Abstract
Background: There are limited data on factors predicting response to azathioprine and uncertainty regarding the optimal duration of treatment.
Patients and methods: The notes of patients attending the Oxford IBD clinic from 1968 to 1999 were reviewed. Remission was defined as no need for oral steroids for at least three months and relapse was defined as active disease requiring steroids.
Results: A total of 622 of 2205 patients were treated with azathioprine (272 Crohn's disease, 346 ulcerative colitis, and four indeterminate colitis). Mean duration of the initial course of treatment was 634 days. The overall remission rates were 45% for Crohn's disease and 58% for ulcerative colitis. For the 424 patients who received more than six months of treatment, remission rates were 64% and 87%, respectively. Factors favouring remission were ulcerative colitis (p=0.0001), lower white blood cell (WBC) or neutrophil count (p=0.0001), higher mean cell volume (p=0.0001), and older age (p=0.05). For Crohn's disease, colonic disease favoured remission (p=0.03). Factors that were not significant were age, sex, lymphocyte count, and dose (mg/kg). The proportion of patients remaining in remission at one, three, and five years was 0.95, 0.69, and 0.55, respectively. The chance of remaining in remission was higher if WBC was less than 5×109 (p=0.03) and in male patients (p=0.01; Crohn's disease only). There was no difference in relapse rates between Crohn's disease and ulcerative colitis. After stopping azathioprine, the proportion of patients remaining in remission at one, three, and five years was 0.63, 0.44, and 0.35 (222 patients). Duration of azathioprine treatment did not affect the relapse rate after stopping treatment (p=0.68).
Conclusions: Azathioprine is effective treatment for ulcerative colitis and Crohn's disease. The efficacy of azathioprine is reasonably well sustained over five years.
Keywords: Crohn's disease, ulcerative colitis, azathioprine, neutropenia, efficacy
Azathioprine is widely used for the treatment of both Crohn's disease and ulcerative colitis. Clinical trial data and a meta-analysis have confirmed the efficacy of azathioprine for Crohn's disease.1–4 There are less efficacy data for ulcerative colitis and there are few data that have compared remission and relapse rates for ulcerative colitis and Crohn's disease.5–8 There are some trial data that found that neutrophil count was a predictor of induction and maintenance of remission.9,10 This needs to be confirmed in a clinical audit as well as identifying other predictive factors for remission. It is unknown if longer duration of azathioprine treatment alters the risk of relapse after stopping treatment. A retrospective study suggested that treatment for longer than three to four years was no better than withdrawal of azathioprine treatment.11 There are no other comparable long term studies of the efficacy of azathioprine. These clinical questions cannot be answered easily by clinical trials but require audits of large clinic populations with careful and long term follow up.
Azathioprine is a purine analogue that competitively inhibits the biosynthesis of purine nucleotides. Its mode of action is not well understood. Once absorbed azathioprine is almost entirely metabolised to 6-mercaptopurine. There are two alternative pathways—one to 6-thiouric acid (mediated by xanthine oxidase) and the other to 6-methylmercaptopurine (mediated by thiopurinemethyltransferase).12 Low levels of thiopurinemethyltransferase lead to toxicity, particularly leucopenia.13 Uncertainty regarding the degree of risk from neutropenia deters some prescribers from using azathioprine at effective doses and for longer treatment durations. Side effects other than leucopenia also appear to limit the usefulness of this drug for a significant proportion of patients. Two types of side effects have been reported. Firstly, “allergic” non-dose related side effects which include pancreatitis, fever, rash, malaise, nausea, diarrhoea, and hepatitis. Secondly, there are “non-allergic” and presumably dose related side effects such as leucopenia and some forms of hepatitis. Although these side effects may be dose related, the genotype of the 6-thiopurinemethyltransferase enzyme is probably a more important determinant of developing leucopenia.14 Clinic data over a long term period of review gives a useful perspective of the clinical risk and toxicity of this drug.
