Abstract
Background: The transcription factor encoded by the intestinal Cdx2 homeobox gene and treatment with sodium butyrate (NaB), a byproduct of fibre fermentation by colonic bacteria, exert similar effects on colon cancer cell lines as they both inhibit cell growth and stimulate cell differentiation and apoptosis.
Aim: To investigate whether NaB regulates expression of the Cdx2 gene in colon cancer cell lines.
Methods: Human adenocarcinoma cell lines Caco2 and HT29 were grown in the presence or absence of NaB. Cells were analysed for Cdx2 mRNA expression by reverse transcription-polymerase chain reaction, for protein expression by western blotting and electromobility shift assays, and for transcriptional activity of the Cdx2 promoter by transfection with luciferase reporter plasmids.
Results: In HT29 and Caco2 cells, NaB stimulated Cdx2 mRNA and protein expression as well as transcriptional activity of the Cdx2 promoter. Stimulation of the activity of the Cdx2 promoter by NaB was dose and time dependent. The Cdx2 promoter contains discrete regions that participate in or inversely that blunt the stimulatory effect exerted by NaB. In addition, NaB stimulated the transcriptional activity of the Cdx2 promoter downregulated by oncogenic ras.
Conclusion: This study is the first report of an intestine specific transcription factor, Cdx2, stimulated by butyrate. Thus it provides a new mechanism whereby butyrate controls proliferation and differentiation of colon cancer cells.
Keywords: fibre, transcription, Cdx2 homeobox gene, butyrate, colon cancer
Diet is an important determinant of the risk of developing colorectal cancers and dietary components are thought to influence gene expression in the gut.1 Although data reported in humans are still controversial,2 evidence has been provided that alimentary fibres exert a protective effect against colon carcinogenesis in rodents.3–5 The protective effect is linked to the capacity of fibres to be metabolised into short chain fatty acid byproducts such as butyrate.6 Direct administration of high doses of butyrate in the lumen of the colon increases cell proliferation at the crypt base and selectively decreases carcinogen induced crypt surface hyperproliferation that is thought to represent an early event in neoplastic transformation.7 In colon cancer cell lines cultured in vitro, sodium butyrate (NaB) acts to oppose the malignant behaviour, inhibiting anchorage independent cell growth while stimulating cell differentiation and apoptosis.8 The cellular effects of NaB have been related (i) to stimulation of cell cycle inhibitors such as p16INK4a and p21WAF together with dephosphorylation of pRb and decline in cyclin D1,9–12 (ii) to decreased expression of the proto-oncogenes c-src and c-myc,13–15 (iii) to a rise in transforming growth factor β1 (TGF-β1) mRNA and modification of chemokine secretion,16,17 and (iv) to a reduced level of the apoptosis inhibitors Bcl-2 and Bc-X-L together with upregulation of proapoptotic Bak and Bax, and caspase-3 mediated cleavage of poly-(ADP-ribose) polymerase.11,18,19
The caudal related Cdx2 gene encodes an intestinal transcription factor of the homeoprotein family that is a key regulator of the development and homeostasis of the intestinal epithelium (for a review see Freund and colleagues20 and references therein). A number of studies performed on several intestinal cell lines21–23 have provided evidence that Cdx2 inhibits cell growth and stimulates overall cell differentiation. In combination with the related Cdx1 homeobox gene, Cdx2 also promotes cell apoptosis. Together, these results indicate that Cdx2 opposes the malignant behaviour of colon cancer cells.23 Apart from the effects reported on cell lines, a link has been proposed between colon cancers and Cdx2 status as human colorectal cancers and chemically induced colon tumours in the rat show reduced Cdx2 levels in relation to tumour grade.24,25 Homozygous Cdx2 deficiency leads to early embryonic lethality which fails to clearly delineate the role played by Cdx2 in the initiation and/or progression of colon cancer in vivo. However, hemizygous Cdx2+/− mice are viable and exhibit intestinal hamartomas and an altered rostrocaudal pattern of epithelial cell differentiation26–28 which suggests that altered Cdx2 expression participates in the broad changes associated with colon cancer.
The mechanisms of Cdx2 regulation are far from being elucidated. We have previously shown that this homeobox gene is downregulated by oncogenic Ras activation in colon adenocarcinoma cells.29 Cdx2 is also negatively targeted by the PI3-kinase pathway that is antagonised by the dual specific phosphatase encoded by the PTEN tumour suppressor gene (unpublished results). On the basis of similar effects exerted by Cdx2 overexpression and by NaB treatment on intestinal cancer cells, we have investigated whether Cdx2 is regulated by NaB.
