Abstract
Background: Few pancreatic cancers are suitable for surgery and few respond to chemoradiation. Photodynamic therapy produces local necrosis of tissue with light after prior administration of a photosensitising agent, and in experimental studies can be tolerated by the pancreas and surrounding normal tissue.
Aims: To undertake a phase I study of photodynamic therapy for cancer of the pancreas.
Patients: Sixteen patients with inoperable adenocarcinomas (2.5–6 cm in diameter) localised to the region of the head of the pancreas were studied. All presented with obstructive jaundice which was relieved by biliary stenting prior to further treatment.
Methods: Patients were photosensitised with 0.15 mg/kg meso-tetrahydroxyphenyl chlorin intravenously. Three days later, light was delivered to the cancer percutaneously using fibres positioned under computerised tomographic guidance. Three had subsequent chemotherapy.
Results: All patients had substantial tumour necrosis on scans after treatment. Fourteen of 16 left hospital within 10 days. Eleven had a Karnofsky performance status of 100 prior to treatment. In 10 it returned to 100 at one month. Two patients with tumour involving the gastroduodenal artery had significant gastrointestinal bleeds (controlled without surgery). Three patients developed duodenal obstruction during follow up that may have been related to treatment. There was no treatment related mortality. The median survival time after photodynamic therapy was 9.5 months (range 4–30). Seven of 16 patients (44%) were alive one year after photodynamic therapy.
Conclusions: Photodynamic therapy can produce necrosis in pancreatic cancers with an acceptable morbidity although care is required for tumours invading the duodenal wall or involving the gastroduodenal artery. Further studies are indicated to assess its influence on the course of the disease, alone or in combination with chemoradiation.
Keywords: photodynamic therapy, pancreatic cancer
Worldwide, pancreatic cancer is one of the top 10 leading causes of cancer death, probably ranking about ninth and killing about 6500 patients per year in the UK.1 In series from specialised centres, over 10% may be resectable at presentation2 but in larger population based studies the number undergoing resection with curative intent can be as low as 2.6%.3 Even after resection, median survival is only 12–18 months and no more than 10–20% of resected patients survive five years.4,5 In skilled hands, the operative mortality can be close to zero although more typical values are about 5% for specialised units and 10% for those who perform less cases, but it is a major procedure with a prolonged recovery period.6,7
Options available for the treatment of inoperable patients are largely limited to radiotherapy, chemotherapy, or some combination of the two. 5-Fluorouracil is probably the most useful single agent for symptomatic relief although no agent has been shown to have a convincing benefit on survival.8 Gemcitabine may also have value for palliation.9 Although one small trial showed a modest improvement in median survival treating locally advanced pancreatic cancer with external radiation in combination with chemotherapy (one year survival increased from 19% to 41%),10 morbidity was high and the benefit has not been confirmed in other studies.
Overall, the long term prognosis of the disease is poor with a one year survival rate of no more than approximately 10%. For non-metastatic disease, median survival is 6–10 months although for those with metastatic disease at presentation, median survival is a dismal 3–6 months.11 A new minimally invasive treatment capable of local destruction of pancreatic cancer with low morbidity may have a place in the treatment of this disease.
Photodynamic therapy (PDT) is a way of producing localised tissue necrosis with light (most conveniently from a laser) after prior administration of a photosensitising agent in the presence of oxygen.12 The cytotoxic intermediary is thought to be singlet oxygen. As the biological effect is photochemical, not thermal, there is little damage to connective tissues such as collagen and elastin, which helps to maintain the mechanical integrity of hollow organs like the gastrointestinal tract.13 Furthermore, as the light used is non-ionising, PDT does not carry the cumulative toxicity associated with radiotherapy. Once a PDT treated area has healed, it can be treated again if necessary. Much of the early interest in PDT centred around the selective retention of photosensitisers in malignant tissue compared with the adjacent normal tissue in which the tumour arose as this raised the possibility of selective destruction of cancers. Unfortunately, although there is some selectivity of uptake, this is rarely enough to make selective tumour destruction feasible and there is essentially always some necrosis in adjacent normal tissue where normal and neoplastic tissue meet. Nevertheless, if necrosis of normal tissue heals safely without loss of the mechanical integrity of the organ, PDT may have an important role to play in the local destruction of a range of cancers.14 In gastroenterology, it has been shown to be of value in treating small localised but inoperable oesophageal and gastric cancers.15,16
Although most work on PDT to date has been on lesions in the wall of hollow organs or on the skin, recent interest has examined more its potential for treating lesions of solid organs such as the pancreas. In view of the close proximity of the pancreas to vital structures such as the stomach, duodenum, biliary tree, and major blood vessels, it is essential to understand how well these structures can tolerate PDT before contemplating clinical studies. We undertook studies on normal hamsters using three photosensitising drugs: aluminium disulphonated phthalocyanine (AlS2Pc; synthesised at Imperial College London, UK),17 5-amino laevulinic acid (ALA; Levulan, DUSA Pharmaceuticals, Valhalla, New York, USA),18 and meso-tetrahydroxyphenyl chlorin (mTHPC, temoporfin; Foscan, Scotia Pharmaceuticals, Stirling, UK).19 The results were broadly similar with all three. Necrosis was produced in the normal pancreas, stomach, duodenum, and the common bile duct but this healed safely with the exception of the duodenum where some free and sealed perforations were seen. There was less duodenal damage with ALA than with AlS2Pc and mTHPC. In the arteries, there was endothelial loss and loss of smooth muscle in the media but the endothelium regenerated within a few days. Separate experiments have shown that there is no risk of thrombosis, no reduction in the mechanical strength of the arterial wall, and no evidence of aneurysm formation.20
Several groups have undertaken experiments on cancers transplanted into the hamster pancreas using haematoporphyrin derivative and its partly purified derivatives, dihaematoporphyrin ether and porfimer sodium (Photofrin; QLT Phototherapeutics, Vancouver)21 and pheophorbide A.22 We have performed these experiments using aluminium sulphonated phthalocyanine (AlSPc),23 ALA,24 and mTHPC.25 All results were broadly similar. It was possible to produce necrosis in the cancer and there was even some selectivity of effect between the cancer and adjacent normal pancreas. This was thought to be due not to selectivity of retention of the photosensitiser but to a constituent of the normal pancreas that reacted with singlet oxygen, perhaps glutathione, that was not present in the cancer. With all of the photosensitisers, some animals had sealed duodenal perforations but these seemed well tolerated, and the one randomised study which used ALA24 showed a significantly increased survival time for PDT treated tumour bearing animals compared with untreated controls.
With these encouraging experimental results, it was felt justified to undertake a pilot clinical study. We decided to use the photosensitiser mTHPC as this gave the largest zone of necrosis around a single treatment fibre (up to 12 mm in diameter) in the animal cancers and also because this drug requires the lowest light doses which would mean a shorter treatment time. This report describes a phase I study using PDT to treat cancers localised to the pancreas and its immediate vicinity in patients who were considered unsuitable for surgery. The aim was to assess technical feasibility, efficacy, and safety.