METHODS
The notes of patients attending the Inflammatory Bowel Clinic at the John Radcliffe Hospital from 1968 to 1999 were reviewed. A clinic patient was defined by attendance at the outpatient clinic over a period of at least 12 months. Patients who had started azathioprine treatment at another hospital were excluded. Patients who received azathioprine primarily for other indications (renal transplant, rheumatoid arthritis, autoimmune liver disease) were excluded. Remission was defined as no need for oral steroids (either prednisolone or budenoside) for at least three months and a Harvey-Bradshaw score of 4 or less. Patients who were well on low doses of steroids were reported as “remission not achieved”. The continued use of oral 5-aminosalicylic acid compounds and steroid or 5-aminosalicylic acid enemas was allowed within the definition of remission. Relapse was defined as the need for reintroduction of steroids or the need for a surgical procedure. Relapse of short duration was defined as needing a course of steroids for less than three months while azathioprine was continued. The efficacy of azathioprine treatment was only assessed if treatment had been continued for six months or more. Patients were considered lost to follow up if there was no clinic visit within the last two years. Data were collected for azathioprine treatment only; 6-mercaptopurine was used sparingly in the Oxford Inflammatory Bowel Disease clinic. The extent of involvement of disease was defined by colonoscopic or radiological examination and not by histological evidence of inflammation. Diagnosis was based on data from the last clinical evaluation. Patients who continued to have a diagnosis of indeterminate colitis at the end of the follow up period were combined with patients with Crohn's disease.
The dose of azathioprine for the efficacy data (mg/kg) was defined as the maintenance dose that induced remission. The initial dose was also recorded to determine if “early onset” side effects were dose related. The definition of leucopenia was a white blood count <3.0×109 and/or a neutrophil count of less than 2.0×109.
Statistical analysis was performed using SPSS version 9.0. The probabilities of relapse were calculated by life table analysis. The influence of concomitant variables on time to relapse was examined by the Cox proportional hazards model. Differences between means for continuous data were tested using analysis of variance.
RESULTS
The clinical notes of 2205 patients were reviewed; azathioprine treatment was given to 622 patients. There were 272 patients with Crohn's disease, four with indeterminate colitis (combined with Crohn's disease data in subsequent analysis), and 346 with ulcerative colitis. Mean duration of follow up from the start of azathioprine treatment was 2518 (1995) days (6.9 (5.5) years; mean (SD)). Mean follow up after diagnosis was 4943 (3395) days (13.5 (9.3) years; 8423 patient years of follow up). Mean duration of initial azathioprine treatment was 634 (771) days (1.7 (2.1) years; 1080 patient years). One hundred and forty two patients had a second or third course of azathioprine. Mean total duration of treatment was 762 days (2.1 years; 1350 patient years).
Reasons for stopping medication
At completion of the review, 517 patients had discontinued treatment. This was after a predetermined period of treatment, usually two years, for 203 patients (39%). These patients were in remission at the time of stopping medication. The other major reason for stopping medication was side effects (152 patients, 28%). The most common side effects were nausea and vomiting (68 patients). Treatment was stopped after a mean of 106 days (range 1–395). Seventeen patients had abnormal liver enzymes; 15 had a raised alkaline phosphatase and gamma-glutamyl transferase and only two patients had elevated transaminases. The elevated liver enzymes returned to normal after stopping the medication for all 17 patients. Treatment was discontinued between 24 and 270 days after onset (mean 82 days). Severe epigastric pain was experienced by six patients although only two patients had a documented elevated serum amylase. Other side effects included generalised warts (two), paraesthesiae (one), flushing (one), and dizziness (one).
