Abstract
Background and aims: Psychological factors have been implicated in the aetiology of irritable bowel syndrome characterised by intestinal altered motility and visceral hypersensitivity. Similar disorders have been found in rats under stressful conditions. The role of tachykinins in bowel dysfunctions caused by stress is not fully documented. Therefore, we investigated the influence of stress on NK1 receptor activation at the colonic level in female rats.
Methods: The stress procedure used consisted of two hours of partial restraint. Histamine release was measured from colonic samples of control and stressed animals and the effect of SR140333, a NK1 receptor antagonist, on substance P induced histamine release was determined. Involvement of steroids has been evaluated in this response.
Results: NK1 receptor antagonist was found to inhibit substance P induced histamine release in samples from stressed female rats but not in samples from control animals. Previous treatment of female rats with RU 486 abolished this effect observed in stressed animals. Similarly, in samples from stressed female rats previously ovariectomised, SR140333 failed to inhibit substance P induced histamine release but previous treatment with both progesterone and oestrogen restored its effect.
Conclusions: Stress induces NK1 receptor activation in the colon, and ovarian steroids are involved in this response.
Keywords: stress, female rats, NK1 receptor, ovarian steroid
Emotional and environmental stressful stimuli are known to affect gastroenterological functions and have been proposed to contribute to the clinical manifestation of irritable bowel syndrome (IBS) of which mainly women are affected.1, 2 These manifestations, such as altered gut motility and sensitivity, are similar to those initiated by stressful stimuli in rats.3, 4 Thus experimental models have been developed to evaluate the effect of stress on lower gut function in rats. Exposure to acute stress such as immobilisation or fear of receiving an electric foot shock was shown to increase intestinal permeability5 and mucin secretion,6 and to stimulate colonic motility and defecation.7 It was shown that immobilisation stress leading to colonic mucin release is associated with gastrointestinal mast cell activation.6 Furthermore, mast cell stabilisation may prevent the stress induced visceral hypersensitivity to rectal distension in female rats.4 Mast cells have been shown to be activated by stress in various organs. Indeed, immobilisation stress induces mast cell degranulation in the dura mater8 or activates ileal mast cells by intragranular changes in rats.9 In addition, restraint stress was found to activate bladder mast cells.10 Intestinal mast cells are closely apposed to afferent nerves endings11 and may be activated by immune or non-immunological stimuli, including neurotransmitters, neuropeptides,12 and nerve stimulation.13 Activated mast cells are known to release preformed or newly generated biologically active molecules such as histamine, proteases, eicosanoids, and cytokines.14 Substance P (SP) released from sensory nerves is one of the neuropeptides that can activate mast cells but relatively high concentrations of SP are required to degranulate mast cells.15 Direct activation of G protein in the mast cell membrane by SP is the currently proposed mechanism involved in triggering histamine release.16 However, there is increasing evidence for a receptor mediated effect of SP on mast cells.17 Several mast cell lines may express specific functional NK1 receptors which can recognise the C terminal sequence of SP peptide. Thus recent findings demonstrated the presence of functional NK1 receptors on RBL-2H2 cells, a mucosal like mast cell line,18 and murine lymphocytes19 have also been reported to exhibit NK1 receptors. Moreover, when injected in vivo, SP can induce oedema formation through a NK1 receptor mediated mechanism, involving mast cells.17 On the other hand, tachykinins are candidate excitatory transmitters participating in the regulation of intestinal motility.20 However, the role of tachykinins in bowel dysfunction caused by stress has not been fully documented in rats. It has been suggested that activation of NK1 receptors by SP released from the intrinsic neurones of the colon may play a major role in stress induced colonic dysfunction in rats.21 While several experimental studies have found an association between stress and levels of SP both at central and peripheral levels,22, 23 no data are available regarding the influence of stress on tachykinin receptor population and/or expression. Stressful events were shown to modulate serum steroid levels in rats and a regulatory role for ovarian steroid hormones was described for tachykinin receptor expression.24–26
Consequently, in this study our aim was to establish at the colonic level in female rats: (i) whether NK1 receptor activation is involved in the response of mast cells to in vitro stimulation with SP, under basal versus acute restraint stress conditions, (ii) if NK1 receptor activation is neuronally mediated, and (iii) whether adrenal or ovarian steroids are involved in stress induced NK1 receptor mediation of the SP effect on mast cell degranulation.
MATERIAL AND METHODS
Partial restraint stress procedure
Female Wistar rats, weighing approximately 250 g, were kept in plastic cages and housed under controlled conditions on a 12:12 hour light-dark cycle. They were provided with food and water ad libitum. All experiments were performed at the same time of day (between 10:00 and 12:00 am) to minimise any influence of circadian rhythms. Stress effects were studied using the wrap partial restraint stress model.4 Animals were lightly anaesthetised with ethyl ether, and their fore shoulders, upper forelimbs, and thoracic trunk were wrapped in a confining harness of paper tape to restrict, but not to prevent, body movements. Then, the animals were placed in their home cage for two hours. The rats recovered from ethyl ether within 2–3 minutes and immediately moved about in their cages, with the restricted mobility of their forelimbs preventing grooming behaviour. Control sham stress animals were anaesthetised but not wrapped and were allowed to move freely in their cages. The restraint stress at room temperature used here is a mild and non-ulcerogenic stressor which reproduces the symptoms associated with stress related colonic dysfunction in humans, suggesting that it may be a suitable model for studying the effect of stress on the gastrointestinal tract.