MATERIAL AND METHODS
Cells cultures
HT29 cells,30 the TC7 subclone of Caco2 cells,31 and control and Ras activated Caco2-H and Caco2-T cell lines32 were cultured under standard conditions. Cells were plated at 1×105 cells/cm2, and grown for 48 hours before addition of NaB or trichostatin A to the culture medium, as indicated below.
Plasmids and transfections
The plasmid pCdx2-1Luc containing the murine Cdx2 promoter inserted into the pGl3-basic luciferase reporter vector (Promega, Charbonniéres, France) has previously been described.29 It contains the promoter fragment −907/+117 with respect to the major Cdx2 transcription start site. Subclones extending respectively to positions −693, −551, −392, −338, and −171 were constructed by deletion with restriction enzymes and religation or by cloning polymerase chain reaction (PCR) subfragments in pGl3-basic. Point mutations were introduced into these plasmids using the GeneEditor in vitro Site-Directed Mutagenesis System, as recommended by the supplier (Promega). For transfection experiments, 1×105 cells/well were plated in triplicate 24 hours prior to addition of DNA. They were then transiently transfected with 0.4 μg of appropriate luciferase reporter plasmids using Exgen500 following the protocol recommended by the manufacturer (Euromedex, Mundolsheim, France). The reporter pCMVβGal (0.04 μg/well) was cotransfected for normalisation. After 9–48 hours of incubation, cells were harvested for luciferase and β-galactosidase measurements using the Dual Light Reporter Gene Assay System (Tropix. Bedford, Massachusetts, USA). At least three independent experiments were performed for each transfection condition.
Semiquantitative RT-PCR
RNA was extracted with Tri-Reagent (MRC, Cincinnati, Ohio, USA). Semiquantitative reverse transcription (RT)-PCR was performed as described previously22 for an increasing number of cycles (24–40 cycles), using the primers cdx2 b/c: dCCCAGCGGCCAGCGGCGAAACCTGT / dTATTTGTCTTTTGTCCTGGTTTTCA. Results were standardised with primers PG193/194: dATGTGAAGTCACTGTGCCAG / dGTGTAATCCGTCTCCACAGA corresponding to the mRNA of the 36B4 ribosomal protein. PCR fragments were run on 3% agarose gels and analysed using an Imaging Densitometer (Bio-Rad Laboratories, Hercules, California, USA).
Western blots and electromobility shift assays (EMSA)
Protein (50 μg) was incubated at 100°C for five minutes in Laemmli buffer containing 2% sodium dodecyl sulphate (SDS) and 100 mM DTT, separated by SDS-polyacrylamide gel electrophoresis (PAGE) on 10% polyacrylamide gels and transferred overnight to nitrocellulose filters by electroblotting in 25 mM Tris HCl, 192 mM glycine, pH 8.2, and 20% methanol. After saturation for one hour at 37°C in phosphate buffered saline (PBS) containing 5% non-fat dried milk, the filters were incubated for four hours at room temperature with anti-Cdx2/3 antibody33 (dilution 1:1000) or with anti-β-actin antibody (Amersham, Orsay, France; dilution 1:2000) in PBS with 1% non-fat milk and 0.1% Tween 20, and subsequently with horseradish peroxidase conjugated secondary antirabbit antibody (Amersham). Detection by chemiluminescence was performed using western blotting detection reagent (Amersham).
Nuclear cell protein fractionation was performed using sucrose density purification.34 Nuclear extracts (10 μg) were incubated with 50 000 cpm of 32P labelled double stranded oligonucleotide probes and 1 μg of poly(dI-dC) in a buffer containing 10% glycerol, 12.5 mM HEPES, pH 7.9, 100 mM KCl, 1 mM DTT, and 1 mM EDTA in a final volume of 20 μl for 20 minutes at room temperature. The DNA-protein complexes were fractionated on a 6% acrylamide gel, run in 0.5× TBE buffer, dried, and exposed to Kodak X-AR film at −70°C. Competition experiments were performed by addition of 100-fold molar excess of the unlabelled oligonucleotide prior to addition of radioactive probe. The sequence of the double stranded probes was: SIF-1: GGCTGGTGAGGGTGCAATAAACTTTATGAGTA; SIF-3: CTGACAGTACAATTACAATTAACTTAGA; SIF-4: CTCGAGCATTTATGTAAACTACTCGAG; and AP-1: CGCTTGATGAGTCAGCCGGAA.