METHODS
Patient selection
Patients were selected from those referred to the pancreatic and biliary service at the Middlesex Hospital, London. All patients had been diagnosed as having a cancer of the pancreas, confirmed by biopsy or cytology as an adenocarcinoma. Ampullary cancers and those having the appearance of a cholangiocarcinoma were excluded. All had presented with obstructive jaundice which had been satisfactorily relieved by insertion of a biliary endoprosthesis. Patients were assessed by contrast enhanced, dual phase spiral computerised tomography (CT) scans and endoscopic retrograde cholangiopancreatography (ERCP), and reviewed by a pancreatic surgeon. Only patients thought to be unsuitable for surgery were considered for PDT. No patient with evidence of metastatic disease outside the immediate vicinity of the pancreas was accepted although those with local involvement of the duodenum or with nodes close to the pancreas were included. Other criteria for inclusion were a Karnofsky status of more than 60% with an anticipated survival of at least three months, being able to attend for follow up assessment, and no previous specific treatment for the cancer. The study was approved by the hospital ethics committee and all patients gave informed written consent.
Photodynamic therapy
The photosensitiser used was mTHPC. This is supplied as dark crystals which are reconstituted in a dedicated solvent containing polyethylene glycol, ethanol, and water just prior to use. It was administered as a single dose of 0.15 mg/kg by slow intravenous injection through a dedicated filter three days prior to light delivery.
Following injection of mTHPC, patients were kept in a darkened room to avoid skin photosensitivity reactions. For the first 24 hours the level of room light was kept below 100 lux (equivalent to a single 60 W bulb). On each subsequent day the permitted light exposure was increased by 100 lux so that by day 3 low level indoor lighting was acceptable and by seven days normal indoor lighting was safe. If more intense exposure was likely during this period, all areas of skin were shielded and dark glasses were worn. After one week, patients were able to go outdoors on dull days but not on bright sunny days. All patients were strongly advised to avoid direct exposure to sunlight or any other intense light source for at least a month after photosensitisation.
Light delivery
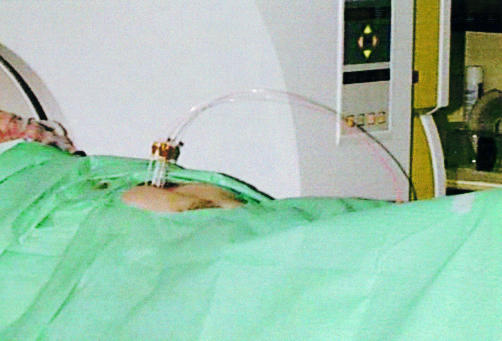
Treatment was undertaken three days after photosensitisation under subdued lighting conditions. The patient was sedated with diazepam or midazolam and given a systemic analgesic (pethidine) and prophylactic antibiotics prior to starting the procedure. The anterior abdominal wall was infiltrated with local anaesthetic. Up to six 19 G needles were inserted into the deepest part of the tumour by the radiologist with their tips separated by about 1.5 cm using a combination of ultrasound and CT guidance, the number being determined by the size and position of the tumour (fig 1 ▶).
Figure 1.
Percutaneous interstitial photodynamic therapy. Four needles were inserted into the pancreas under ultrasound guidance and their positions checked with a computerised tomography scan. A laser fibre was passed through each needle to deliver red light at 652 nm.
The light source used was a diode laser delivering red light at 652 nm (Applied Optronics Corporation, New Jersey, USA). All laser procedures were carried out in accordance with the local laser safety rules as laid down by the hospital laser protection advisor. Using a beam splitter (Diomed Ltd, Cambridge, UK) the light from the laser was divided equally between up to four 0.4 mm core diameter optical fibres. When all of the needles had been confirmed as correctly sited in the tumour, a fibre was passed down to the tip of each needle to leave 3 mm of bare fibre in direct contact with the tumour during delivery of the therapeutic light. In patients requiring six needles, the last two sites were illuminated after the first four rather than concurrently. Prior to use, the system was calibrated to deliver 100 mW at the tip of each fibre. This power setting was used to minimise photocoagulation of blood around the fibre tips which can reduce the amount of light delivered to the target site. After delivery of the planned light dose at the initial sites, under CT control, the needles and fibres were pulled back in approximately 1 cm steps as required to cover the entire tumour and the same light dose delivered at each position. The light dose delivered at each site varied from 20 to 40 J between patients although it was kept constant for all treated sites in individual patients.
Follow up
Following treatment patients were closely monitored on the ward. They were kept nil by mouth and on intravenous fluids and antibiotics until bowel sounds returned, after which oral intake and subsequently solids were slowly resumed. Contrast enhanced spiral CT scans were performed 3–5 days after PDT with flexible duodenoscopy or ERCP being performed approximately one week after treatment, prior to discharge from hospital. Subsequent CT scans were scheduled for one and three months after PDT with other investigations as clinically indicated.
All patients were followed closely for the remainder of their life, the frequency of visits depending on their clinical condition. Subsequent interventions were undertaken either in our hospital or in the referring hospital. Further clinical problems related to PDT or to progression of the disease were treated as they arose. Patients in a good general condition with evidence of progressive disease who so wished were considered for chemotherapy when they had recovered from PDT.
RESULTS
Patient selection
Sixteen patients with cancers localised in the region of the head of the pancreas referred to the Middlesex Hospital between November 1996 and March 1999 met the criteria for treatment with PDT and agreed to participate in this study. All presented with obstructive jaundice. Associated symptoms were weight loss (11) abdominal pain (6) diabetes (four requiring medication), and epigastric tenderness, lethargy, cholangitis, steatorrhoea, and anorexia (one of each). Patients were staged using the UICC TNM classification. Two patients had stage 1 cancers, eight had stage 2, and six had stage 3 (documented to have nodes of over 1 cm in diameter on CT scans at presentation).
In 14 cases the main reason that surgery was not felt appropriate was tumour involvement with or proximity to major blood vessels. The vessels most commonly involved at the time of PDT were the superior mesenteric vein (12 cases), the superior mesenteric artery (three cases), the portal vein (six cases), and the gastroduodenal artery (two cases). In two cases with small tumours without obvious vascular involvement or other spread at presentation, surgery was not undertaken because of the poor general condition of the patient. No patient had evidence of distant metastases. Endoscopically, 10 had a normal duodenum at presentation although on CT scans involvement of the duodenum below the mucosa was detected in six of these so that only four had an entirely normal duodenum prior to PDT.