Leucopenia was observed during treatment in 29 patients (4.6%). The mean dose of azathioprine at which leucopenia was observed was 1.77 mg/kg. The azathioprine dose was ≤100 mg for 19 patients and >100 mg for 10 patients. The medication was stopped because of leucopenia in 21 patients. Other patients were managed by dose reduction or by observation (four patients in each group). Two patients had significant pancytopenia. Mean duration of treatment before the onset of leucopenia was 421 days (range 47–1514). Five patients developed leucopenia in less than three months, seven patients in 3–6 months, three patients in 6–12 months, eight patients in 12–24 months, and five patients developed leucopenia after 24 months of treatment (the highest duration of treatment was 50 months). Nine patients had episodes of sepsis during azathioprine treatment that could be related to immunosuppression. Only four episodes of sepsis were related to neutropenia. Three patients required treatment with intravenous antibiotics and there was no mortality. Five patients had infective complications but did not have neutropenia. One patient presented with a sore throat and a large mouth ulcer with a nadir of neutrophils of only 2.3×109. One patient had cytomegalovirus hepatitis, another had sacral herpes zoster infection, and two patients had generalised warts. Three patients (out of the 2205 patients with inflammatory bowel disease) had neutropenic related sepsis related to other medications. Two patients had sulphasalazine induced pancytopenia (one patient had life threatening Pseudomonas septicaemia). Another patient died from neutropenic sepsis eight years after completing a four year course of azathioprine. Neutropenia was considered to be due to chlorpromazine.
Other reasons for discontinuation of medication were that the medication was considered to be ineffective (46), surgery become necessary (68), the patient was uneasy about the potential side effects and requested stopping the medication (41), or the patient conceived or wished to become pregnant while off the medication (seven).
Induction of remission
A total of 424 patients completed six months of azathioprine treatment. For these patients remission was achieved in 64% of patients with Crohn's disease and 87% with ulcerative colitis (p=0.0001). Overall remission rates (including all patients treated with azathioprine) were 45% and 58% for Crohn's disease and ulcerative colitis, respectively. Factors predictive of achieving remission are listed in table 1 ▶. Significant factors were a lower white blood count (p=0.0001), lower neutrophil count (p=0.0001), higher mean cell volume (p=0.0001), and an older age when treatment was given (p=0.05). Factors that were not significant were weight, dose of azathioprine (mg/kg), age at diagnosis, and lymphocyte count. For Crohn's disease patients analysed separately, colonic disease was associated with a higher rate of remission (p=0.03). By multiple logistic regression the independent factors in the model were white blood count (or neutrophil count) (p=0.0001), diagnosis (Crohn's disease or ulcerative colitis; p=0.001), and mean cell volume (p=0.004). Mean cell volume and white blood count (or neutrophil count) were closely correlated (r=0.8, p=0.01) but were still independent factors in the model.
Table 1.
Factors predicting remission on azathioprine treatment (mean and 95% confidence intervals)
| Remission achieved | Remission not achieved | p Value | |
| Neutrophil count (×109) | 3.83 (3.63; 4.02) | 5.85 (5.28; 6.42) | 0.0001 |
| WBC (×109) | 5.86 (5.63; 6.10) | 7.91 (7.32; 8.51) | 0.0001 |
| MCV | 93.2 (92.1; 94.2) | 88.8 (87.2; 90.4) | 0.0001 |
| Age when Rx given | 38.0 (36.4; 39.7) | 34.7 (32.0; 37.5) | 0.05 |
| Weight (kg) | 69.8 (68.1; 71.6) | 66.6 (63.6; 69.7) | 0.07 |
| Age at diagnosis | 31.1 (29.6; 32.7) | 28.7 (25.9; 31.4) | 0.13 |
| Dose (mg/kg) | 1.65 (1.57; 1.74) | 1.64 (1.59; 1.68) | 0.74 |
| Lymphocyte count (×109) | 1.33 (1.26; 1.40) | 1.33 (1.15; 1.50) | 0.96 |
WBC, white blood cell count; MCV, mean cell volume.
