Abstract
Background: Little is known about secretory immunity—the major defence mechanism at mucosal surfaces—in human immunodeficiency virus (HIV) infected patients, especially in the early stages of the disease.
Aims: The aim of the study was to analyse mucosal immunoglobulin production and simian immunodeficiency virus (SIV) specific antibody response in the intestinal mucosa during the course of SIV infection in comparison with serum and saliva.
Animals and methods: IgG, IgA, and IgM concentrations were determined in supernatants of short term cultured duodenal biopsies, serum, and saliva from SIV infected rhesus macaques (n=8) and controls (n=2) by ELISA at defined times before and after infection. Specific antibodies to SIV were detected by western blot and/or dot blot analysis. In addition, rectal swabs from two uninfected and 12 SIV infected rhesus macaques (seven without and five with enteritis) were analysed for albumin and IgG concentrations.
Results: An increase in total intestinal IgG and a decrease in IgA were observed. SIV specific IgG or IgA responses were detectable as early as one week after SIV infection in the serum of seven of eight animals. In contrast, intestinal SIV specific IgG production was detected only four weeks after infection in six of eight animals, and intestinal SIV specific IgA was not produced in the intestine at any time point. In saliva, the secretory component on SIV specific IgA was only detected in one animal at week 24 after infection. Enteritis is frequent in SIV infected animals and results in a significant increase in albumin and IgG secretion into the intestinal lumen.
Conclusion: Despite modest quantitative changes in mucosal immunglobulin production there was a total lack of SIV specific IgA synthesis in the intestine during SIV infection. This lack or disturbed secretory SIV specific IgA response at mucosal surfaces may explain the rapid and high HIV/SIV replication in this compartment. In addition, our investigations indicate secretion of serum proteins into intestinal fluids during SIV infection. Previous investigations using intestinal secretions or swabs for analysing quantitative and specific immunglobulins therefore should be interpreted with caution.
Keywords: mucosal immunity, SIV infection, intestinal antibodies, immunodeficiency
The intestine is a major portal of entry for a variety of infectious pathogens, including human immunodeficiency virus (HIV).1 Indeed, several investigators demonstrated a high concentration of HIV/simian immunodeficiency virus (SIV) proteins in the intestinal mucosa with a peak early after infection.2–6 Immune exclusion by secretory IgA in mucosal secretions plays a key role in the protection against inflammation and infection of the intestinal mucosa. Apart from its function in blocking adherence of agents to epithelial cells, secretory IgA can also inhibit virus assembly and release intracellularly.7 This latter mechanism also seems to be important for a reduction in HIV transmission by the mucosal route, as demonstrated recently by in vitro studies.8,9 Therefore, secretory mucosal immune responses to HIV are not only important issues for vaccination strategies but may also significantly affect the pathogenesis of HIV infection in the mucosal immune system which comprises the largest lymphoid compartment of the body.
Despite the crucial role of mucosal antibody production for the pathogenesis of HIV/SIV infection and the development of vaccines, little is known about the mucosal humoral immune response, especially in the early phase of infection. HIV/SIV specific antibodies have been demonstrated in several body fluids from HIV infected patients and SIV infected animals, such as saliva, duodenal secretions, rectal swabs, and stool.10–13 However, it is not clear whether salivary immunoglobulins correctly mirror intestinal secretory immunity, and exudation of serum proteins as well as enzymatic digestion may confound analysis of mucosal immunoglobulin production in intestinal fluids. Therefore, we used short term culture of duodenal biopsies as described previously14 to analyse SIV specific intestinal humoral immunity in comparison with saliva and serum in SIV infected animals. Furthermore, intraindividual development of mucosal antibody production cannot be regularly studied in humans. Therefore, we used the SIV model, which is the most appropriate animal model for this disease, to determine quantitative changes in intestinal antibody production as well as the appearance and follow up of SIV specific intestinal antibodies in comparison with saliva and serum.