Measurement of in vitro histamine release
Fifteen minutes after the end of the stress session, animals were sacrificed and exsanguinated, as approved by the local ethics committee. The colon was rapidly removed and cut longitudinally. Colonic segments (0.5 cm length) were then placed in Ringer's solution where tested compound had been added. After an incubation period, histamine concentration was evaluated in the supernatant with a radioimmunoassay kit using polyclonal histamine antibodies (Immunotech, Marseille, France). Histamine levels were expressed in nanomoles per gram of tissue and histamine values under SP stimulation were corrected for spontaneous histamine release in the absence of any degranulating agent.
Ovariectomy procedure
Animals were anaesthetised using acepromazine (0.6 mg/kg intraperitoneally) and ketamine (120 mg/kg intraperitoneally) and a bilateral ovariectomy was performed. Briefly, the fallopian tubes were ligated below the ovaries which were then excised, and muscle and skin wounds closed using silk suture. Sham surgery consisted of externalising the ovaries from the abdominal cavity and replacing them without being excised. Animals were allowed a two week period of rest before the beginning of the protocol.
Vaginal smear
The hormonal status of each animal was evaluated by a vaginal smear after Haris Shorr staining. Only the female in proestrus, which corresponds to a hormonal status combining both oestradiol and progesterone impregnation, were selected in our studies. The effectiveness of ovariectomy and hormonal progesterone or oestrogen treatments were systematically controlled by vaginal smear. Sham operated rats also underwent evaluation by vaginal smear, based on microscopic readings, to assess the phase of their ovarian cycle.
Experimental design
Six series of experiments were performed.
In the first series of experiments, two groups of eight female rats were used. One group was subjected to partial restraint stress (two hours) and the second group was used as a control (sham stress). As described above, measurement of in vitro histamine release was performed on colonic samples from these animals. Mast cell histamine release was triggered by SP (3×10−5 M) or GR73632 (1×10−8, 1×10−6, 1×10−5 M), a selective NK1 receptor agonist, each incubated for one hour.
In the second series of experiments, two groups of eight female rats were used: one group was subjected to the partial restraint stress procedure and another group was used as controls (sham stress). Similarly, measurement of in vitro histamine release was performed on colonic samples from these animals. Mast cell histamine release was triggered by one hour of incubation with SP (3×10−5 M) in the absence or presence of SR140333 (3×10−5 M), a non-peptide tachykinin NK1 receptor antagonist, added to the buffer solution two hours before SP. This experiment investigated whether restraint stress can influence the potential involvement of NK1 receptor in SP induced histamine release.
In the third series of experiments, one group of eight female rats subjected to the stress procedure was used. The effects of in vitro pretreatment of colonic samples with the neuronal blocker tetrodotoxin (TTX) at a dose of 10−6 M (incubation period 15 minutes) was evaluated on histamine release induced by SP (3×10−5 M).
Three additional groups of female rats, including a control (sham stress) group and two stressed groups, were used in the fourth series. One of the stressed group received the glucocorticoid receptor antagonist RU 486 (4 mg/0.2 ml olive oil/rat subcutaneously) 30 minutes before the stress session. The second stressed group and the control groups received only olive oil (0.2 ml/rat subcutaneously). As previously described, animals were sacrificed 15 minutes after the end of the stress session, and the colon was rapidly removed and placed in Ringer's solution. Then, mast cells were stimulated in vitro with SP (3×10−5 M) and the ability of SR140333 (3×10−5 M) to antagonise histamine release was evaluated.
In the fifth series of experiments, three groups of eight female rats were used. One group was bilaterally ovariectomised and two groups underwent a sham operation. The stress protocol was applied to one sham ovariectomised group and to the ovariectomised animals. Then, the effects of SP in the absence or presence of SR140333 on histamine release from colonic samples were evaluated in vitro.
In the final set of experiments, five groups of eight female Wistar rats were used, including four groups that underwent bilateral ovariectomy and one sham ovariectomised group. All were subjected to a restraint stress session. Ovariectomised animals received oestradiol 5 μg, progesterone 500 μg dissolved in 0.2 ml of olive oil, or both oestradiol and progesterone, subcutaneously one hour before the stress session. Sham ovariectomised animals and the last ovariectomised group received vehicle (0.2 ml olive oil/rat subcutaneously) before stress. As documented above, in vitro mast cell stimulation was performed with SP (3×10−5 M) with or without previous treatment with the NK1 receptor antagonist SR140333 (3×10−5 M).
All experimental procedures were approved by the local animal care and use committee.