Relapse
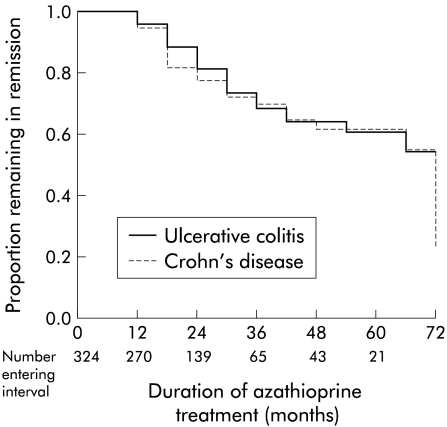
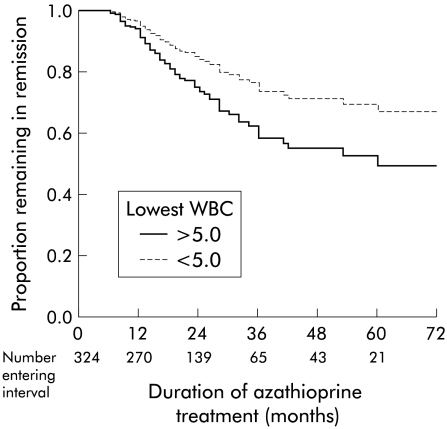
For the 324 patients who achieved remission, 250 patients remained in remission during the treatment period (28 were still continuing treatment at the time of follow up) (table 2 ▶). Using a strict definition of relapse (including patients with a short relapse), the proportion of patients still in remission at 12, 24, 36, 48, and 60 months was 0.95, 0.90, 0.69, 0.63, and 0.62, respectively (life table analysis; data for Crohn's disease and ulcerative colitis combined). If patients with “short relapse” are included as “no relapse”, the proportion of patients still in remission at 12, 24, 36, 48, and 60 months was 0.99, 0.92, 0.85, 0.81, and 0.81, respectively. Factors predictive of remaining in remission (while still on treatment) were determined by the Cox proportion hazards model. The relapse rates were similar for ulcerative colitis and Crohn's disease (p=0.5) (fig 1 ▶). Patients with a minimum white blood count of less than 5.0×109 had a lower risk of relapse (p=0.03) (fig 2 ▶). There was a trend for patients aged more than 36 years at the time when azathioprine treatment was started to have a lower risk of relapse (p=0.057). For patients with Crohn's disease, male sex was associated with a lower risk of relapse (p=0.01). There was no sex difference for patients with ulcerative colitis. The time taken to achieve remission was not a significant factor for predicting relapse (p=0.6).
Table 2.
Outcome while on azathioprine treatment for the 424 patients who were given treatment for more than six months
| Crohn's disease | Ulcerative colitis | |
| No relapse | 91 | 159 |
| Short relapse | 16 | 26 |
| Relapse | 15 | 17 |
| Remission not achieved | 70 | 30 |
| Total | 192 | 232 |
Figure 1.
Cox regression analysis of the proportion of patients remaining in remission during azathioprine treatment related to diagnosis of inflammatory bowel disease (324 patients). There was no difference in relapse rate between patients with ulcerative colitis and Crohn's disease.
Figure 2.
Cox regression analysis of the proportion of patients remaining in remission during azathioprine treatment related to minimum observed white blood cell (WBC) count during treatment (324 patients). Patients with a WBC count of less than 5.0×109 were more likely to remain in remission (p=0.03).
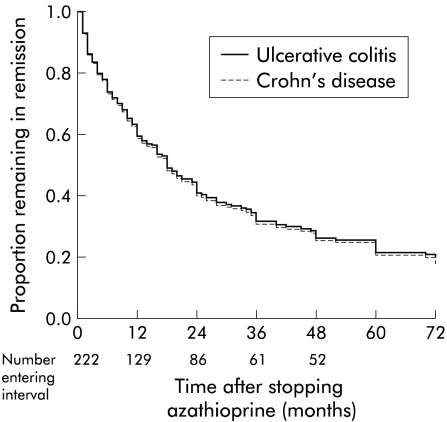
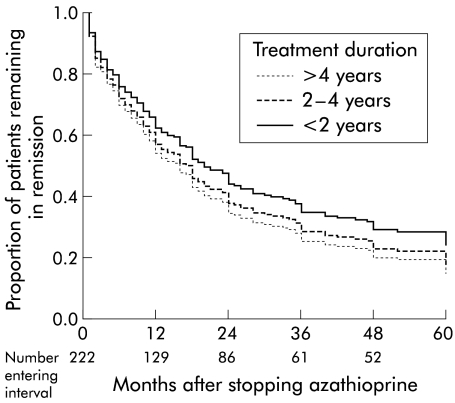
A total of 222 patients stopped azathioprine while still in remission and therefore could be evaluated for relapse rates after stopping medication (table 3 ▶). The proportion of patients still in remission after 12, 24, 36, 48, and 60 months was 0.63, 0.44, 0.34, 0.28, and 0.25, respectively (fig 3 ▶). There were no significant predictive factors. One hundred and fifteen patients had been treated with azathioprine for more than two years, 79 patients for 2–4 years, and 36 patients for more than four years. Duration of azathioprine treatment before stopping medication did not affect the chance of staying in remission after stopping medication (p=0.40) (fig 4 ▶).