MATERIAL AND METHODS
Animals
Eight healthy, colony bred, juvenile (aged 2–3 years), male rhesus macaques (Macaca mulatta) were evaluated prospectively. All animals were imported from the Laboratory Animal Breeders and Services (Jemassee, North Carolina, USA), and caged individually at the German Primate Centre during the study. Animals received a standardised commercially available dry feed (Purina Mills monkey chow; Purina, St Louis, Missouri, USA) supplemented with fresh fruit twice a day. During six weeks of quarantine before infection, none of the animals developed symptoms of disease. All animals were seronegative for simian T lymphotropic virus 1, D-type virus, and SIV. Rectal swabs identified Campylobacter as the only pathogen in eight of 10 animals. Campylobacter positive animals were treated with gyrase inhibitor for five days to eradicate the pathogen. Eradication was verified by repeated rectal swabs.
Rectal swabs were obtained from an additional two healthy, uninfected, colony bred, juvenile (aged 2–3 years), male rhesus macaques (Macaca mulatta) and 12 SIV infected animals at different time points after infection to determine mucosal barrier dysfunction during infection with SIV. These animals also underwent rectosigmoidoscopy to determine the presence of macroscopic signs of inflammation. In all cases rectal biopsies were taken for histological work up. Seven animals had no macroscopic or histological signs of mucosal inflammation in the rectum or sigma, and five monkeys had enteritis in this area based on macroscopic and histological assessment.
Experimental design
Eight animals underwent venepuncture and upper endoscopy (Fujinon, UGI-FP7) twice before and at defined times after infection (one, two, four, 12, 24, 36, and 48 weeks post infection) under ketamine/xylazine sedation to obtain whole blood, serum, saliva, and duodenal biopsy specimens.
Infection with SIV
Four animals were inoculated intravenously with 100 MID50 (50% monkey infectious doses) cell free SIVmac251 grown in monkey peripheral blood mononuclear cells (kindly provided by Dr Anne Marie Aubertin, Strasbourg, France). Four animals were inoculated atraumatically by insertion of a silicon tube intrarectally and application of 2 ml of virus containing solution representing 20 MID50 of the same pathogenic strain (approximately 500-fold higher virus dose than required for intravenous infection). To confirm infection, polymerase chain reaction analysis for virus RNA and monitoring of specific antibody responses by western blot analysis of sera were performed.
Sample collection
Serum was collected from peripheral venous blood. Saliva collection was done by the standard Salivette (Sarstedt, Nümbrecht, Germany) as described previously.15 Rectal swabs were collected according to Israel and Marx16 and heat inactivated for 45 minutes at 60°C prior to freezing. Serum, saliva samples, and rectal swabs were stored at −70°C until assay. Three biopsies were taken from macroscopically normal areas of the distal duodenum in all animals.
Measurement of albumin and IgG in rectal swabs
Albumin and IgG in rectal swabs were assayed by immunoturbidimetric procedures, as described previously.17 Standard plots of albumin and IgG were prepared from Human Reference Serum (ALB plus; Roche, Mannheim, Germany CRM 470 Standard; IgG N antiserum, CRM 470 Standard).
Short term culture of intestinal biopsy samples
Preparation of culture supernatants of intestinal biopsies for the detection of local antibody production was done using a modification of the method described by Schneider and colleagues.14,18 Biopsies were immediately placed into phosphate buffered saline (PBS) and washed five times in 30 ml of PBS. All biopsies were weighed. This was followed by incubating three biopsies in 1.5 ml RPMI 1640 medium containing 10% fetal calf serum (Gibco BRL, Berlin, Germany), 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamycin, and 2.5 μg/ml amphotericin (Seromed Biochrom KG, Berlin, Germany) at 37°C in a humidified 5% carbon dioxide, 95% air atmosphere for 48 hours. Supernatants were stored at −70°C until assay. Optimal duration of culture for detection of SIV specific antibodies in biopsy supernatants was determined in previous experiments comparing incubation periods of one, eight, 24, 48, and 72 hours for biopsies from the same animal. In these experiments a maximum number and intensity of SIV specific bands was found after 48 hours of incubation (data not shown).