Ten men and six women were included, aged 46–77 years (median 66). Time from diagnosis to first PDT varied from one to five months (median 2.5). Fifteen patients were treated initially with PDT using mTHPC and one using ALA. As the treatment with ALA was ineffective, PDT was repeated five weeks later in this patient using mTHPC. Three other patients had repeat treatments using mTHPC, one of whom had two further treatments. In 11 cases the tumour arose in the head of the pancreas. In three cases it was thought to arise in the periampullary region although in two of these it involved the head of the pancreas at presentation. In the other two cases, it was not possible to be sure of the origin. All treatments except one were undertaken percutaneously using ultrasound and CT guidance. Up to six needles were used for each patient with up to four fibre positions for each needle track. For all but three treatments, the light dose delivered was 20 J per site. For the other three treatments, the light dose was 25, 30, and 40 J per site. This did not increase the volume of necrosis around the site of the fibre tip and in one case (40 J per site) may have contributed to multiple subsequent complications (see below) and therefore later treatments used only 20 J per site. The total light energy delivered per treatment ranged from 40 to 480 J (median 240). The one treatment that was not carried out percutaneously was in the patient with a predominantly periampullary cancer. His first treatment was undertaken percutaneously but the fibres slipped after insertion and the treated area was predominantly normal pancreas at the side of the cancer. In view of the location of the cancer, treatment was repeated a few weeks later endoscopically by inserting a diffuser fibre into the distal common bile duct. Tumour sizes and details of treatment are given in table 1 ▶.
Table 1.
Details of patients, tumour size, treatment, and volume of photodynamic therapy (PDT) induced necrosis
| Patient No | Age/sex | Pre PDT maximum tumour diameter (cm) | Pre PDT tumour volume (cm3) | No of sites (No of fibres) | Total energy (J) (energy per site) | Volume of necrosis (cm3) |
| 1 | 77/F | 4.5 | 25 | 12 (4) | 240 (20) | 36 |
| 2 | 66/M | 5.0 | 63 | 16 (6) | 320 (20) | 51 |
| 3 | 62/F | 3.9 | 24 | 12 (4) | 480 (40) | 30 |
| 4 | 55/M | 6.0 | (A) 49 | 8 (4) | 160 (20) | 19 |
| (B) n/a | 8 (4) | 200 (25) | n/a | |||
| 5 | 67/F | 5.2 | 56 | 12 (4) | 240 (20) | 33 |
| 6 | 74/M | 4.9 | 43 | 8 (4) | 160 (20) | 52 |
| 7 | 77/F | 2.8 | 8.8 | 4 (1) | 80 (20) | 9 |
| 8 | 70/M | 2.5 | (A) 3.0 | 4 (4) | 80 (20) | 21 |
| (B) 4.2 | Diff* | 40 (20/cm) | 14 | |||
| 9 | 59/M | 4.9 | 47 | 16 (6) | 320 (20) | 55 |
| 10 | 75/M | 4.0 | 29 | 12 (4) | 240 (20) | 60 |
| 11 | 65/M | 4.5 | 44 | 16 (4) | 320 (20) | 23 |
| 12 | 46/F | 4.0 | 13 | 16 (4) | 320 (20) | 39 |
| 13 | 46/F | 3.0 | 14 | 12 (6) | 240 (20) | 35 |
| 14 | 75/M | 3.5 | (A) n/a | 12 (4) | 360 (30) | n/a |
| (B) 21 | 16 (6) | 320 (20) | n/a | |||
| (C) 49 | 16 (4) | 320 (20) | 55 | |||
| 15 | 74/M | 3.3 | 14 | 8 (4) | 160 (20) | 36 |
| 16 | 61/M | 5.7 | 60 | 14 (4) | 280 (20) | 54 |
*Diff, treatment carried out using a 2 cm diffuser fibre inserted through the ampulla endoscopically.
n/a, data not available.
General response
Patients were advised that they may have some pain at the site of drug injection and this occurred in 15 individuals but resolved without specific treatment in all cases. Eight patients had some skin photosensitivity to light, all within one month of injection, but no specific treatment was required.
There were no treatment related deaths. All patients had abdominal pain after the procedure, most requiring opiate analgesia for the first few days, but none had clinical evidence of pancreatitis and most resumed oral intake after approximately 48 hours. The maximum increase in amylase documented after PDT was 2.8 times the upper limit of normal and this was in the patient in whom the fibres slipped so the treated area was mostly normal pancreas. Nine of the 10 patients tested prior to PDT had abnormal pancreatic exocrine function on a pancreolauryl test. Five had diarrhoea after PDT (of whom three had intermittent diarrhoea before PDT) but all were reasonably controlled on pancreatic supplements. In all, 11 patients received pancreatic supplements. Using the definition of diabetes as fasting glucose >6.7 mmol/l and/or postprandial glucose >10 mmol/l, 14 patients were diabetic prior to PDT although only four required oral hypoglycaemics and none needed insulin.26 After PDT, only one required insulin for more than a few days and one other required oral hypoglycaemics that had not been necessary prior to PDT.
Median hospital stay after PDT was seven days (range 5–9) with three further days between drug injection and light delivery, with two exceptions: one had a major bleed (admission 26 days, as discussed below) and one whose general condition was poor required gastrostomy feeding due to her continuing anorexia (admission 30 days).
Tumour response
Early
The documented maximum tumour diameter prior to PDT was 2.5–6.0 cm (median 4.0) and tumour volume was 3–63 cm3 (median 27). In 12 cases the last pretreatment contrast enhanced CT was taken within one month of PDT but in the other four it was longer (6–11 weeks) so the cancer may have been larger at the time of treatment than indicated in table 1 ▶.
In all cases, contrast enhanced CT scans taken a few days after PDT with mTHPC showed new areas of non-enhancement, interpreted as zones of PDT induced necrosis (fig 2 ▶). In one patient, this area was biopsied and showed necrotic cancer (fig 3 ▶). However, the procedure was uncomfortable and caused a small haematoma. This resolved spontaneously but it was not considered justifiable to undertake post PDT biopsies in other patients. Overall, needle insertion caused a haematoma in six cases. All resolved spontaneously although transfusion was required in two patients. The volume of necrosis produced by PDT treatment ranged from 9.0 to 60.0 cm3 (median 36) and the volume of necrosis around individual fibre sites (averaged for individual patients) ranged from 1.4 to 5.1 cm3 (median 2.9). Assuming roughly spherical geometry, the typical radius of PDT necrosis around each treatment point was thus approximately 9 mm (range 7–11). The ratio of the volume of PDT induced necrosis to the volume of the tumour being treated ranged from 3.4 to 0.4 (median 1.1).
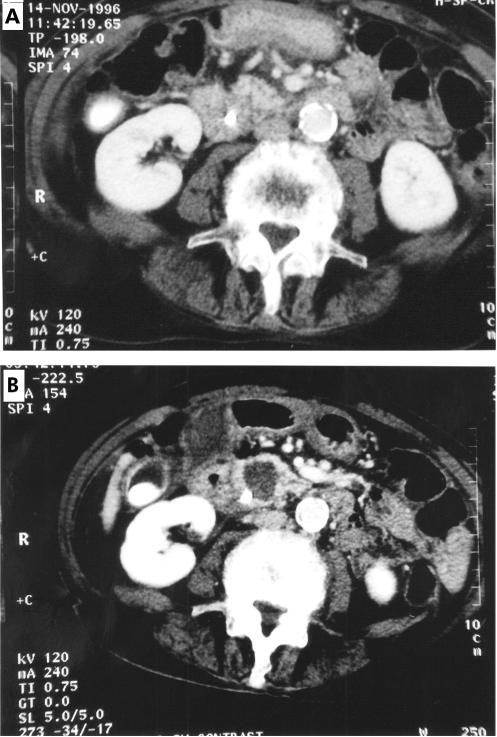
Figure 2.