Drugs
Progesterone (4-pregnene-3, 20-dione), oestrogen (1, 3, 5[10]-estratriene-3, 17β-diol, 3-benzoate), and the neuronal blocker TTX were purchased from Sigma Chemical (St Quentin Fallavier, France). The antiprogestin RU 486 (mifepristone) was a gift from Roussel-Uclaf (Paris, France).
The tachykinin NK1 agonist GR73632 (δ-amino valeryl (L-Pro9, N-MeLeu10)-substance P(7-11)) was obtained from RBI (Bioblock, Illkirch, France) and the non-peptide NK1 antagonist SR140333 ((S)1-(2[3-(3, 4-dichlorophenyl)-1-(3-isopropoxyphenylacetyl) piperidin-3-yl]ethyl)-4-phenyl-1-azoniabicyclo[2.2.2]octane chloride) was a gift from Dr Emonds-Alt (Sanofi Research, Montpellier, France). Substance P (acetate salt) was purchased from Bachem (Voisins le Bretonneux Cedex, France).
The doses used for each compound were selected after extended review of the literature.
Statistical analysis
Histamine release was expressed in nanomoles per gram of tissue and presented as mean (SEM). After one way analysis of variance, differences in histamine levels were assessed using the paired Student's t test, and unpaired t test for control versus stress comparisons. Differences were considered significant at p<0.05.
RESULTS
Effect of stress on in vitro histamine release induced by SP and GR73632
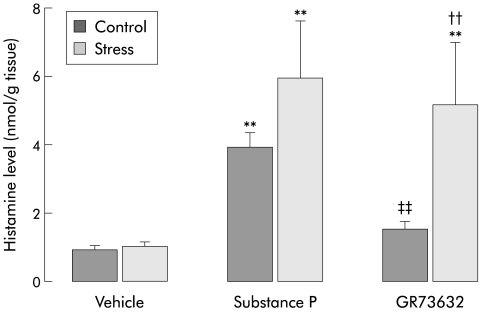
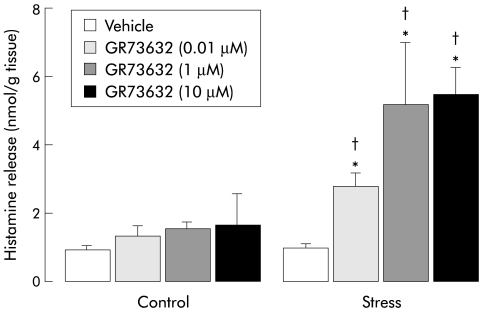
Spontaneous histamine release from colonic samples was found to be very low and similar in both the control and stress groups (0.9 (0.1) v 1 (0.1) nmol/g tissue). From these basal values, SP significantly increased in vitro histamine release in the control and stress groups (3.9 (0.4) v 0.9 (0.1) (p<0.01) and 5.9 (1.7) v 1 (0.1) nmol/g tissue; p<0.01) with a higher level in stressed compared with control animals (5.9 (1.7) v 3.9 (0.4) nmol/g tissue; p>0.05) (fig 1 ▶). In the control group, in contrast with SP, GR73632 at doses of 10−8 to 10−5 M did not cause a significant increase in in vitro histamine discharge compared with spontaneous release. However, GR73632 dose dependently (from 10−8 to 10−6 M) increased histamine release in samples from stressed animals but the highest dose of 10−5 M did not further increase histamine release. Also, GR73632 induced histamine release was found to be significantly higher in the stress group compared with controls (fig 2 ▶).
Figure 1.
Histamine levels after in vitro stimulation with vehicle, substance P (3×10−5 M), or GR 73632 (1×10−6 M) in colonic samples from control or stressed female rats. Values are mean (SEM), n=8. *p<0.05, **p<0.01, significantly different from vehicle values; ††p<0.01, significantly different from control values; ‡‡p<0.01, significantly different from control SP value.
Figure 2.
Dose dependent effect of GR73632 on in vitro histamine release in samples from control and stressed female rats. Values are mean (SEM), n=8. *p<0.05, significantly different from vehicle values; †p<0.05, significantly different from control values.