Measurement of immunoglobulin concentration
Immunoglobulin concentrations were determined by ELISA. Ninety six well plates (Micro Test III; Falcon, Oxnard, California, USA) were coated overnight at 4°C with 2 μg/well goat antimonkey Ig antibody (Nordic, Tilburg, the Netherlands) and blocked with 5% dry milk powder (Merck, Darmstadt, Germany) in PBS for two hours at 37°C in a humidified atmosphere. Samples were added in 10-fold serial dilutions and incubated in a humidified atmosphere for two hours at 37°C. Horseradish peroxidase conjugated goat antibodies to monkey IgG (Nordic, Tilburg, the Netherlands), to monkey IgA (Nordic), and to monkey secretory component (SC) (Nordic) were used to detect IgG, IgA, and secretory IgA, respectively.
Western blot analysis
To compare the immune response in different body fluids, serum and saliva from each individual were adjusted to the same immunoglobulin concentration by dilution of serum samples.
Minimal total IgA and IgG concentrations for the detection of specific SIV antigens (glycoprotein (gp)160, gp148, p27, and nef) were evaluated by serial dilution of serum from an animal with known total amount of IgA and IgG. On the basis of total IgA and IgG concentrations, a cut off level for detection of the specific SIV antigens was determined. In all experiments for determination of an IgA and IgG response to SIV antigens, the total immunoglobulin concentration was at least twice this minimal concentration.
SIV specific antibodies were analysed by western blot analysis. Goat antibodies to monkey IgG, monkey IgA, and monkey SC followed by an alkaline phosphatase conjugated mouse antigoat antibody (Jackson ImmunoResearch, West Grove, Pennsylvania, USA) were used to detect SIV specific IgG, IgA, and secretory IgA. When all bands were visible on the positive control strip, the staining reaction was stopped by extensive washing with water.
Dot blot analysis
To compare antibody production in different body fluids, serum and biopsy supernatants from each individual were adjusted to the same immunoglobulin concentration by dilution of serum samples. The minimum detection (cut off) levels of each immunoglobulin isotype (IgA, secretory IgA, IgG) were determined by measuring half log serial dilutions (1:100–1:6400) of standard controls with known IgA, secretory IgA, and IgG antibody concentrations.
SIV proteins gp160 (Dr R Randall/MRC AIDS Reagent Project), gp148 (Drs G Gilljam, B Wahren/MRC AIDS Reagent Project), p27 (Dr I Jones/MRC AIDS Reagent Project), and nef (Dr R Randall/MRC AIDS Reagent Project) were coated in 96 well plates with mixed cellulose ester membrane bottom (Millipore, Eschborn, Germany) by adding 1 μg of protein followed by air drying for 30 minutes. The cellulose ester membrane was blocked with 5% (w/v) dry milk powder/0.2% Triton X-100 (Serva, Heidelberg, Germany) in PBS and incubated with samples overnight at 4°C. Goat antibodies to monkey IgG, monkey IgA, and monkey SC followed by an alkaline phosphatase conjugated mouse antigoat antibody were used to detect SIV specific IgG, IgA, and secretory IgA. When all bands were visible in the positive control, the staining reaction was stopped by extensive washing with water.
Statistical analysis
Quantitative data are presented as median (range). The non-parametric two tailed Mann-Whitney U test for unpaired data was used to evaluate comparative statistical significance; p values less than 0.05 were considered significant.
RESULTS
Course of infection
The animals survived 4–18 (median 7) months after infection with SIV. In six of the eight animals severe intestinal involvement (cytomegalovirus enteritis in three, crypotosporidiosis in two, and intestinal lymphoma in one animal) was found to be responsible for death.
