromolecular permeability. Crit Care Med 1995;23:1801–6. [DOI] [PubMed] [Google Scholar]
- 34.Di Paolo MC, Merrett MN, Crotty B, et al. 5-Aminosalicylic acid inhibits the impaired epithelial barrier function induced by gamma interferon. Gut 1996;38:115–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKay DM, Brattsand R, Wieslander E, et al. Budesonide inhibits T cell-initiated epithelial pathophysiology in an in vitro model of inflammation. J Pharmacol Exp Ther 1996;277:403–10. [PubMed] [Google Scholar]
- 36.Nagel E, Bartels M, Pichlmayr R. Scanning electron-microscopic lesions in Crohn's disease: relevance for the interpretation of postoperative recurrence. Gastroenterology 1995;108:376–82. [DOI] [PubMed] [Google Scholar]
- 37.Fries W, Mazzon E, Squarzoni S, et al. Experimental colitis increases small intestine permeability in the rat. Lab Invest 1999;79:49–57. [PubMed] [Google Scholar]
- 38.Pantzar N, Ekstrom GM, Wang Q, et al. Mechanisms of increased intestinal [51Cr]EDTA absorption during experimental colitis in the rat. Dig Dis Sci 1994;39:2327–33. [DOI] [PubMed] [Google Scholar]
- 39.Davis G, Santa Ana C, Morawki S, et al. Permeability characteristics of human jejunum, ileum, proximal colon and distal colon: results of potential difference measurements and unidirectional fluxes. Gastroenterology 1982;83:844–50. [PubMed] [Google Scholar]
- 40.Schmitz H, Barmeyer C, Fromm M, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999;116:301–9. [DOI] [PubMed] [Google Scholar]
- 41.Madara JL. Intestinal absorptive cell tight junctions are linked to the cytoskeleton. Am J Physiol 1987;253:C171–5. [DOI] [PubMed] [Google Scholar]
- 42.Somasundaram S, Rafi S, Hayllar J, et al. Mitochondrial damage: a possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut 1997;41:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soboll S, Gründel S, Schwabe U, et al. Influence of fatty acids on energy metabolism. 2. Kinetics of changes in metabolic rates and changes in subcellular adenine nucleotide contents and pH gradients following addition of octanoate and oleate in perfused rat liver. Eur J Biochem 1984;141:231–6. [DOI] [PubMed] [Google Scholar]
- 44.Nusrat A, Giry M, Turner JR, et al. Rho regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA 1995;92:10629–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol 1999;276:G1279–88. [DOI] [PubMed] [Google Scholar]
- 46.Ferruzza S, Scarino ML, Rotilio G, et al. Copper treatment alters the permeability of tight junctions in cultured human intestinal Caco-2 cells. Am J Physiol 1999;277:G1138–48. [DOI] [PubMed] [Google Scholar]
- 47.Tomita M, Hayashi M, Awazu S. Absorption-enhancing mechanism of sodium caprate and decanoylcarnitine in Caco-2 cells. J Pharmacol Exp Ther 1995;272:739–43. [PubMed] [Google Scholar]
- 48.Shimazaki T, Tomita M, Sadahiro S, et al. Absorption-enhancing effects of sodium caprate and palmitoyl carnitine in rat and human colons. Dig Dis Sci 1998;43:641–5. [DOI] [PubMed] [Google Scholar]
- 49.Sakai M, Imai T, Ohtake H, et al. Effects of absorption enhancers on cytoskeletal actin filaments in Caco-2 cell monolayers. Life Sci 1998;63:45–54. [DOI] [PubMed] [Google Scholar]
- 50.Turner JR, Angle JM, Black ED, et al. PKC-dependent regulation of transepithelial resistance: roles of MLC and MLC kinase. Am J Physiol 1999;277:C554–62. [DOI] [PubMed] [Google Scholar]
- 51.Turner JR, Rill BK, Carlson SL, et al. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol 1997;273:C1378–85. [DOI] [PubMed] [Google Scholar]
- 52.Söderholm JD, Wirén M, Franzén L, et al. “Topical” phase effects of acetylsalicylic acid on human small bowel epithelium: inhibition of oxidative phosphorylation and increased tight junction permeability. Gastroenterology 2000;118:A811. [Google Scholar]
- 53.Schurmann G, Bruwer M, Klotz A, et al. Transepithelial transport processes at the intestinal mucosa in inflammatory bowel disease. Int J Colorectal Dis 1999;14:41–6. [DOI] [PubMed] [Google Scholar]
- 54.Söderholm JD, Perdue MH. Stress and intestinal barrier function. Am J Physiol 2001;280:G7–13. [DOI] [PubMed] [Google Scholar]
- 55.Berin MC, Kiliaan AJ, Yang PC, et al. Rapid transepithelial antigen transport in rat jejunum: impact of sensitization and the hypersensitivity reaction. Gastroenterology 1997;113:856–64. [DOI] [PubMed] [Google Scholar]
- 56.Sartor RB. Role of the enteric microflora in the pathogenesis of intestinal inflammation and arthritis. Aliment Pharmacol Ther 1997;11(suppl 3):17–23. [DOI] [PubMed] [Google Scholar]