Influence of SR140333 and TTX on SP induced histamine release
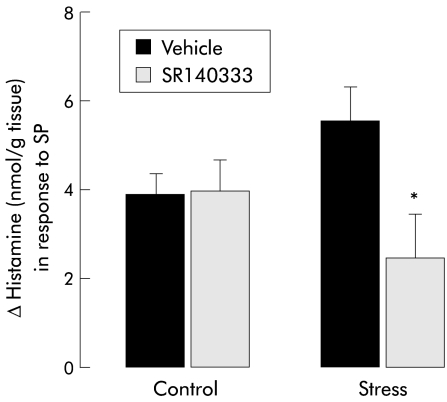
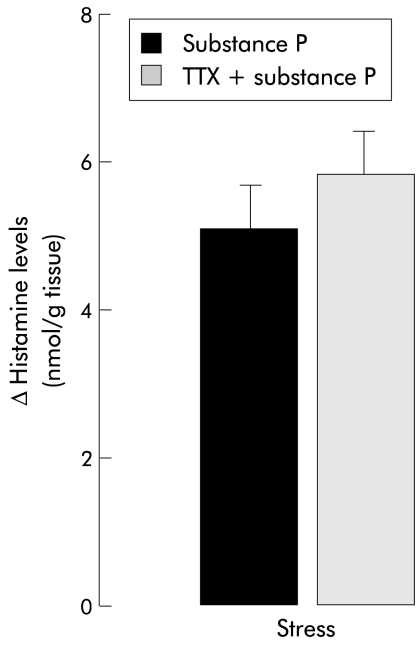
In colonic samples from control female rats, in vitro histamine release induced by mast cell activation with SP was not modified by in vitro pretreatment with the non-peptide NK1 receptor antagonist SR140333. Interestingly, in samples from stressed female rats, SP induced histamine release was found to be significantly reduced by previous treatment with SR140333 (2.5 (0.6) v 5.6 (0); p<0.05) (fig 3 ▶). Furthermore, TTX failed to modify SP induced histamine release from colonic samples of stressed animals (fig 4 ▶).
Figure 3.
Histamine levels after in vitro stimulation with substance P (SP) (3×10−5 M) in colonic samples from control or stressed female rats: incidence of previous incubation with SR140333 (3×10−5 M). Values are mean (SEM), n=8. *p<0.05, significantly different from vehicle values.
Figure 4.
Histamine levels after in vitro stimulation with substance P (3×10−5 M) in colonic samples from stressed female rats: incidence of previous incubation with the neuronal blocker tetrodotoxin (TTX 1×10−6). Values are mean (SEM), n=8.
Influence of RU 486 and ovariectomy on blockage of SP induced histamine release by SR140333
An inhibitory effect of SR140333 on SP induced histamine release was found in stressed animals pretreated with vehicle (olive oil) (3.8 (0.6) v 5.7 (0.3); p<0.05). In contrast, in colonic samples from stressed animals pretreated with the glucocorticoid/progesterone receptor antagonist RU 486, SR140333 failed to reduce histamine release induced by SP (fig 5 ▶).
Figure 5.
Histamine levels after in vitro stimulation with substance P (SP) (3×10−5 M) in colonic samples from control or stressed female rats: incidence of pretreatment with the glucocorticoid/progesterone receptor antagonist RU 486 (4 mg/0.2 ml/rat) or vehicle (olive oil 0.2 ml/rat) on the ability of SR140333 (3×10−5 M) to inhibit SP induced histamine release. Values are mean (SEM), n=8. *p<0.05, significantly different from vehicle values.
In colonic samples from stressed female rats submitted to sham ovariectomy, previous incubation with SR140333 significantly reduced SP induced histamine release (2.85 (0.5) v 6 (0.7); p<0.05). Interestingly, in samples from ovariectomised stressed rats, previous treatment with SR140333 failed to reduce histamine release triggered by SP (fig 6 ▶).
Figure 6.
Histamine levels after in vitro stimulation with substance P (SP) (3×10−5 M) in colonic samples from control or stressed female rats previously ovariectomised (OVX) or not (sham OVX): incidence of ovariectomy on the ability of SR140333 (3×10−5 M) to inhibit SP induced histamine release. Values are mean (SEM), n=8. *p<0.05, significantly different from vehicle values.
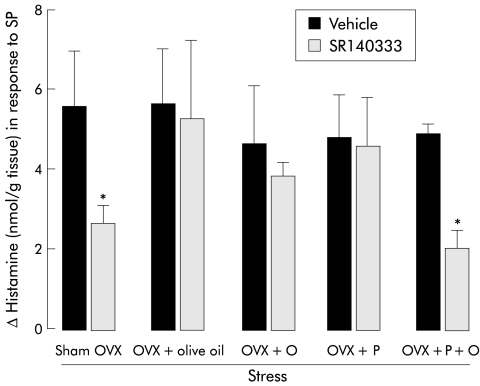
A similar inability of SR140333 to antagonise SP induced histamine release has been observed in colonic samples from stressed females previously ovariectomised and pretreated with oestradiol, progesterone, or vehicle alone (fig 7 ▶). However, the efficacy of SR140333 in reversing SP induced histamine release from colonic samples was restored when ovariectomised animals were given combined treatment with oestradiol and progesterone (fig 7 ▶).
Figure 7.
Histamine levels after in vitro stimulation with substance P (SP) (3×10−5 M) in colonic samples from stressed female rats previously ovariectomised (OVX) or not (sham OVX): incidence of previous treatment with ovarian hormones oestrogen (O) (5 μg/0.2 ml/rat), progesterone (P) (500 μg/0.2 ml/rat), or both (P+O) on the ability of SR140333 (3×10−5 M) to inhibit SP induced histamine release. Values are mean (SEM), n=8. *p<0.05, significantly different from vehicle values.