Measurement of albumin and IgG in rectal swabs
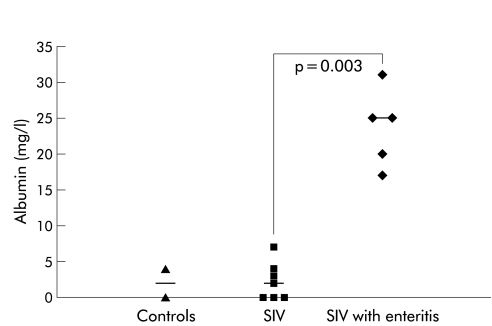
To determine mucosal barrier dysfunction during infection with SIV, we compared albumin and IgG concentrations in rectal swabs of uninfected animals, SIV infected monkeys without mucosal inflammation in the colon (macroscopic and histological), and animals with enteritis (macroscopic and histological). IgG levels in rectal swabs of uninfected animals were 0.68 mg/dl (0.45–0.9), in SIV infected rhesus macaques 0.6 mg/dl (0.3–1.7), and were clearly elevated in SIV infected animals with enteritis (8.4 mg/dl (0.9–42.2)) (p=0.002 v controls, p=0.002 v without enteritis). Similar results were found for albumin levels in rectal swabs, as shown in fig 1 ▶. Mucosal barrier dysfunction was found independently of duration of SIV infection (16–24 weeks).
Figure 1.
Quantitative analysis of albumin in rectal swabs from non-infected macaques (controls), simian immunodeficiency virus infected animals without enteritis (SIV), and SIV infected animals with enteritis (SIV with enteritis), displayed as single values. Horizontal bars represent the median.
Quantitative immunoglobulin production in intestine, and antibody concentrations in saliva and serum
Short term cultures of duodenal biopsies
Intestinal IgA production in animals before SIV infection was about 20-fold higher than that of IgG (840 (350–2200) v 40 (30–58) μg/mg biopsy (median and range)) and about fivefold higher than IgM production (180 (130–280) μg/mg biopsy).
During infection with SIV, no clear difference in IgA production in the duodenum was found. Only in three animals who survived one year after SIV infection was there a decrease in total IgA production in the intestine compared with preinfection values (400 (240–600) v 840 (350–2200) μg/mg biopsy).
Total IgG production increased and was elevated late after infection compared with preinfection levels (141 (40–226) v 40 (30–58) μg/mg biopsy). IgM production in the intestine was not substantially altered by SIV infection.
Thus only in the late stages of SIV infection were changes in total intestinal immunoglobulin production registered.
Saliva
Salivary IgA concentration in animals before SIV infection was about sixfold higher than that of IgG (60.1 (34.3–126.5) v 10.4 (9.6–16.5) mg/dl) and about sevenfold higher than IgM concentrations (8.2 (4.2–27.2) mg/dl).
During infection with SIV, IgA concentration showed a tendency to decrease but the decrease did not reach statistical significance (20.6 (15.2–41.2) v 60.1 (34.3–126.5) mg/dl). IgG concentration in saliva did not show significant changes during infection with SIV (5.6 (3.3–63.0) v 10.4 (9.6–16.5) mg/dl)). No changes in IgM concentration were demonstrated. Thus quantitative immunoglobulin concentrations in saliva were not altered by SIV infection.
Serum
Serum IgG concentration in animals before SIV infection (1744; 1185–2550 mg/dl) was approximately sevenfold higher than those of IgA (237; 160–572 mg/dl) and IgM (235.5; 103.5–273.8 mg/dl) concentrations.
IgA concentration in serum showed no significant changes during infection with SIV (data not shown). Except for an early decrease in IgG concentration two weeks (1062; 816–1182 mg/dl; p=0.002) and four weeks (1026; 666–1297 mg/dl; p=0.026) after infection with SIV compared with preinfection levels, there was no alteration in total serum IgG concentration during SIV infection.
During infection with SIV, IgM concentration was not altered except for a decrease in IgM 36 weeks after infection with SIV (98; 79–117 mg/dl; p=0.002) compared with preinfection levels which increased to normal values by 48 weeks after infection (144; 118–165 mg/dl; p=0.308).
SIV specific immunoglobulin production in short term cultures of duodenal biopsies
Because of the limited amount of duodenal biopsy specimens and the small volume of culture supernatants, western blot analysis could not be performed in the majority of cases. Therefore, we used dot blot analysis for detection of specific antibodies to the SIV proteins gp160, gp148, p27, and nef in the intestine and serum. To exclude the possibility that other SIV specific antigens not tested for in the dot blot analysis are relevant for the mucosal SIV specific IgA response, one animal with a strong IgG and IgA response to all SIV antigens in serum was tested for the presence of SIV specific IgA in culture supernatant by western blot. As expected, in the animal tested by western blot, no SIV specific IgA bands were found (data not shown).