RESULTS
NaB stimulates Cdx2 expression in colon cancer cells
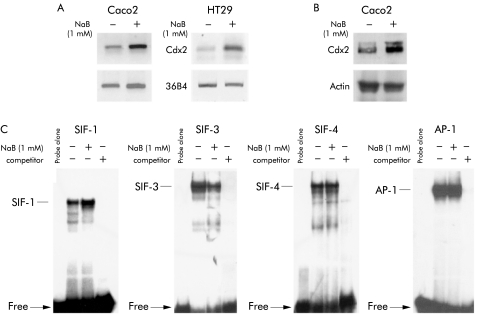
Several studies, including our own, have shown that treatment with NaB at 1–2 mM promotes differentiation of intestinal cell lines. At higher doses (above 5 mM) NaB causes massive apoptosis, followed by increased proliferation of the remaining cells. Unless otherwise stated, we used NaB at 1 or 2 mM throughout the study. To investigate the effect of NaB on expression of the Cdx2 homeobox gene, human colon adenocarcinoma Caco2 cells were treated with NaB for 48 hours. Semiquantitative RT-PCR using Cdx2 specific primers, similar to western blot analysis with anti-Cdx2 antibody, indicated that mRNA and protein expression of Cdx2 were both stimulated in NaB treated Caco2 cells compared with control cells incubated with vehicle alone (fig 1A, B ▶). In another human colon adenocarcinoma cell line, HT29, NaB treatment also increased levels of Cdx2 mRNA detected by RT-PCR (fig 1A ▶). The intestinal specific sucrase-isomaltase gene is a typical target of the Cdx2 protein which binds the SIF-1 element in the proximal promoter.35 Nuclear proteins extracted from HT29 cells were used for EMSA with the double stranded SIF-1 oligonucleotide. Figure 1C ▶ (left panel) shows a higher amount of DNA-protein complex in NaB treated HT29 cells compared with controls. Preincubation of nuclear proteins with anti-Cdx2 antibody largely prevented the formation of the DNA-protein complex, confirming the identity of the Cdx2 protein in the complex (not shown). Taken together, these results provide evidence that Cdx2 expression is stimulated in human colon cancer cell lines by NaB treatment at a dose that promotes cell differentiation.
Figure 1.
Stimulation of Cdx2 mRNA and protein expression by butyrate (NaB). Caco2 or HT29 cells were plated on culture dishes and treated for 48 hours with 1 mM NaB or vehicle alone. (A) RNA was used for reverse transcription-polymerase chain reaction analysis with the cdx2 b/c primers to detect Cdx2 mRNA and with PG193/194 primers to detect mRNA of the ribosomal protein 36B4. (B) Cellular proteins from Caco2 cells separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to nitrocellulose filters were probed with anti-Cdx2 antibody and anti-β-actin antibody. (C) Nuclear cell extracts from HT29 cells were assayed for Cdx2 DNA binding activity using the labelled SIF-1 oligonucleotide (left panel), and for HNF-1, E4BP4, and AP-1 DNA binding activity with the oligonucleotides SIF-3, SIF-4, and AP-1, respectively. For competition experiments, 100-fold molar excess of unlabelled oligonucleotide was incubated with the nuclear extracts prior to addition of the labelled probe.
To investigate if NaB causes a general increase in DNA binding activity or if the effects are specific to Cdx2, EMSAs were performed with double stranded oligonucleotides for a series of other DNA binding factors. The SIF-3 and SIF-4 oligonucleotides correspond to cis elements of the sucrase-isomaltase promoter that have been shown to bind, respectively, the homeoproteins of the HNF1 family and transcription repressor E4BP4.36,37 As shown in fig 1C ▶, SIF-3 binding activity was reduced in NaB treated HT29 cells compared with controls whereas SIF-4 binding activity was unchanged. EMSA also revealed that the DNA binding activity of the AP-1 complex was not modified on cell treatment with NaB (fig 1C ▶). Hence these data indicate that NaB has distinct effects on specific nuclear factors, including an increase in Cdx2 DNA binding activity.