DISCUSSION
This study provides the first evidence that in female rats, stress affects the involvement of colonic NK1 receptors in SP induced histamine release. In this report, we showed that GR73632, a specific NK1 receptor agonist, induced slight histamine release in samples from control animals while a greater response was found in samples from female rats subjected to restraint stress. Furthermore, SP was shown to trigger histamine release with a similar level in both control and stressed animals. This last result contrasts with previous findings which showed that in acutely stressed rats, the level of histamine release in response to compound 48/80 was significantly increased compared with a control group.4 However, the cationic amphilic 48/80 compound acts differently than the neuropeptide SP in inducing histamine release and this may explain the differences in their effects on stress. Indeed, 48/80 compound is known to act on connective mast cells while the effect of SP is directed towards mucosal mast cells. In addition, whereas SP but not GR73632 caused significant histamine release in the basal state, comparable levels of histamine release were observed in samples from stressed animals after SP and GR73632 stimulation. Taken together, these results suggest that stress may enhance the reactivity to the NK1 agonist GR73632 in the colon. Similarly, we can hypothesise a switch from a non-receptor to a NK1 receptor mediated mechanism in the effect of SP on histamine release under basal compared with stress conditions, respectively. A direct action of SP on G protein in the mast cell membrane can be proposed under basal conditions while the NK1 receptor would be the predominant mechanism for SP after stress.
Hence we investigated whether NK1 receptors are involved in in vitro histamine release induced by SP under basal and stress conditions using the selective NK1 receptor antagonist SR140333. This compound has a high affinity for NK1 binding sites and acts as a competitive inhibitor of NK1 receptor mediated responses in various in vitro and in vivo tests.27 Concerning the antagonistic effects of SR140333 on SP induced histamine release, our findings indicate that histamine release triggered by SP in colonic samples from control rats is non-receptor mediated while NK1 receptors are involved in this process after restraint stress. These results suggest that stress may have a regulatory role in NK1 receptor expression or functionality at the colonic level in female rats. Several studies have previously reported regulation of SP and NK1 receptor expression in stimulated situations such as in inflamed tissue28, 29 but no previous study has shown that stress affects NK1 receptor expression or activation. Conflicting reports on changes in SP content or NK1 receptor expression in the gut in inflammatory conditions have been published. Indeed, Manthy et al showed a dramatic increase in SP levels and binding sites in resected bowels from patients suffering from active Crohn's disease and ulcerative colitis.30 In contrast, other findings have shown that expression of SP receptors is altered but not increased during colitis induced by trinitrobenzene sulphonic acid in rats.31 Furthermore, several animal studies have found an association between stress and SP levels. Restraint stress in rats was found to be associated with increased levels of SP in the periaqueductal grey area of the brain32 and electric footshocks were shown to increase release of SP from rat adrenomedullary cells.33 In humans, a recent study demonstrated that peripheral blood levels of SP may be influenced by anxiety.34 Moreover, stress also increases immunoreactive SP levels in peritoneal fluid in mice.35 Considering the similar occurrence of SP elevation in immune stimulated conditions such as inflammation and stress, it is possible that NK1 receptor expression is increased during stress, as described during inflammation. This hypothesis is supported by previous reports suggesting that stress may affect gene expression. For example, sustained stressors can alter the responsiveness of feedback systems by downregulation of adrenergic or serotoninergic receptors.36, 37 Moreover, restraint stress was described as changing gene expression of neuronal nitric oxide synthase in areas related to stress reactions.38 Similarly, preproenkephalin mRNA levels within the limbic system and hypothalamus are regulated by acute stress.39
As our results suggest the involvement of NK1 receptor in SP induced histamine release after stress, the question remains whether nerves are involved in these in vitro SP effects. Our study showed that previous incubation with the neuronal blocker TTX failed to abolish the response of SP under stress conditions suggesting a non-neuronally mediated mechanism. These results contrast with a recent finding in human colonic mucosa which indicates that mucosal nerves are involved in SP induced histamine release related to mast cell stimulation.40 These conflicting results must be considered in terms of the stress level of the animals used in our study and species differences (rats v humans) in the two studies. Considering our data and because mast cells are a major source of histamine in the colonic mucosa, a direct effect of SP on mast cells can be proposed. Indeed, many studies demonstrated the ability of SP to promote mast cell activation resulting in histamine release.40 A receptor independent pathway through direct activation of G protein in mast cell membranes has been proposed as the mechanism by which SP acts on mast cells.41 On the other hand, there is evidence for a possible presence of NK1 receptors on mast cells. For example, recent findings demonstrated the presence of functional NK1 receptors on RBL-2H3 cells, a mucosal like mast cell line.18