To exclude sample dilution as responsible for the detection of specific antibodies, we determined the minimum detection (cut off) levels for each SIV protein (gp160, gp148, p27, nef) and each immunoglobulin isotype (IgA, IgG) in the dot blot assay prior to analysis. In all experiments for determination of an IgA and IgG response to SIV antigens, the concentration was at least twice this cut off value. In the following dot blot analysis, serum samples were diluted to the same immunoglobulin concentrations as in the culture supernatants of the duodenal biopsy specimens (preinfection values).
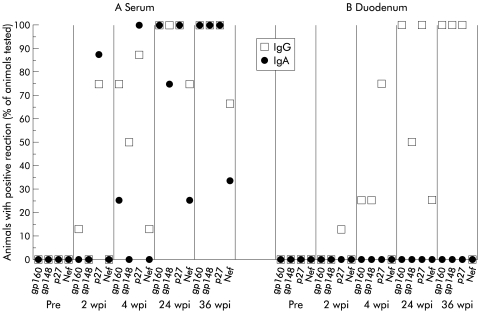
Only one animal showed an isolated IgG response to p27 in the intestine one and two weeks after infection. At four weeks most animals showed an IgG response at least against p27. All four animals who survived to week 24 after infection had an intestinal IgG response to at least two SIV antigens (fig 2B ▶). The intestinal IgG response against p27 was weak in all animals at any time after infection. In contrast, the IgG response was detected late after infection and was much stronger (fig 3 ▶). An intestinal IgA immune response against SIV antigens could not be detected at any time after infection (fig 2B, 3 ▶ ▶).
Figure 2.
Proportion of animals with serum or intestinal IgG or IgA reactive with the simian immunodeficiency virus (SIV) proteins gp160, gp148, p27, and Nef, indicated by dot blot analysis (Pre, 2 wpi and 4 wpi, n=8; 24 wpi, n=4; 36 wpi, n=3). Pre, preinfection; wpi, weeks postinfection.
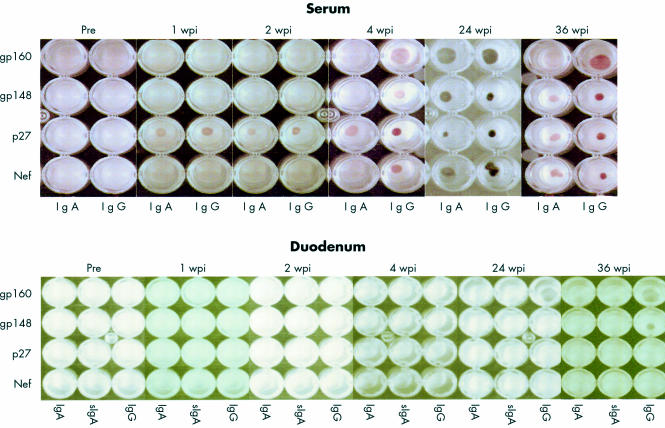
Figure 3.
Typical example of simian immunodeficiency virus (SIV) specific immunoglobulin isotype in serum and culture supernatants of duodenal biopsy specimens of one SIV infected animal during the time course of infection indicated by dot blot analysis. The SIV proteins used are indicated on the left. Pre, preinfection; wpi, weeks postinfection.
Dot blot analysis of SIV specific immunmoglobulins in serum
In contrast with the intestine, in 6/8 animals a strong SIV specific IgG and IgA response to p27 was detected as early as one week after infection in serum (fig 3 ▶). Four weeks after infection, 7/8 animals showed an antibody response to two or more different SIV antigens (fig 2A ▶). In all four animals who survived to week 24 after infection with SIV, an IgG response against three or all antigens tested was detectable and two to four of all antigens tested were also recognised by serum IgA (fig 2A ▶).
Western blot analysis of SIV specific immunoglobulins in saliva and serum
To compare the SIV specific antibody response in serum and saliva of SIV infected animals, serum IgG and IgA were adjusted to the same concentration and processed as described above for the dot blot analysis.