Table 3.
Outcome after stopping azathioprine for 222 patients who were in remission at the time of stopping azathioprine
| Crohn's disease | Ulcerative colitis | |
| No relapse | 16 | 36 |
| Short relapse | 14 | 28 |
| Relapse | 49 | 79 |
| Total | 79 | 143 |
Figure 3.
Cox regression analysis of the proportion of patients remaining in remission after stopping azathioprine treatment related to diagnosis of inflammatory bowel disease (222 patients). There was no difference in relapse rate between Crohn's disease and ulcerative colitis.
Figure 4.
Cox regression analysis of the proportion of patients remaining in remission after stopping azathioprine related to duration of azathioprine treatment (222 patients). There was no difference in relapse rate according to duration of treatment before stopping azathioprine.
DISCUSSION
This study confirms the safety and efficacy of azathioprine for the treatment of inflammatory bowel disease. This was a retrospective review and hence has some limitations but long term data are critical for clinical decision making and are unlikely to be obtained from prospective data.
There was no drug related mortality over a 30 year period. Neutropenic sepsis was not a major problem and in fact the most serious episode of sepsis was related to sulphasalazine. In general, the clinic followed the guidelines for follow up and blood testing suggested by St Marks Hospital (two monthly blood tests after the first three months).15 The proportion of patients with leucopenia was similar to previous reports (2–3.8%).15,16 The incidence of other side effects was also similar to previous reports except that pancreatitis appeared to be less common (although serum amylase level was not obtained in all patients).16,17 Epigastric pain requiring hospitalisation but without evidence of pancreatitis (normal amylase) was more common. Occasionally, retreatment at a later time and/or at a lower dose was successful. Nausea and vomiting did not appear to be dose related and dose reduction was successful only for a minority of patients.
This study confirms the efficacy of azathioprine for both Crohn's disease and ulcerative colitis. The remission rates achieved and acceptable maintenance of remission with ongoing treatment make azathioprine a very valuable part of the treatment of inflammatory bowel disease. This result is consistent with clinical trial data.1–8 A meta-analysis of randomised studies of azathioprine in Crohn's disease gave an odds ratio of 3.1 for inducing remission and an odds ratio of 2.3 for maintaining remission.4 Candy et al randomised patients with Crohn's disease to treatment with azathioprine plus prednisone or prednisone alone. The remission rates at 12 weeks were the same but after 15 months 42% of patients receiving azathioprine achieved and maintained remission compared with 7% on placebo (p=0.001).3
The white blood and neutrophil counts were both good predictors of achieving and maintaining remission but the lymphocyte count had no value for predicting remission. White blood count and mean cell volume were closely correlated but were independent variables for predicting remission (logistic regression analysis). These data have modest clinical use because of the variable onset of fall in white blood count and significant overlap between responders and non-responders. In the first few months there may be no change in white blood count possibly because of the inflammatory activity and also because of steroid treatment. Many patients with a normal or “high normal” white blood count had a good response to azathioprine. It is debatable whether dose increases should be based on achieving a fall in white count or a rise in mean cell volume but the presence of either of these two markers is an encouraging sign for the patient and physician. In the study of Candy et al, leucopenia requiring dose reduction was associated with sustained remission. Median white blood count at completion of 15 months of treatment was 4.9×109 (interquartile range 3.9–5.7) for responders compared with 6.8×109 (5.1–9.0) for non-responders (p=0.005).3 Colonna and Korelitz also found a strong positive correlation between the extent of drug induced leucopenia and clinical outcome.9 A low white blood count was also a significant variable for prediction of remaining in remission. The better outcome for older patients and male sex was also found in a French study of 157 patients with Crohn's disease in remission for more than six months.11 The site of disease involvement for patients with Crohn's disease was not significant in the French study although another study of 95 patients showed a better outcome for patients with colonic disease (similar to this study).18