Contrast enhanced computerised tomography scans of patient No 7. (A) Prior to photodynamic therapy (PDT), showing a 2.8 cm carcinoma in the head of the pancreas. (B) Four days after PDT, showing a large new area of non-enhancement. This patient had a plastic biliary stent in place at the time of treatment. Technically, this tumour was thought to be operable but the general condition of the patient was considered to be too poor.
Figure 3.
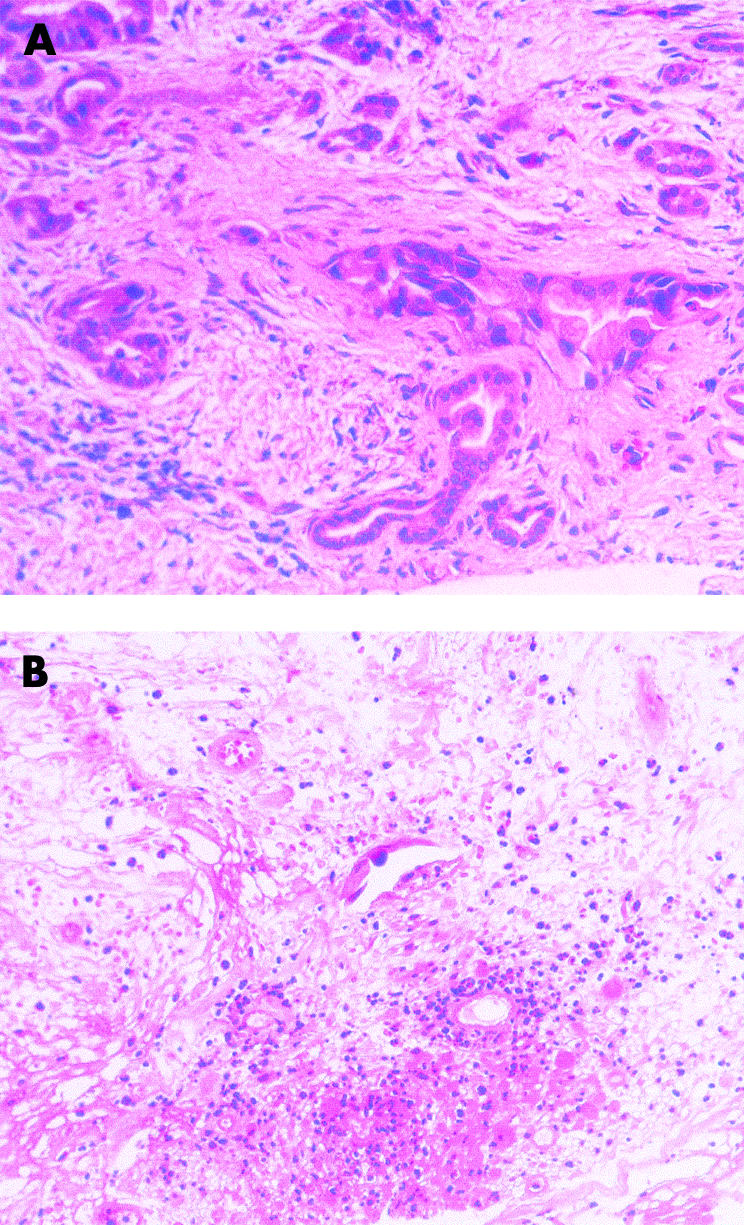
Percutaneous biopsies from patient No 7. (A) Biopsy taken before treatment confirming adenocarcinoma of the pancreas. (B) Biopsy taken four days after photodynamic therapy from an area that had become non-enhancing on computerised tomography after treatment. There was extensive necrosis. Residual glandular structures are lined by degenerative appearing, probably neoplastic epithelium. Prominent fibroblast cells are also noted (haematoxylin-eosin). Photomicrographs by Dr M Novelli.
In the early post PDT scans, the zone of necrosis was usually well defined although it was sometimes difficult to be sure whether remaining enhancing areas were normal or neoplastic. Oedema around the treated area was noted in 10 cases. In three cases no definite cancer could be seen in the head of the pancreas in the early follow up scans and in three others only tiny areas of viable cancer were seen.
Late
In most cases, the necrosed area of tumour healed safely without changing in size. In three cases the area of necrosis (and the overall size of the head of the pancreas) shrunk as it healed due to resorption of necrotic material. There was no CT or ERCP evidence of a pseudocyst, abscess, or pancreatic duct leak in any patient at any time after PDT. As healing proceeded it became much more difficult to identify the sites of previous PDT necrosis. In 14 cases the late stages of the disease were dominated by local tumour invasion and lymphadenopathy, often merging into each other, around the duodenum, around major blood vessels, up to the hilum, and retroperitoneally. Tumour did not regrow at the site of PDT necrosis but often regrew from the edges of the treated areas. In two patients multiple liver metastases were detected soon after PDT and their subsequent clinical course was dominated by this development. Two patients developed gross ascites in the terminal stages of their illness.
Three patients had chemotherapy when they had recovered from PDT. They survived 11, 12, and 15 months after PDT. One of these had several small tumour seedlings along a needle track in the anterior abdominal wall. These were treated with local excision and radiotherapy and did not recur. The only documented metastases outside the abdomen were in one patient with supraclavicular node involvement.
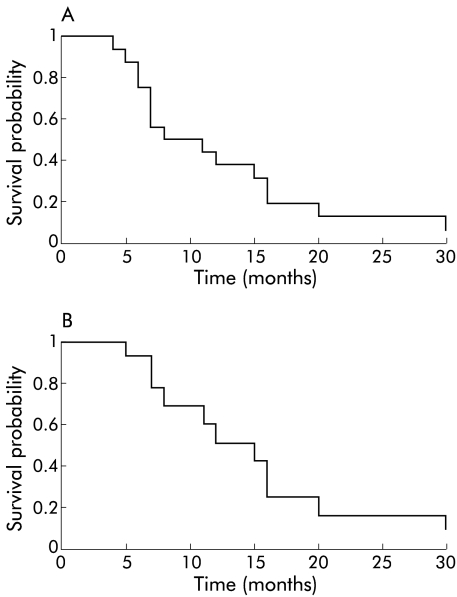
Survival time from PDT for all patients in the study ranged from 4 to 30 months (median 9.5), with one patient still alive at 31 months. As the prognosis of patients with cancers of the head of the pancreas is, in general, worse than that of periampullary cancers, data for those with definite cancers of the head of the pancreas were also analysed separately. This subgroup had a median survival from PDT of 12 months (range 5–20) with one patient still alive at 31 months. Kaplan-Meier survival plots are shown in fig 4 ▶ for all patients in the study (fig 4A ▶) and for those with cancers of the head of the pancreas (fig 4B ▶). Median survival for all patients from the time of diagnosis was 12.5 months (range 6–34) with one patient alive at 35 months.