Transcription from the Cdx2 promoter is activated by NaB
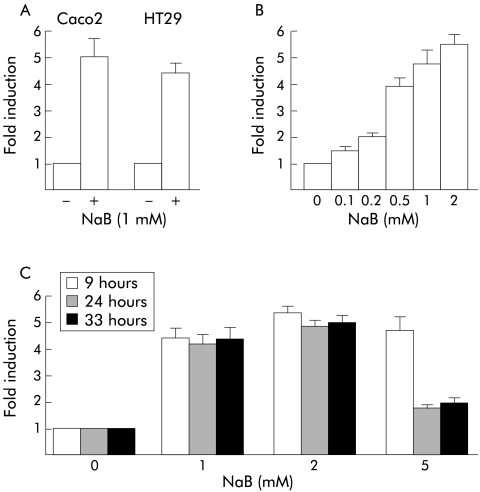
The reporter luciferase plasmid pCdx2-1Luc containing the Cdx2 promoter fragment between positions −907 to +117 with respect to the major transcription start site was transfected into Caco2 or HT29 cells, and cells were then treated with 1 mM NaB or vehicle alone for 48 hours. Figure 2A ▶ shows that NaB stimulated the transcriptional activity of the Cdx2 promoter in both cell types by a factor of 4–5. No effect was observed using the empty luciferase vector pGl3-basic. Transcriptional stimulation of the Cdx2 promoter by NaB was dose dependent, and a faint effect was observed at concentrations as low as 0.1 mM NaB (fig 2B ▶). We next treated pCdx2-1Luc transfected Caco2 cells with NaB at 0–5 mM, and luciferase activity was measured after NaB treatment for 9–33 hours (fig 2C ▶). Maximal transcriptional activation was already observed nine hours after treatment, irrespective of the concentration of NaB. This activation was maintained throughout the time course of the experiment for NaB at 1 and 2 mM whereas stimulation was largely reduced after 24 hours and 33 hours of incubation with 5 mM NaB. No significant stimulation was observed with 10 mM NaB (not shown). Thus increased expression of the Cdx2 homeobox gene by NaB was related to dose and time dependent stimulation of the transcriptional activity of its promoter.
Figure 2.
Stimulation of the transcriptional activity of the Cdx2 promoter by butyrate (NaB). (A) Caco2 or HT29 cells were transfected with the pCdx2-1Luc reporter plasmid and pCMV-βGal for normalisation. They were then treated for 48 hours with 1 mM NaB or vehicle alone. (B) Caco2 cells were transfected with pCdx2-1Luc and pCMV-βGal and treated for 48 hours with 0.1–2 mM NaB or vehicle alone. (C) pCdx2-1Luc and pCMV-βGal transfected Caco2 cells were treated with 1, 2, or 5 mM NaB or vehicle alone for nine, 24, or 33 hours. In all experiments, luciferase activity was related to β-galactosidase and the results are expressed as fold induction compared with control cells treated with vehicle alone. The results are means of three independent experiments.
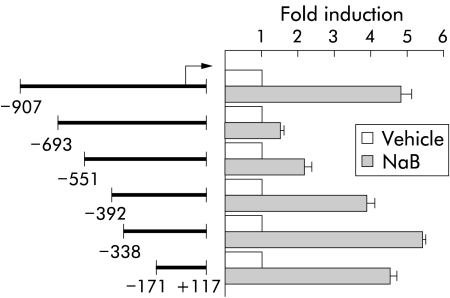
We have constructed a series of reporter plasmids containing different segments of the Cdx2 promoter, all starting at position +117, and the transcriptional activity of these constructs was analysed under the influence of 1 mM NaB in Caco2 cells (fig 3 ▶). The plasmids ending at position −171, −338, or −392 exhibited a level of activation by NaB similar to the full length pCdx2-Luc reporter. However, the plasmids ending at position −551 or −693 showed a lower level of activation than either the shorter reporter plasmids or the full length pCdx2-1Luc extending to −907. These results revealed that the Cdx2 promoter contains discrete regions that exhibit opposite effects on the transcriptional activation by NaB: the segment +117/−171 contains positive response element(s) to NaB, the activity of which is partially inhibited by element(s) located between −392 and −693; in addition, the sequence between −693 and −907 blunts the negative effect exerted by the −392/−693 element(s).
Figure 3.
Cdx2 promoter analysis for butyrate (NaB) stimulation. Caco2 cells were transfected with the pCdx2-1Luc reporter plasmid containing the promoter fragment between nucleotides −907 to +117 or with the shorter promoter fragments extending to positions −693, −551, −392, −338, or −171. The arrow indicates the transcription start site. pCMV-βGal was cotransfected for normalisation. Cells were then treated for 48 hours with 1 mM NaB or vehicle alone. Luciferase activity was related to β-galactosidase and the results are expressed as fold induction compared with control cells treated with vehicle alone. The results are means of three independent experiments.