In this study, azathioprine was more likely to achieve remission in patients with ulcerative colitis than Crohn's disease but was equally effective for maintenance of remission. There are fewer data on the efficacy of azathioprine for the treatment of ulcerative colitis and no good comparative data with Crohn's disease. Two controlled trials have shown a steroid sparing effect for chronic active disease and an earlier trial gave equivocal results.5–8 Hawthorne et al studied 79 patients with ulcerative colitis who had been receiving treatment for more than six months and were randomised to continuing treatment or withdrawal of treatment (67 patients were off steroids completely). The one year relapse rate was 36% (12/33) for patients continued on azathioprine and 59% (20/34) for patients who discontinued treatment.8 For patients in remission for more than six months the relapse rate on treatment was 31% (8/26) compared with 61% (17/28) for patients discontinuing treatment. In a similar retrospective review using 6-mercaptopurine in 105 patients with ulcerative colitis the remission rate was 65% (similar to this study).19
Life table analysis shows that maintenance azathioprine treatment is effective for up to five years of treatment. There is a gradual but acceptable increase in the proportion of patients who have relapsed over time. There is no suggestion that the effectiveness of treatment “wears out” after a specific duration. There is no support for the concept that treatment should be stopped after 3–4 years (because it is no better than placebo). A French study of 157 patients with Crohn's disease in remission for at least six months compared the relapse rate of 115 patients who continued treatment with 42 patients who stopped treatment. The proportion remaining in remission at 12, 36, and 60 months was 0.89, 0.78, and 0.68, respectively. For the 42 patients who stopped treatment the proportion of patients still in remission at 12, 36, and 60 months was 0.62, 0.39, and 0.25, respectively.11 These data are remarkably similar to our data. The authors concluded that azathioprine was effective for at least four years but observed that the two year relapse rate after four years of treatment appeared to be similar whether treatment was continued or stopped. However, this observation was based on small numbers (only nine patients). O'Donoghue et al also reported a similar relapse rate of 41% one year after stopping treatment (proportion in remission 0.61).2 Data from our study using Cox proportional hazards modelling showed that there was no difference in relapse rates for patients treated for <2 years, 2–4 years, or >4 years duration.
Azathioprine is an established medication for the treatment of inflammatory bowel disease but there are many ways in which greater benefit can be obtained. Prescribing by a strict mg/kg schedule (at least 2 mg/kg) may increase the likelihood of giving a dose that will induce remission. The lack of dose-response seen in this study may be because treatment was given over a relatively narrow dose range and few patients were treated with recommended doses—up to 2.5 mg/kg. Patients with Crohn's disease frequently required surgery before completing a six month course of treatment. Many patients were operated on for obstructive symptoms that are less likely to respond to medical treatment. This is a justification for starting treatment early before irreversible fibrosis necessitates an operation for obstruction.
Increasing the duration of treatment will keep patients in remission for longer. A survey of British gastroenterologists showed that there was a marked variation in duration of use of azathioprine. Forty six per cent of gastroenterologists were using azathioprine for less than two years and only 17% were continuing treatment for four years or longer. Consultants with more experience of azathioprine in ulcerative colitis used azathioprine at higher maintenance doses for longer periods, and in patients with less extensive disease.20 The main argument against longer durations of treatment is the long term risk of malignancy. Connell et al observed no increased risk of malignancy in 755 patients with inflammatory bowel disease followed for a median of nine years from the start of azathioprine treatment.21 These are reassuring data but further studies from similar large clinic populations are required.