Figure 4.
Kaplan-Meier curves showing survival times after photodynamic therapy. (A) All patients in study; median survival 9.5 months. (B) Patients with cancers of the head of the pancreas; median survival 12 months.
Biliary tract intervention after PDT
All patients in this study presented with obstructive jaundice. This was treated by insertion of a biliary stent before they were considered for PDT. In 14 cases the stent was inserted endoscopically and in two cases with a combined endoscopic and percutaneous procedure. To minimise the risk of stent problems after treatment, whenever practical, plastic stents were replaced with an expanding metal stent prior to PDT. The stents in situ at the time of PDT are shown in table 2 ▶.
Table 2.
Survival times and biliary interventions after photodynamic therapy (PDT)
| Patient No | Tumour location | Stent type at PDT | Time from 1st PDT to intervention | Procedure | Time from 1st PDT to death (months) | Time from diagnosis to death (months) | |
| No biliary intervention after PDT | 1 | HOP | Plastic | N/A | 7 | 9 | |
| 2 | HOP | Metal | N/A | 5 | 7 | ||
| 3 | Periamp | Metal | N/A | 6 | 8 | ||
| 4* | HOP | (A) Metal | N/A | 2nd PDT 14 weeks after 1st | 11 | 13 | |
| (B) Metal | |||||||
| Single prophylactic intervention | 5 | Periamp | Metal | 1 week | Stent trawled | 4 | 6 |
| 6 | HOP | Metal | 4 months | Stent trawled | 7 | 12 | |
| 7 | HOP | Plastic | 11 months | Routine change of plastic stent | 16 | 17 | |
| 8 | Periamp | (A) Plastic | 2nd PDT 10 weeks after 1st | 30 | 34 | ||
| (B) Nil† | 10 months | Biliary stent inserted prior to duodenal stent | |||||
| 9* | HOP | Plastic | 6 weeks | CBD duod-fistula. Metal to replace plastic stent | 15 | 18 | |
| 10 | SNC | Plastic | 6 weeks | CBD duod-fistula. Metal to replace plastic stent | 6 | 9 | |
| Intervention for cholangitis | 11 | SNC | Metal | 6 weeks | Plastic stent placed through metal | 7 | 10 |
| 12 | HOP | Plastic | 16 months | Plastic stent replaced | 20 | 24 | |
| 13* | HOP | Plastic | 6 weeks | Plastic stent replaced with metal | 12 | 14 | |
| 14 weeks | Plastic stent positioned through mesh of metal | ||||||
| Intervention for recurrent jaundice (not infected) | 14 | HOP | (A) Metal | 1 month | Metal stent trawled×4 in 4 months | 31 (alive) | 35 (alive) |
| (B) Metal | 2nd PDT, 5 weeks after 1st | ||||||
| (C) Metal | 5 months | Plastic stent inserted | |||||
| 9 months | Plastic stent replaced | ||||||
| 10 months | Plastic stent replaced | ||||||
| 11 months | 3rd PDT 46 weeks after 1st and plastic stent replaced | ||||||
| 15 | HOP | Metal | 6 months | Plastic stent placed through metal | 16 | 20 | |
| 8 months | Plastic stent removed. ALA-PDT to tumour in metal stent | ||||||
| 10 months | 2nd metal stent inside 1st | ||||||
| 16 | HOP | (A) Plastic | 5 weeks | Inadequate 1st PDT (ALA). Stent changed to metal for 2nd PDT, 5 weeks after 1st | 8 | 11 | |
| (B) Metal | 19 weeks | Percutaneous stent to relieve jaundice as duodenal stent in situ | |||||
| 29 weeks | Percutaneoius stent to relieve jaundice as duodenal stent in situ | ||||||
*Patients received chemotherapy.
HOP, head of pancreas; Periamp, periampullary; SNC, site of origin not clear; CBD, common bile duct.
†Light delivered endoscopically.
Biliary interventions required after PDT are summarised in table 2 ▶. Four patients required no further biliary intervention and six had single prophylactic stent changes. Six patients had more troublesome biliary problems. Three developed cholangitis requiring antibiotics. In two of these the distal end of a plastic stent was noted to be impacted against the opposite wall of a stenosed duodenum. In both of these cases the stent replacements were technically difficult. Three other patients developed obstructive jaundice which was difficult to treat. One required two percutaneous procedures as he had a duodenal stent in place and hence there was no endoscopic access to his ampulla.
Duodenal wall involvement
Abnormalities in the duodenal wall of these patients, as seen endoscopically, are summarised in table 3 ▶. They are divided into those present before PDT, those occurring or getting worse within six weeks of PDT (and likely to be related to PDT, at least in part), and those only appearing later in the course of the illness (which were less likely to have been related to PDT).
Table 3.
Duodenal effects of photodynamic therapy (PDT). Endoscopic findings are shown prior to PDT, in the first six weeks after PDT, and at longer follow up times, together with any intervention required for stenosis or haemorrhage
| Early effects (up to 6 weeks) | Late effects | |||||||
| Patient No | Endoscopy pre 1st PDT | Endoscopy | Symptoms | Treatment | Time after 1st PDT | Endoscopy | Symptoms | Treatment |
| 1 | Normal | Normal | — | — | — | — | — | — |
| 2 | Normal | Duodenal necrosis (small area) | — | — | — | — | — | — |
| 3 | Normal | Duodenal necrosis | (A) Bleeding | Embolisation | — | — | — | — |
| (B) Obstruction | duodenal stent | |||||||
| 4 | Stenosed | (A) Duodenal stent before PDT | — | — | 2 months | Tumour ingrowth in stent | Obstruction | 2nd duodenal stent |
| (B) Duodenal stent in situ | — | — | — | — | — | — | ||
| 5 | Ulcerated ampulla | More extensive ulceration, duodenal stenosis | — | — | — | — | — | — |
| 6 | Normal | Duodenal necrosis | Bleeding | Endoscopic injection | — | — | — | — |
| 7 | Normal | Normal | — | — | — | — | — | — |
| 8 | Ulcerated ampullary tumour | n/a | — | — | (A) 10 months | Stenosis | Obstruction | Duodenal stent |
| (B) 26 months | Tumour ingrowth | Obstruction | 2nd duodenal stent | |||||
| 9 | Normal | Duodenal necrosis. CBD—duodenal fistula | — | Biliary stent | — | — | — | — |
| 10 | Ulcerated duodenum and ampulla | CBD—duodenal fistula | — | Biliary stent | 5 months | Stenosis | Obstruction | Duodenal stent |
| 11 | Ampullary tumour | Necrosis around stent | — | — | — | — | — | — |
| 12 | Normal | Duodenal necrosis (small area) | — | — | 16 months | Stenosis | — | — |
| 13 | Normal | Necrosis around ampulla. Stenosis | — | — | 3 months | Stenosis | — | — |
| 14 | Normal | (A) Duodenal necrosis | — | — | 13 months (3 months after last PDT) | Tumour in duodenal wall | Bleeding | Transfusion |
| (B) CBD—duodenal fistula | ||||||||
| (C) Further duodenal necrosis | ||||||||
| 15 | Normal | Normal | — | — | 14 months | Stenosis | Obstruction | Duodenal stent |
| 16 | Haemorrhagic, distorted duodenum | Ulcerated duodenum | Obstruction | Duodenal stent | 4 months | Stenosis | Obstruction | Gastroenterostomy |
n/a, data not available; CBD, common bile duct
Ten patients had a normal duodenum endoscopically prior to PDT although CT scans on six of these individuals showed that the duodenum was involved with the cancer even though the mucosa was intact. All those noted to be abnormal endoscopically before PDT were also abnormal on CT.