The present study showed that SR140333 was inefficient in antagonising SP induced histamine release in colonic samples from control animals, suggesting a non-NK1 receptor mediated mechanism under basal conditions. In the stress situation, our data suggest the involvement of NK1 receptor, possibly located on mast cells, in SP induced histamine release. This result suggests an increase in NK1 receptor expression related to stress stimulated conditions. However, we cannot exclude involvement of mediators released from other immunocytes that are resident or recruited during stress in colonic tissues, which may further induce mast cell activation and histamine release in response to SP stimulation. Concurrently, the hypothesis of stress induced regulation of NK1 receptor desensitisation may be proposed. Phosphorylation of NK1 receptors or interaction with β-arrestin may be affected by stress which consequently could attenuate desensitisation of the NK1 receptor.42 In addition, stress could promote NEP (cell surface protease neutral endopeptidase) inhibition and then potentiate the effect of exogenous SP on NK1 receptors.43
Because stress stimulates the hypothalamic-pituitary-adrenal axis,44 we investigated the possible modulatory role of corticosteroids in stress induced activation of NK1 receptors using RU 486, a specific glucocorticoid/progesterone receptor antagonist.45 Our results showed that in samples from stressed animals previously treated with RU 486, involvement of NK1 receptor in SP induced histamine release was abolished. These data suggest that under these experimental conditions, endogenous glucocorticoids are necessary for induction of NK1 receptors following restraint stress. These results are consistent with previous studies showing the regulatory potency of stress induced glucocorticoid release in modulating gene expression. For example, a direct mediatory role of glucocorticoids has been established for the increase in proenkephalin mRNA induced by stress in rats.46 In addition, glucocorticoids were shown to be important for liver metallothionein protein synthesis during restraint stress.47 However, to date, no data are available concerning the regulatory potency of glucocorticoids on tachykinin receptors. Furthermore, RU 486 acts as an antiprogestin on progesterone receptors suggesting the possible involvement of ovarian steroids in the occurrence of functional NK1 receptors on colonic mast cells. This hypothesis is confirmed by the inability of SR140333, an NK1 receptor antagonist, to block SP induced histamine release in colonic samples from ovariectomised animals subjected to restraint stress. A similar absence of blockade of SP induced histamine release by SR140333 was obtained when ovariectomised rats were treated with either progesterone or oestradiol before the stress session. In contrast, an inhibitory effect of SR140333 on SP induced histamine release was restored in samples from ovariectomised stressed females pretreated with combined oestradiol and progesterone. Regarding these data, we can conclude that ovarian steroids have a regulatory role in NK1 receptor induction during restraint stress at the colonic level and that both oestradiol and progesterone are needed to trigger this effect. A recent study demonstrated that exposure to an acute stressful event immediately and persistently enhances serum oestradiol.24 The contribution of sex hormones to transcriptional regulation of genes encoding different components has already been described. Recent data indicate that oestrogens regulate kinin B2 receptor gene expression and function in female rats.48 Furthermore, the hypothesis of NK1 receptor regulation by steroid hormone is supported by a previous study describing the regulation of tachykinin receptor expression by ovarian steroids in the rat uterus.25 Other studies demonstrated that expression of the SP receptor gene is regulated by oestrogens by way of an oestrogen receptor mediated genomic control.26 In other respects, testosterone was found to influence the binding of SP on human IM-9B lymphoblasts49 suggesting a regulatory role for androgens on SP receptors.
In conclusion, the present findings provide evidence that restraint stress promotes NK1 receptor expression at the colonic level, as shown by the ability of an NK1 receptor antagonist to abolish in vitro SP induced histamine release in stressed animals only. Furthermore, activation of NK1 receptor by SP was found to be non-neuronally mediated which suggests direct involvement of mast cells or other immune cells triggering mast cell degranulation. Lastly, this study highlighted the regulatory potency of steroids and more particularly ovarian hormones in stress induced induction of NK1 receptors. Therefore, these data suggest that NK1 receptors, activated by SP, may play a major role in stress induced colonic dysfunction in rats, such as colonic or rectal hypersensitivity to distension.4 The data also provide evidence for the role of sex hormones in the regulation of immune processes and support the role of these hormones in disease states highly represented in women where mast cells are involved.