NaB restores the transcriptional activity of the Cdx2 promoter downregulated by oncogenic Ras
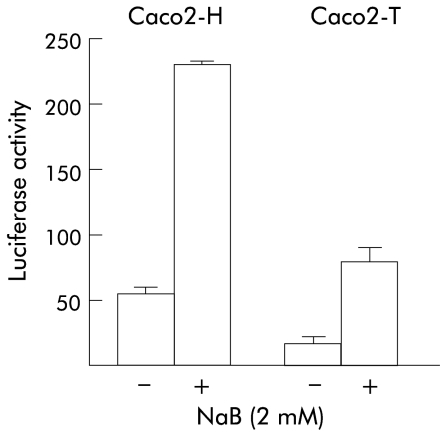
In previous work, we showed that Cdx2 was downregulated by oncogenic Ras activation in Caco2 cells.29 Thus we investigated whether NaB can abolish the negative effect of oncogenic Ras on the transcriptional activity of the Cdx2 promoter. For this purpose, we used the Caco2-T cell line stably transfected with the pHO6T1 plasmid that encodes oncogenic Val12 Ha-Ras and the control Caco2-H cells transfected with the empty vector pHO6.32 The reporter pCdx2-1Luc plasmid was introduced into both cell lines, and luciferase activity was measured after 48 hours of treatment with 2 mM NaB or vehicle alone (fig 4 ▶). As shown earlier, basal activity of the Cdx2 promoter was threefold lower in Ras activated cells compared with controls. NaB stimulated by fourfold the Cdx2 promoter in control Caco2-H cells, in a similar manner to standard Caco2 cells shown above. In the Ras activated Caco2-T cells, NaB also resulted in fivefold stimulation of the transcriptional activity of pCdx2-1Luc which reached a level similar to that measured in control Caco2-H cells without NaB. Consistent with the results described above, the −693/+117 reporter plasmid was significantly less stimulated by NaB than the full length pCdx2-1Luc in both Caco2-H and Caco2-T cells whereas the −171/+117 reporter gave similar results as pCdx2-1Luc (not shown).
Figure 4.
Blunting effect of butyrate (NaB) on Cdx2 downregulation by oncogenic Ras. Control Caco2-H and Ras activated Caco2-T cells were transfected with the pCdx2-1Luc and pCMV-βGal plasmids and treated for 48 hours with 2 mM NaB or vehicle alone. Luciferase activity was related to β-galactosidase and the results are expressed as arbitrary units of luciferase activity. The results are means of three independent experiments.
DISCUSSION
Butyrate, a fermentation byproduct of dietary fibre by the colonic microflora, is the major metabolic substrate of colonocytes and a potent regulator of gene expression. Here we showed that NaB stimulated mRNA and protein expression of the Cdx2 homeobox gene through transcriptional activation of its promoter. This study is the first report of an intestine specific transcription factor targeted by NaB in colon cancer cells.
The Cdx2 promoter contains discrete regions required for or blunting the stimulatory effect exerted by NaB, which indicates that multiple DNA-protein interactions participate in the regulation of Cdx2 by butyrate. Several mechanisms of action of butyrate have been proposed, including chromatin changes through inhibition of histone deacetylases,38 indirect effects mediated by the TGF-β1 pathway,16,39 and transcription regulation via Sp1/Sp3 binding sites40,41 or butyrate response elements.9 During the course of this study, we have examined these pathways and found no evidence that they are involved in Cdx2 regulation by butyrate (unpublished results). For example, unlike butyrate, the histone deacetylase inhibitor trichostatin A did not stimulate Cdx2 expression. Interestingly, trichostatin A has been shown to mimic the cell growth inhibitory effect of butyrate in HT29 cells whereas it failed to mimic the cell differentiation effect.42 Additional experiments like those reported in the case of the osteocalcin gene43 are required to investigate the consequences of butyrate on the chromatin structure at the Cdx2 locus. Our results also suggest that the butyrate effect on Cdx2 is not dependent on the TGF-β pathway as cell transfection with plasmids encoding members of the Smad family (Smad-2, -3, -4, -7 alone or in combination) did not stimulate the transcription activity of the Cdx2 promoter. This is further supported by the fact that we did not observe any significant change in Cdx2 expression in TGF-β responding colon cancer cells treated with TGF-β1 or in fetal intestinal explants grafted under the skin of nude mice in the presence of TGF-β1 soaked beads (Foltzer-Jourdainne and Freund, unpublished results). Finally, although the Cdx2 promoter contains a consensus binding site for Sp1/Sp3 factors, mutation of this site as well as mutations of adjacent nuclear factor κB sites required for Cdx2 regulation by the PI3-kinase pathway (unpublished results) did not abolish the NaB effect, suggesting that neither Sp1/Sp3 nor nuclear factor κB participate in Cdx2 stimulation by butyrate. The Cdx2 promoter contains additional GC rich segments yet without a high degree of similarity with the butyrate responsive element described in the promoter of several genes such as cyclin D1.9 At present, we cannot rule out that butyrate acts through atypical butyrate responsive elements.