In the first six weeks after PDT only three patients had a normal appearing duodenum endoscopically. In three patients there was a breakdown of the wall between the duodenum and common bile duct with loss of 2–3 cm of the common bile duct wall but there were no free duodenal perforations. Two patients had haemodynamically significant bleeds requiring transfusion 2–3 weeks after PDT. One was controlled by endoscopic injection of adrenaline but the other required embolisation of the gastroduodenal artery.
Beyond the first six weeks, small areas of PDT induced necrosis in the duodenal wall healed without sequelae although larger areas of necrosis may have contributed to later duodenal stenosis in two patients.
Quality of life
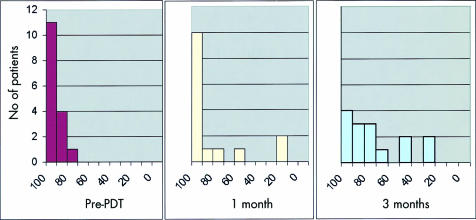
All patients in this study presented with obstructive jaundice and therefore their main symptom was relieved by biliary stenting undertaken prior to PDT. Six had abdominal pain at presentation. Four of these required analgesics (moderate in three and opiates in one). Most patients required opiates in the first few days after PDT but by one month only six required moderate analgesics and three required opiates. At 3–4 months, six were receiving moderate analgesics and seven were on opiates. All except two patients were discharged from hospital less than 10 days after PDT with a satisfactory oral intake and feeling comfortable. The Karnofsky performance status of patients immediately prior to treatment and at one and three months after PDT is shown in fig 5 ▶. Eleven had a Karnofsky performance status of 100 at the time of PDT and in 10 it returned to 100 one month after PDT.
Figure 5.
Karnofsky performance status of patients before photodynamic therapy (PDT) and at one and three months after PDT.
All patients were able to spend at least some time at home with their families after PDT. The percentage of time spent in hospital from the time of PDT (including admission for PDT but excluding admissions for chemotherapy or for terminal care in the last month of life) ranged from 5% to 38% (median 11%; only three patients spent more than 20% of their survival time in hospital). In addition, two patients had repeated admissions to hospices for respite care and several others had shorter hospice admissions, particularly for terminal care. Two of the patients who had chemotherapy spent more time in hospital due to complications from the chemotherapy than they did for PDT. Both had received 5-fluorouracil and mitomycin whereas the one who received gemcitabine tolerated chemotherapy better and had a longer survival time (15 months from PDT).
Although all patients required at least one further admission to hospital, in the majority of cases they felt comfortable at home until the problem arose, which was most frequently jaundice, with or without cholangitis (six cases), which resolved during a short hospital admission. The patient who required seven endoscopies over a period of a year for trawling or replacing his biliary stent and had PDT on three occasions led a reasonably active life at home and felt well. He is still alive more than two years after PDT.
In three cases the demand on hospital services and patient time was greater and the quality of life worse. The clinical course of the female patient who required a gastrostomy was dominated by pain, requiring multiple hospital and hospice admissions. She also developed supraclavicular metastases which responded well to radiotherapy. In the other two particularly difficult cases, the dominant clinical problem was duodenal obstruction.
DISCUSSION
This study confirms the feasibility of applying mTHPC mediated photodynamic therapy to cancers of the pancreas using an image guided percutaneous technique. It has proved possible to produce extensive areas of tumour necrosis that in some cases covered all the tumour visible on CT. There was no treatment related mortality, most patients were out of hospital in less than 10 days after treatment, and morbidity was considerably less than would be expected after surgery. The most important question to ask is how much it helps patients to have local tumour destruction of this nature in terms of either survival time or quality of life. The survival values are encouraging with a median of 9.5 months from PDT. Seven of 16 (44%) patients were alive one year after PDT; nine (56%) were alive one year after diagnosis. Two were alive two years after PDT. These values compare favourably with the median survival of 6–10 months from diagnosis in patients with non-metastatic locally advanced disease reported in other series11 although the true influence of PDT on survival can only be established in a randomised controlled study.
The cancers we treated were not all small, with a maximum diameter at the time of treatment of 2.5–6.0 cm, comparable with the size of those resected in larger surgical series. The three patients in whom no viable tumour was seen in the first scans after treatment did particularly well, surviving 16, 20, and 30 months after PDT. None had chemotherapy. Two of these had fairly small cancers (2.5, 2.8 cm) but the other was 4.0 cm in diameter at the time of PDT. Treatment may have influenced the course of their disease. The two patients with multiple liver metastases detected soon after PDT did poorly, surviving only four and five months after treatment. It is unlikely that PDT influenced their survival. For the other 11 patients in whom viable tumour was seen in the first scans after PDT and whose subsequent clinical course was dominated by local tumour spread in and around the head of the pancreas, it is not possible to comment on whether PDT influenced the course of the disease. All 16 of our cases were confirmed on histology or cytology to be adenocarcinomas although some of these may have been relatively slowly growing tumours. Those with rapidly growing lesions would be less likely to be suitable for PDT if there was an interval of more than a few weeks between diagnosis and treatment. There were many technical and organisational problems setting up these treatments and some patients came to us after a lengthy search for possible treatment options, and hence there was often a considerable delay between diagnosis and PDT. Although one patient with a periampullary tumour did particularly well (survival 30 months after PDT) as might be expected, others in this group did not, and therefore median survival was longer in those with cancers in the head of the pancreas than in the group as a whole.
With any new treatment, the benefits must be balanced against the risks. There are so many vital structures in the vicinity of the pancreas that it is essential to be sure that PDT does not cause any unacceptable damage to the normal pancreas or other adjacent tissues.