Abbreviations
IBS, irritable bowel syndrome
SP, substance P
TTX, tetrodotoxin
REFERENCES
- 1.Drossman DA, Creed FH, Olden KW, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gut 1999;45(suppl 2):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins S, Barbara G, Vallance B. Stress, inflammation and the irritable bowel syndrome. Can J Gastroenterol 1999;13:47–49A. [DOI] [PubMed] [Google Scholar]
- 3.Williams CL, Villar RG, Peterson JM, et al. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology 1988;94:611–21. [DOI] [PubMed] [Google Scholar]
- 4.Gue M, Del Rio-Lacheze C, Eutamene H, et al. Stress-induced visceral hypersensitivity to rectal distension in rats: role of CRF and mast cells. Neurogastroenterol Motil 1997;9:271–9. [DOI] [PubMed] [Google Scholar]
- 5.Saunders PR, Kosecka U, Mc Kay DM, et al. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol 1989;256:G739–46. [DOI] [PubMed] [Google Scholar]
- 6.Castagliuolo I, Lamont JT, Qiu B, et al. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol 1996;271:G884–92. [DOI] [PubMed] [Google Scholar]
- 7.Monnikes H, Schmidt BG, Taché Y. Psychological stress-induced accelerated colonic transit in rats involves hypothalamic corticotropin-releasing factor. Gastroenterology 1993;104:716–23. [DOI] [PubMed] [Google Scholar]
- 8.Theoharides TC, Spanos C, Pang X, et al. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology 1995;136:5745–50. [DOI] [PubMed] [Google Scholar]
- 9.Theoharides TC, Letourneau R, Patra P, et al. Stress-induced rat intestinal mast cell intragranular activation and inhibitory effect of sulphated proteoglycans. Dig Dis Sci 1999;44:87–93S. [PubMed] [Google Scholar]
- 10.Spanos C, Pang X, Ligris K, et al. Stress-induced bladder mast cell activation: implication for interstitial cystitis. J Urol 1997;157:669–72. [PubMed] [Google Scholar]
- 11.McKay DM, Bienenstock J. The interaction between mast cells and nerves in the gastrointestinal tract. Immunol Today 1994;15:533–7. [DOI] [PubMed] [Google Scholar]
- 12.Theoharides TC. The mast cell: a neuroimmunoendocrine master player. Int J Tissue React 1996;18:1–21. [PubMed] [Google Scholar]
- 13.Gottwald T, Becker HD, Stead RH. Sex differences in neuromodulation of mucosal mast cells in the rat jejunum. Langenbecks Arch Chir 1997;382:157–63. [PubMed] [Google Scholar]
- 14.Metcalfe DD, Baram D, Meroki Y. Mast cells. Physiol Rev 1997;77:1033–79. [DOI] [PubMed] [Google Scholar]
- 15.Janiszewski J, Bienenstock J, Blennerhassett MG. Picomolar doses of substance P trigger electrical responses in mast cells without degranulation. Am J Physiol 1994;267:C138–45. [DOI] [PubMed] [Google Scholar]
- 16.Chahdi A, Mousli M, Landry Y. Substance P-related inhibitors of mast cell exocytosis act on G-proteins or on cell surface. Eur J Pharmacol 1998;341:329–35. [DOI] [PubMed] [Google Scholar]
- 17.Cao T, Gerard NP, Brain SD. Use of NK1 knockout mice to analyse substance P-induced edema formation. Am J Physiol 1999;277:476–81. [DOI] [PubMed] [Google Scholar]
- 18.Cooke HJ, Fox P, Alferes L, et al. Presence of NK1 receptors on a mucosal-like mast cell line, RBL-2H3 cells. Can J Physiol Pharmacol 1998;76:188–93. [PubMed] [Google Scholar]
- 19.Stanisz AM, Sciccitano R, Dazin P, et al. Distribution of substance P receptors on murine spleen and Peyer's patch T and B cells. J Immunol 1987;139:749–54. [PubMed] [Google Scholar]
- 20.Holzer P, Holzer-Petsche U, Leander S. A tachykinin antagonist inhibits gastric emptying and gastrointestinal transit in the rat. Br J Pharmacol 1986. ;89:453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda K, Miyata K, Orita A, et al. RP67580, a neurokinin1 receptor antagonist, decreased restraint stress-induced defecation in rat. Neurosci Lett 1995;198:103–6. [DOI] [PubMed] [Google Scholar]
- 22.Culman J, Klee S, Ohlendorf C, et al. Effect of tachykinin receptor inhibition in the brain on cardiovascular and behavioral responses to stress. J Pharmacol Exp Ther 1997;280:238–46. [PubMed] [Google Scholar]
- 23.Fehder WP, Sachs J, Uvaydova M, et al. Substance P as an immune modulator of anxiety. Neuroimmunomodulation 1997;4:42–8. [DOI] [PubMed] [Google Scholar]
- 24.Shors TJ, Pickett J, Wood G, et al. Acute stress persistently enhances estrogen levels in the female rat. Stress 1999;3:163–71. [DOI] [PubMed] [Google Scholar]
- 25.Pinto FM, Armesto CP, Magraner J, et al. Tachykinin receptor and neutral endopeptidase gene expression in the rat uterus: characterization and regulation in response to ovarian steroid treatment. Endocrinology 1999;140:2526–32. [DOI] [PubMed] [Google Scholar]
- 26.Villablanca AC, Hanley MR. 17beta estradiol stimulates substance P receptor gene expression. Mol Cell Endocrinol 1997;135:109–17. [DOI] [PubMed] [Google Scholar]