We found that NaB restored the transcriptional activity of the Cdx2 promoter downregulated by oncogenic Ras activation. We have previously shown that the effect of Ras is linked to changes in AP-1 DNA binding activity.29 Two different results indicate that NaB does not rescue Ras inhibition of Cdx2 via AP-1. Firstly, the reporter plasmid containing the Cdx2 promoter region −171/+117 was stimulated by NaB in common with the full length reporter, although it lacked the Cdx2 AP-1 site located at position −177. Secondly, we showed that NaB does not change AP-1 binding by EMSA. Thus we conclude that the opposite effects of NaB and oncogenic Ras on Cdx2 promoter activity involve distinct molecular mechanisms.
Together with previous data showing that Cdx2 stimulates cell differentiation while inhibiting cell growth,21–23 the present study indicates that Cdx2 stimulation is an important factor of cytodifferentiation and cell growth arrest provoked by butyrate in colon cancer cells. A correlation could be drawn between the opposite effects of butyrate versus oncogenic Ras on Cdx2 expression and the protective role of butyrate against azoxymethane induced colon cancerogenesis in rodents in which mutational and non-mutational activation of Ras is observed.44 In summary, through stimulation of the intestinal Cdx2 gene, this study describes a new way whereby butyrate acts to oppose the broad changes associated with colon tumour initiation and/or progression, and opens up the possibility that the Cdx2 homeobox gene is a direct target of regulation by nutrients in intestinal cells.
Acknowledgments
The authors are grateful to Dr C Gespach (INSERM U.482, Paris) for providing the Caco2-H and Caco2-T cell lines, and Dr M German (UCSF, CA) for the anti-Cdx2/3 antibody. We thank E Martin for skilful technical assistance. CD-D is a recipient of a fellowship of the Ligue Nationale contre le Cancer. This work was supported by INSERM and the Association pour la Recherche sur le Cancer.
Abbreviations
NaB, sodium butyrate
TGF-β1, transforming growth factor β1
RT-PCR, reverse transcription-polymerase chain reaction
SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis
PBS, phosphate buffered saline
EMSA, electromobility shift assay
REFERENCES
- 1.Sanderson IR, Naik S. Dietary regulation of intestinal gene expression. Annu Rev Nutr 2000;20:311–38 [DOI] [PubMed] [Google Scholar]
- 2.Kim YI. AGA technical review: impact of dietary fiber on colon cancer occurrence. Gastroenterology 2000;118:1235–57. [DOI] [PubMed] [Google Scholar]
- 3.Pierre F, Perrin P, Champ M, et al. Short-chain fructo-oligosaccharides reduce the occurrence of colon tumors and develop gut-associated lymphoid tissue in Min mice. Cancer Res 1997;57:225–8. [PubMed] [Google Scholar]
- 4.Hioki K, Shivapurkar N, Oshima H, et al. Suppression of intestinal polyp development by low-fat and high-fiber diet in Apc(delta716) knockout mice. Carcinogenesis 1997;18:1863–5. [DOI] [PubMed] [Google Scholar]
- 5.Compher CW, Frankel WL, Tazelaar J, et al. Wheat bran decreases aberrant crypt foci, preserves normal proliferation, and increases intraluminal butyrate levels in experimental colon cancer. JPEN J Parenter Enteral Nutr 1999;23:269–77. [DOI] [PubMed] [Google Scholar]
- 6.Perrin P, Pierre F, Patry Y, et al. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut 2001;48:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velazquez OC, Zhou D, Seto RW, et al. In vivo crypt surface hyperproliferation is decreased by butyrate and increased by deoxycholate in normal rat colon: associated in vivo effects on c-Fos and c-Jun expression. JPEN J Parenter Enteral Nutr 1996;20:243–50. [DOI] [PubMed] [Google Scholar]
- 8.Basson MD, Turowski GA, Rashid Z, et al. Regulation of human colonic cell line proliferation and phenotype by sodium butyrate. Dig Dis Sci 1996;41:1989–93. [DOI] [PubMed] [Google Scholar]