In summary, this study has shown good efficacy for azathioprine treatment sustained over at least five years with minimal toxicity and no mortality from neutropenic related sepsis over a 30 year review period.
REFERENCES
- 1.Present DH, Korelitz BI, Wisch N, et al. Treatment of Crohn's disease with 6-mercaptopurine. A long-term randomised double-blind study. N Eng J Med 1980;402:981–7. [DOI] [PubMed] [Google Scholar]
- 2.O'Donoghue DP, Dawson AM, Powell-Tuck K, et al. Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn's disease. Lancet 1978;ii:955–7. [DOI] [PubMed] [Google Scholar]
- 3.Candy S, Wright J, Gerber M, et al. A controlled double blind study of azathioprine in the management of Crohn's disease. Gut 1995;37:674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson DC, May GR, Fick GH, et al. Azathioprine and 6-mercaptopurine in Crohn's disease. A meta-analysis. Ann Intern Med 1995;123:132–42. [DOI] [PubMed] [Google Scholar]
- 5.Kirk AP, Lennard-Jones JE. Controlled trial of azathioprine in chronic ulcerative colitis. BMJ 1982;284:1291–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg JL, Wall AJ, Levin B, et al. A controlled trial of azathioprine in the management of chronic ulcerative colitis. Gastroenterology 1975;69:96–9. [PubMed] [Google Scholar]
- 7.Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: final report of a controlled therapeutic trial. BMJ 1974;4:627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawthorne AB, Logan RFA, Hawkey CJ, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ 1992;305:20–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colonna T, Korelitz BI. The role of leukopenia in the 6-mercaptopurine induced remission of refractory Crohn's disease. Am J Gastroenterol 1994;89:362–6. [PubMed] [Google Scholar]
- 10.Berg PS, George J, Present DH, et al. 6MP—is leukopenia required to induce remission in the treatment of ulcerative colitis. Gastroenterology 1996;110:A863. [Google Scholar]
- 11.Bouhnik Y, Lémann M, Mary J-Y, et al. Long-term follow-up of patients with Crohn's disease treated with azathioprine or 6-mercaptopurine. Lancet 1996;347:215–19. [DOI] [PubMed] [Google Scholar]
- 12.Sandborn WJ. Azathioprine: State of the art in inflammatory bowel disease. Scand J Gastroenterol 1998;33(suppl 225):92–9. [DOI] [PubMed] [Google Scholar]
- 13.Yates CR, Krynetski EY, Loennechen T, et al. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprie and mercaptopurine intolerance. Ann Intern Med 1997;126:608–14. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, Simmon JD, Orchard TR, et al. Allelic variants of thiopurine methyl transferase are associated with azathioprine-induced leucopenia. Gastroenterology 2000;119:A338. [Google Scholar]
- 15.Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut 1993;34:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Present DH, Meltzer SJ, Krumholtz MP, et al. 6-Mercaptopurine in the management of inflammatory bowel disease: short and long-term toxicity. Ann Intern Med 1989;111:641–9. [DOI] [PubMed] [Google Scholar]
- 17.Kirschner BS. Safety of azathioprine and 6-mercaptopurine in pediatric patients with inflammatory bowel disease. Gastroenterology 1998;115;813–21. [DOI] [PubMed] [Google Scholar]
- 18.Doménech E, Aldeguer X, Cabré E, et al. Azathioprine in inflammatory bowel disease: an 8-year study of response predictors. Gastroenterology 1998;114:967. [Google Scholar]
- 19.George J, Present DH, Pou R, et al. The long-term outcome of ulcerative colitis treated with 6-mercaptopurine. Am J Gastroenterol 1996;91:1711–14. [PubMed] [Google Scholar]
- 20.Stack WA, Williams D, Stevenson M, et al. Immunosuppressive therapy for ulcerative colitis: Results of a nation-wide survey among consultant physician members of the British Society of Gastroenterology. Aliment Pharmacol Ther 1999;5:569–75. [DOI] [PubMed] [Google Scholar]
- 21.Connell WR, Kamm MA, Dickson M, et al. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet 1994;343:1249–52. [DOI] [PubMed] [Google Scholar]