- 27.Emonds Alt X, Doutrmepuich JD, Heaulme M, et al. In vitro and in vivo biological activities of SR140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. Eur J Pharmacol 1993;250:403–13. [DOI] [PubMed] [Google Scholar]
- 28.Abbadie C, Brown JL, Mantyh PW, et al. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience 1996;70:201–9. [DOI] [PubMed] [Google Scholar]
- 29.Goode T, O'Connell J, Anton P, et al. Neurokinin-1 receptor expression in inflammatory bowel disease: molecular quantitation and localization. Gut 2000;47:387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manthy CR, Vigna SR, Bollinger RR, et al. Differential expression of substance P recepors in patients with Crohn's disease and ulcerative colitis. Gastroenterology 1995;109:850–60. [DOI] [PubMed] [Google Scholar]
- 31.Evangelista S, Maggi M, Renzetti AR. Down-regulation of substance P receptors during colitis induced by trinitrobenzene sulfonic acid in rats. Neuropeptides 1996;30:425–8. [DOI] [PubMed] [Google Scholar]
- 32.Rosen A, Brodin K, Eneroth P, et al. Short-term restraint stress and s.c. saline injection alter the tissue levels of substance P and cholecystokinin in the peri-aqueductal grey and limbic regions of rat brain. Acta Physiol Scand 1992;146:341–8. [DOI] [PubMed] [Google Scholar]
- 33.Vaupel R, Jarry H, Schlomer H, et al. Differential response of substance P-containing subtypes of adrenomedullary cells to different stressors. Endocrinology 1988;123:2140–5. [DOI] [PubMed] [Google Scholar]
- 34.Schedlowski M, Fluge T, Richter S, et al. Beta-endorphin, but not substance-P, is increased by acute stress in humans. Psychoneuroendocrinology 1995;20:103–10. [DOI] [PubMed] [Google Scholar]
- 35.Chancellor-Freeland C, Zhu GF, Kage R, et al. Substance P and stress-induced changes in macrophages. Ann N Y Acad Sci 1995;771:472–84. [DOI] [PubMed] [Google Scholar]
- 36.Lopez JF, Liberzon I, Vazquez DM, et al. Serotonin 1A receptor messenger RNA regulation in the hippocampus after acute stress. Biol Psychiatry 1999;45:934–7. [DOI] [PubMed] [Google Scholar]
- 37.Flugge G. Alterations in the central nervous alpha 2-adrenoceptor system under chronic psychosocial stress. Neuroscience 1996;75:187–96. [DOI] [PubMed] [Google Scholar]
- 38.De Oliveira RM, Aparecida Del Bel E, Mamede-Rosa ML, et al. Expression of neuronal nitric oxide synthase mRNA in stress-related brain areas after restraint in rats. Neurosci Lett 2000;289:123–6. [DOI] [PubMed] [Google Scholar]
- 39.Sinchak K, Eckersell C, Quezada V, et al. Preproenkephalin mRNA levels are regulated by acute stress and estrogen stimulation. Physiol Behav 2000;69:425–32. [DOI] [PubMed] [Google Scholar]
- 40.Riegler M, Castagliuolo I, So PTC, et al. Effect of substance P on human colonic mucosa in vitro. Am J Physiol 1999;276:G1473–83. [DOI] [PubMed] [Google Scholar]
- 41.Mously M, Bronner C, Landry Y, et al. Direct activation of GTP-binding regulatory proteins (G-proteins) by substance P and compound 48/80. FEBS Lett 1990;259:260. [DOI] [PubMed] [Google Scholar]
- 42.Garland AM, Grady EF, Lovett M, et al. Mechanisms of desensitization and resensitization of G protein-coupled neurokinin1 and neurokinin2 receptors. Mol Pharmacol 1995;49:438–46. [PubMed] [Google Scholar]
- 43.Maa J, Grady EF, Kim H, et al. NK1 recptor desensitization and neutral endopeptidase terminate SP-induced pancreatic plasma extravasation. Am J Physiol 2000;279:G726–32. [DOI] [PubMed] [Google Scholar]
- 44.Pignatelli D, Magalhaes MM, Magalhaes MC. Direct effect of stress on adrenocortical function. Horm Metab Res 1998;30:464–74. [DOI] [PubMed] [Google Scholar]
- 45.Philibert D, Moguilewsky M, Mary I, et al. Pharmacological profile of RU 486 in animals. In: Beaulieu EE, Segal SJ, eds. The antiprogestin steroid RU 486 and human fertility control. New York: Plenum Press, 1985:46–68.
- 46.Garcia-Garcia L, Harbuz MS, Manzanares J, et al. RU-486 blocks stress-induced enhancement of proenkephalin gene expression in the paraventricular nucleus of rat hypothalamus. Brain Res 1998;786:215–18. [DOI] [PubMed] [Google Scholar]
- 47.Hernadez J, Carrasco J, Belloso E, et al. Metallothionein induction by restraint stress: role of glucocorticoids and IL-6. Cytokine 2000;12:791–6. [DOI] [PubMed] [Google Scholar]
- 48.Madeddu P, Emmanueli C, Varoni MV, et al. Regulation of bradykinin B2-receptor expression by oestrogen. Br J Pharmacol 1997;121:1763–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parnet P, Payan DG, Kerdelhue B, et al. Neuro-endocrine interaction on lymphocytes. Testosterone-induced modulation of the lymphocyte substance P receptor. J Neuroimmunol 1990;28:185–8. [DOI] [PubMed] [Google Scholar]