- 9.Lallemand F, Courilleau D, Sabbah M, et al. Direct inhibition of the expression of cyclin D1 gene by sodium butyrate. Biochem Biophys Res Commun 1996;229:163–9. [DOI] [PubMed] [Google Scholar]
- 10.Archer SY, Meng S, Shei A, et al. p21(WAF1) is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci USA 1998;95:6791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litvak DA, Evers BM, Hwang KO, et al. Butyrate-induced differentiation of Caco-2 cells is associated with apoptosis and early induction of p21Waf1/Cip1 and p27Kip1. Surgery 1998;124:161–9. [PubMed] [Google Scholar]
- 12.Schwartz B, Avivi-Green C, Polak-Charcon S. Sodium butyrate induces retinoblastoma protein dephosphorylation, p16 expression and growth arrest of colon cancer cells. Mol Cell Biochem 1998;188:21–30. [PubMed] [Google Scholar]
- 13.Foss FM, Veillette A, Sartor O, et al. Alterations in the expression of pp60c-src and p56lck associated with butyrate-induced differentiation of human colon carcinoma cells. Oncogene Res 1989;5:13–23. [PubMed] [Google Scholar]
- 14.Heruth DP, Zirnstein GW, Bradley JF, et al. Sodium butyrate causes an increase in the block to transcriptional elongation in the c-myc gene in SW837 rectal carcinoma cells. J Biol Chem 1993;268:20466–72. [PubMed] [Google Scholar]
- 15.Souleimani A, Asselin C. Regulation of c-myc expression by sodium butyrate in the colon carcinoma cell line Caco-2. FEBS Lett 1993;326:45–50. [DOI] [PubMed] [Google Scholar]
- 16.Barnard JA, Warwick G. Butyrate rapidly induces growth inhibition and differentiation in HT-29 cells. Cell Growth Differ 1993;4:495–501. [PubMed] [Google Scholar]
- 17.Fusunyan RD, Quinn JJ, Fujimoto M, et al. Butyrate switches the pattern of chemokine secretion by intestinal epithelial cells through histone acetylation. Mol Med 1999;5:631–40. [PMC free article] [PubMed] [Google Scholar]
- 18.Ruemmele FM, Dionne S, Qureshi I, et al. Butyrate mediates caco-2 cell apoptosis via up-regulation of pro-apoptotic BAK and inducing caspase-3 mediated cleavage of poly-(ADP-ribose) polymerase (PARP). Cell Death Differ 1999;6:729–35. [DOI] [PubMed] [Google Scholar]
- 19.Mandal M, Olson DJ, Sharma T, et al. Butyric acid induces apoptosis by up-regulating Bax expression via stimulation of the c-Jun N-terminal kinase/activation protein-1 pathway in human colon cancer cells. Gastroenterology 2001;120:71–8. [DOI] [PubMed] [Google Scholar]
- 20.Freund JN, Domon-Dell C, Kedinger M, et al. The Cdx-1 and Cdx-2 homeobox genes in the intestine. Biochem Cell Biol 1998;76:957–69. [DOI] [PubMed] [Google Scholar]
- 21.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol 1996;16:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorentz O, Duluc I, Arcangelis AD, et al. Key role of the Cdx2 homeobox gene in extracellular matrix-mediated intestinal cell differentiation. J Cell Biol 1997;139:1553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallo GV, Soubeyran P, Lissitzky JC, et al. Expression of the Cdx1 and Cdx2 homeotic genes leads to reduced malignancy in colon cancer-derived cells. J Biol Chem 1998;273:14030–6. [DOI] [PubMed] [Google Scholar]
- 24.Ee HC, Erler T, Bhathal PS, et al. Cdx-2 homeodomain protein expression in human and rat colorectal adenoma and carcinoma. Am J Pathol 1995;147:586–92. [PMC free article] [PubMed] [Google Scholar]
- 25.Mallo GV, Rechreche H, Frigerio JM, et al. Molecular cloning, sequencing and expression of the mRNA encoding human Cdx1 and Cdx2 homeobox. Down-regulation of Cdx1 and Cdx2 mRNA expression during colorectal carcinogenesis. Int J Cancer 1997;74:35–44. [DOI] [PubMed] [Google Scholar]
- 26.Chawengsaksophak K, James R, Hammond VE, et al. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature 1997;385:84–7. [DOI] [PubMed] [Google Scholar]