Our experimental work had suggested that PDT to the region of the pancreas was tolerated well. Nevertheless, we were concerned that extensive necrosis to the head of the pancreas might lead to severe pancreatitis. We found no clinical evidence of this. There was also concern that producing a large area of necrosis in the head of the pancreas might cause obstruction of the main pancreatic duct in the body and tail of the gland. However, there was no evidence that treatment exacerbated dilatation of the main pancreatic duct documented prior to PDT in 11 cases (associated with atrophy of the body of the gland in seven). The area of PDT necrosis produced inadvertently in the normal pancreas in one patient healed well with no complications. Nevertheless, most patients had poor exocrine pancreatic function before treatment which was worse after PDT requiring pancreatic supplements in 11. PDT did not exacerbate any abnormalities of glucose tolerance other than in the first few days after treatment. There was no evidence of leakage from the pancreatic duct or of pancreatic infection or an abscess. One of the major complications of resection or palliative surgery for pancreatic cancer is anastomotic breakdown27 but there does not seem to be a comparable risk after PDT.
In our hamster studies, the peripancreatic organ most vulnerable to PDT was the duodenum and a sealed duodenal perforation was a common finding.19 The human duodenum also seems to be vulnerable although we had no free perforations. In all three patients in whom the wall between the duodenum and common bile duct broke down, there was CT evidence of involvement of the duodenal wall in that area with tumour. Even so, these fistulae were all asymptomatic. Small areas of PDT induced necrosis in normal duodenal wall healed safely without sequelae but more extensive PDT induced duodenal necrosis may have contributed to stenosis.
Clinically significant duodenal stenosis was seen at some stage of the illness in six of our 16 patients (37%). This compares with a value in the literature of 15–20% of patients with pancreatic cancer who develop duodenal obstruction at some stage during their illness.28 In a series from this hospital, 19 of 100 patients developed symptomatic duodenal stenosis after biliary stenting for pancreatic cancer during a median survival period of five months,27 about half the median survival in the present study. Although the data available are limited, it is likely that the risk of duodenal stenosis increases with tumour size and length of survival.
In three of our cases, stenosis was clearly due just to the cancer, one patient presenting with obstruction prior to PDT and two others who obstructed several months after PDT in the terminal phase of the disease. Stenosis was only related to treatment in three cases and PDT was probably the major cause in no more than two cases. The three patients who developed duodenal stenosis from tumour progression alone had good symptomatic relief from an expanding metal stent placed in the duodenum endoscopically, as did the patient with the periampullary tumour who stenosed 10 months after PDT, most likely from duodenal scarring. The only major problems encountered were in the female patient who developed duodenal obstruction after PDT and subsequent embolisation of her gastroduodenal artery (in whom the obstruction may have been partly due to scarring following her embolisation) and in the male patient with tumour encircling the pylorus with probable partial obstruction prior to PDT. Although both had enteral stents inserted, these functioned poorly, possibly because the stents had a strong tendency to straighten after insertion and were unable to maintain their curvature sufficiently to provide adequate recanalisation around the curves of the duodenum. Future designs of enteral stent may overcome this problem. A gastric neuropathy may also contribute to gastric outlet delay in these patients but in this situation even a surgical gastroenterostomy may not work, as in our case with partial obstruction from tumour surrounding the pylorus prior to PDT.
There was concern that treatment of a tumour that encased or was in close proximity to a major blood vessel might lead to intra-abdominal or gastrointestinal tract haemorrhage. In six patients, a haematoma was documented immediately after needle insertion (prior to light delivery) although all six resolved without further intervention. The only two clinically significant bleeds associated with PDT induced tumour necrosis were into the gastrointestinal tract and were from the gastroduodenal artery, which was documented to course through the treated cancer in both cases. One of these patients was also one of the two individuals requiring transfusion for a needle related haematoma. In all patients in this study, tumour was seen to encase, compress, or distort other vessels at some stage of the illness, particularly the superior mesenteric vein and the portal vein, but it was rare for these vessels to be completely occluded even in the late stages of the disease and there was no evidence of blood loss from them. It is well documented that although PDT can destroy the endothelium and kill smooth muscle cells in the medial layer of the wall of normal arteries, there is little risk of thrombosis or perforation.20 Veins seem equally resistant. There is likely to be more risk if tumour actually invades the vessel wall but our experience suggests that for PDT in the region of the pancreas, we only need to worry about tumour involvement of arteries as large as the gastroduodenal artery. Furthermore, the patient who had the most severe bleed was treated with a higher light dose per fibre site than any other patient and this may have contributed to her complications.
All patients with stents in the common bile duct may have problems with obstruction, with or without infection, although metal stents usually remain patent for longer periods than plastic ones.29 In the present study, plastic stents were replaced by metal stents prior to PDT in several cases in an attempt to reduce the need for subsequent biliary intervention, although half of the patients had plastic stents in situ at the time of their first PDT. Both types of stent played an important part in follow up procedures. PDT is not a thermal technique and there is no evidence that treatment damaged either type of stent in any way. Stent blockages during follow up were due to tumour progression in the biliary tree or duodenum or accumulation of debris in the stent. Blocked metal stents could often be cleared by trawling, but when this was not possible, patency could usually be restored by inserting a plastic stent inside the metal one. If this became occluded, it could be replaced. On one occasion, a second metal stent was placed inside the first. Although most stent changes were straightforward, on two occasions the endoscopic procedure was technically difficult and one patient required two percutaneous procedures as he already had a duodenal stent in place. Managing biliary obstruction when the ampulla is not endoscopically accessible as in patients with duodenal stents is likely to be a difficult challenge in the future.
The data available are limited but the period of stent patency seemed comparable with that in other patients with pancreatic malignancies not receiving PDT who survived for similar periods of time. None of the episodes of stent blockage appeared to be related to PDT with the exception of one case in which PDT scarring around the ampulla may have caused the distal end of the plastic stent to impact on the opposite wall of the duodenum.
We were reassured by the relatively low incidence of serious treatment related complications in this study, the absence of any treatment related mortality, and how well the procedure was tolerated. PDT is simpler and has a shorter recovery time than any form of pancreatic resection. Although full quality of life assessments were not done in this pilot study, it was most encouraging to see that only three patients had a lower Karnofsky performance status one month after PDT than they did prior to PDT and that at this time, 10 patients had a status of 100%.
This was a phase I study, aiming to deliver treatment just to the tumour area in the pancreas. The light doses used were based on results from animal studies. There was some variation in the volume of necrosis around each treatment site, even with the standard light dose of 20 J, for which there are several possible explanations. An unexpectedly small volume of necrosis may be due to a low tissue concentration of photosensitiser, or a small amount of blood around the fibre tip may have reduced light transmission into the target tissue, as has been shown to occur in other organs.30,31 In future studies, it may be necessary to develop ways of monitoring drug levels and light intensity in tissue during PDT, perhaps by inserting thin fibreoptic sensors into strategic points in the area being treated. The one patient in whom we used a light dose of 40 J per site had the most serious acute complications (major haemorrhage and duodenal stenosis), and therefore this dose is probably too high. Nevertheless, the treatment conditions used in this study seemed appropriate for proceeding with further clinical trials, especially as PDT can be repeated if important areas of tumour are missed. The typical radius of PDT necrosis around each treatment site was about 9 mm, and hence a fibre separation of 12–15 mm seems appropriate to achieve confluent necrosis.