- 27.Tamai Y, Nakajima R, Ishikawa T, et al. Colonic hamartoma development by anomalous duplication in Cdx2 knockout mice. Cancer Res 1999;59:2965–70. [PubMed] [Google Scholar]
- 28.Beck F, Chawengsaksophak K, Waring P, et al. Reprogramming of intestinal cell differentiation and intercalary regeneration in Cdx2 mutant mice. Proc Natl Acad Sci USA 1999;96:7318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorentz O, Cadoret A, Duluc I, et al. Downregulation of the colon tumour-suppressor homeobox gene Cdx-2 by oncogenic ras. Oncogene 1999;18:87–92. [DOI] [PubMed] [Google Scholar]
- 30.Pinto M, Appay MD, Simon-Assmann P, et al. Enterocytic differentiation of cultured human colon cancer cells by replacement of glucose with galactose in the medium. Biol Cell 1982;44:193–6. [Google Scholar]
- 31.Chantret I, Rodolosse A, Barbat A, et al. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2:evidence for glucose-dependent negative regulation. J Cell Sci 1994;107:213–25. [DOI] [PubMed] [Google Scholar]
- 32.Chastre E, Empereur S, Di Gioia Y, et al. Neoplastic progression of human and rat intestinal cell lines after transfer of the ras and polyoma middle T oncogenes. Gastroenterology 1993;105:1776–89. [DOI] [PubMed] [Google Scholar]
- 33.German MS, Wang J, Chadwick RB, et al. Synergistic activation of the insulin gene by a LIM-homeo domain protein and a basic helix-loop-helix protein: building a functional insulin minienhancer complex. Genes Dev 1992;6:2165–76. [DOI] [PubMed] [Google Scholar]
- 34.Han Y, Meng T, Murray NR, et al. Interleukin-1-induced nuclear factor-kappaB-IkappaBalpha autoregulatory feedback loop in hepatocytes. A role for protein kinase calpha in post-transcriptional regulation of ikappabalpha resynthesis. J Biol Chem 1999;274:939–47. [DOI] [PubMed] [Google Scholar]
- 35.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol 1996;16:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu GD, Chen L, Forslund K, et al. Hepatocyte nuclear factor-1 alpha (HNF-1 alpha) and HNF-1 beta regulate transcription via two elements in an intestine-specific promoter. J Biol Chem 1994;269:17080–5. [PubMed] [Google Scholar]
- 37.Traber PG, Silberg DG. Intestine-specific gene transcription. Annu Rev Physiol 1996;58:275–97. [DOI] [PubMed] [Google Scholar]
- 38.Doenecke D, Gallwitz D. Acetylation of histones in nucleosomes. Mol Cell Biochem 1982;44:113–28. [DOI] [PubMed] [Google Scholar]
- 39.Schroder O, Hess S, Caspary WF, et al. Mediation of differentiating effects of butyrate on the intestinal cell line Caco-2 by transforming growth factor-beta 1. Eur J Nutr 1999;38:45–50. [DOI] [PubMed] [Google Scholar]
- 40.Nakano K, Mizuno T, Sowa Y, et al. Butyrate activates the WAF1/Cip1 gene promoter through Sp1 sites in a p53-negative human colon cancer cell line. J Biol Chem 1997;272:22199–206. [DOI] [PubMed] [Google Scholar]
- 41.Sowa Y, Orita T, Minamikawa-Hiranabe S, et al. Sp3, but not Sp1, mediates the transcriptional activation of the p21/WAF1/Cip1 gene promoter by histone deacetylase inhibitor. Cancer Res 1999;59:4266–70. [PubMed] [Google Scholar]
- 42.Siavoshian S, Segain JP, Kornprobst M, et al. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut 2000;46:507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montecino M, Frenkel B, van Wijnen AJ, et al. Chromatin hyperacetylation abrogates vitamin D-mediated transcriptional upregulation of the tissue-specific osteocalcin gene in vivo. Biochemistry 1999;38:1338–45. [DOI] [PubMed] [Google Scholar]
- 44.Bissonnette M, Khare S, von Lintig FC, et al. Mutational and nonmutational activation of p21ras in rat colonic azoxymethane-induced tumors: effects on mitogen-activated protein kinase, cyclooxygenase-2, and cyclin D1. Cancer Res 2000;60:4602–9. [PubMed] [Google Scholar]