In most cases, tumour regrew around the edges of the PDT treated area and so in future studies it would be desirable to extend the treated area beyond the tumour margins identified on the pretreatment scans. Our results suggest that it is likely to be safe to treat around most major blood vessels although caution is required if vessels as large as the gastroduodenal artery pass through the tumour bulk. Caution is also required if the cancer involves or is close to more than a small area of the wall of the duodenum.
This is the first report of the use of PDT to treat cancers of the pancreas. It has shown efficacy with a low morbidity and mortality. The technique may be of value for treating localised cancers in patients who are poor candidates for definitive surgery or in whom the location of the tumour makes pancreatic resection inappropriate. PDT can be used in conjunction with chemotherapy or radiotherapy. These promising early results justify larger trials to assess PDT either as a single therapy or in combination with chemotherapy and/or radiotherapy.
Acknowledgments
We would like to express our sincere thanks to the Association for International Cancer Research (St Andrews, UK) who funded this study and the preceding experimental work. Additional funding and supply of the photosensitising drug mTHPC for this work was provided by Scotia Pharmaceuticals (Stirling, UK). We would also like to thank Mr RCG Russell for his surgical advice on the patients in the study.
Abbreviations
ALA, 5-amino laevulinic acid
AlSPc, aluminium sulphonated phthalocyanine
AlS2Pc, aluminium disulphonated phthalocyanine
CT, computerised tomography
ERCP, endoscopic retrograde cholangiopancreatography
mTHPC, meso-tetrahydroxyphenyl chlorin
PDT, photodynamic therapy
REFERENCES
- 1.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin 1999;49:33–64, 1. [DOI] [PubMed] [Google Scholar]
- 2.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med 1992;326:455–65. [DOI] [PubMed] [Google Scholar]
- 3.Bramhall SR, Allum WH, Jones AG, et al. Treatment and survival in 13,560 patients with pancreatic cancer, and incidence of the disease, in the epidemiological study. Br J Surg 1995;82:111–15. [DOI] [PubMed] [Google Scholar]
- 4.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995;221:721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 1995;221:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Russell RC, Bramhall S, et al. Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. Br J Surg 1997;84:1370–6. [PubMed] [Google Scholar]
- 7.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990;211:447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. American Gastroenterological Association. Gastroenterology 1999;117:1464–84. [DOI] [PubMed] [Google Scholar]
- 9.Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403–13. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst 1988;80:751–5. [PubMed] [Google Scholar]
- 11.Hawes RH, Xiong Q, Waxman I, et al. A multispecialty approach to the diagnosis and management of pancreatic cancer. Am J Gastroenterol 2000;95:17–31. [DOI] [PubMed] [Google Scholar]
- 12.Dougherty TJ, Gomer CJ, Henderson BW, et al. Photodynamic therapy. J Natl Cancer Inst 1998;90:889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr H, Tralau CJ, Boulos PB, et al. The contrasting mechanisms of colonic collagen damage between photodynamic therapy and thermal injury. Photochem Photobiol 1987;46:795–800. [DOI] [PubMed] [Google Scholar]
- 14.Bown SG. Photodynamic therapy to scientists and clinicians—one world or two? J Photochem Photobiol B 1990;6:1–12. [DOI] [PubMed] [Google Scholar]
- 15.Sibille A, Lambert R, Souquet JC, et al. Long-term survival after photodynamic therapy for esophageal cancer. Gastroenterology 1995;108:337–44. [DOI] [PubMed] [Google Scholar]
- 16.Ell C, Gossner L, May A, et al. Photodynamic ablation of early cancers of the stomach by means of mTHPC and laser irradiation: preliminary clinical experience. Gut 1998;43:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nuutinen PJ, Chatlani PT, Bedwell J, et al. Distribution and photodynamic effect of disulphonated aluminium phthalocyanine in the pancreas and adjacent tissues in the Syrian golden hamster. Br J Cancer 1991;64:1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravi B, Regula J, Buonaccorsi GA, et al. Sensitization and photodynamic therapy of normal pancreas, duodenum and bile ducts in the hamster using 5-aminolaevulinic acid. Lasers Med Sci 1996;11:11–21. [Google Scholar]
- 19.Mlkvy P, Messmann H, Pauer M, et al. Distribution and photodynamic effects of meso-tetrahydroxyphenylchlorin (mTHPC) in the pancreas and adjacent tissues in the Syrian golden hamster. Br J Cancer 1996;73:1473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant WE, Buonaccorsi G, Speight PM, et al. The effect of photodynamic therapy on the mechanical integrity of normal rabbit carotid arteries. Laryngoscope 1995;105(8 Pt1):867–71. [DOI] [PubMed] [Google Scholar]
- 21.Schroder T, Chen IW, Sperling M, et al. Hematoporphyrin derivative uptake and photodynamic therapy in pancreatic carcinoma. J Surg Oncol 1988;38:4–9. [DOI] [PubMed] [Google Scholar]
- 22.Evrard S, Keller P, Hajri A, et al. Experimental pancreatic-cancer in the rat treated by photodynamic therapy. Br J Surg 1994;81:1185–9. [DOI] [PubMed] [Google Scholar]
- 23.Chatlani PT, Nuutinen PJO, Toda N, et al. Selective necrosis in hamster pancreatic tumours using photodynamic therapy with phthalocyanine photosensitization. Br J Surg 1992;79:786–90. [DOI] [PubMed] [Google Scholar]
- 24.Regula J, Ravi B, Bedwell J, et al. Photodynamic therapy using 5-aminolevulinic acid for experimental pancreatic-cancer—prolonged animal survival. Br J Cancer 1994;70:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mlkvy P, Messman H, MacRobert AJ, et al. Photodynamic therapy of a transplanted pancreatic cancer model using meta-tetrahydroxyphenylchlorin (mTHPC). Br J Cancer 1997;76:713–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar P, Clark M. Clinical medicine, 4th edn. Edinburgh: WB Saunders, 1998.
- 27.Smith AC, Dowsett JF, Russell RC, et al. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet 1994;344:1655–60. [DOI] [PubMed] [Google Scholar]
- 28.Singh SM, Longmire WPJ, Reber HA. Surgical palliation for pancreatic cancer. The UCLA experience. Ann Surg 1990;212:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc 1998;47:1–7. [DOI] [PubMed] [Google Scholar]
- 30.Feather JW, Driver I, King PR, et al. Light delivery to tumour tissue through implanted optical fibres during photodynamic therapy. Lasers Med Sci 1990;5:345–50. [Google Scholar]
- 31.Lee LK, Whitehurst C, Pantelides ML, et al. An interstitial light assembly for photodynamic therapy in prostatic carcinoma. BJU Int 1999;84:821–6. [DOI] [PubMed] [Google Scholar]