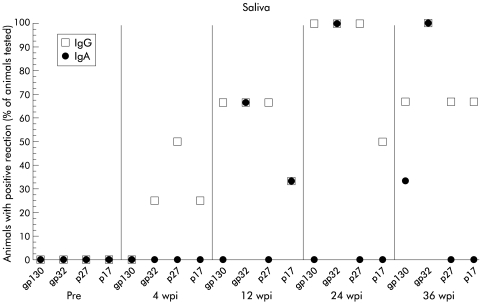
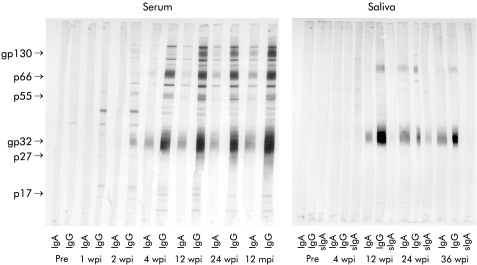
Four weeks after infection, 2/8 animals showed an IgG response to two different SIV antigens (fig 4 ▶). Twelve weeks after infection an IgA response against one or two antigens was also detectable (fig 4 ▶). None of the SIV infected animals except one showed any secretory IgA response in saliva. SC was demonstrated on SIV specific IgA in saliva only in 1/8 animals and only 24 weeks after infection (fig 5 ▶).
Figure 4.
Proportion of animals with salivary IgG or IgA reactive with the simian immunodeficiency virus (SIV) proteins gp130, gp32, p27, and p17, indicated by western blot (Pre, 2 wpi, and 4 wpi, n=8; 24 wpi, n=4; 36 wpi, n=3). Pre, preinfection; wpi, weeks postinfection.
Figure 5.
Representative example of simian immunodeficiency virus (SIV) specific immunoglobulin isotype in serum and saliva of one SIV infected animal during the time course of infection indicated by western blot. SIV specific polypeptides are indicated on the left. Pre, preinfection; wpi, weeks postinfection; mpi, months postinfection.
The antibody response detected by western blot was identical to the dot blot analysis for the antigens gp120, gp41, and p27. In addition, most animals also had IgG and IgA antibodies to p17, p55, and p66 beginning four weeks after infection (fig 5 ▶).
DISCUSSION
As expected, intestinal IgA production in uninfected animals was about 20-fold higher than IgG and approximately fivefold higher than IgM production.
In spite of substantial intraindividual and interindividual variability in local intestinal IgG, IgA, and IgM production, total IgG production increased while IgA production decreased late after infection. Similar findings in HIV infected patients have also been reported.13,19–21 In contrast with these reports, our method conducted previously in HIV infected patients14 and now in SIV infected animals reflects local intestinal immunoglobulin production and is not confused by transudation of antibodies of other origin. This is a crucial point in investigating mucosal antibody responses in HIV/SIV infection because it was shown that even early after infection mucosal inflammation is present,2,3 and epithelial barrier defects are demonstrable.22 The increase in local IgG production later after infection is in agreement with our studies in HIV infected patients14 and may further increase mucosal inflammation by complement activation.
To characterise the extent of transudation, proteins of plasma origin, such as albumin and IgG, were measured in rectal fluids at different disease stages. As shown, IgG and albumin were secreted in intestinal fluids in greater amounts in 5/12 SIV infected animals. Therefore, previous studies10–13 using intestinal fluids to demonstrate the intestinal immune response against SIV proteins were not able to discriminate between transudation of plasma antibodies and local immune responses.
In this study, we demonstrated a complete lack of SIV specific IgA production in the intestinal mucosa of SIV infected animals at any time, independent of the route of infection. In contrast, in serum, as early as one week after infection IgG and IgA were detectable against the SIV core protein p27 and three weeks later against most of the other SIV antigens. The lack of intestinal SIV specific IgA production was not due to a dilution artefact as prior dilution experiments were performed for calculating the minimal concentrations of IgA and IgG to detect the different SIV antigens. In contrast with SIV specific IgA, SIV specific IgG was detected in the supernatants of duodenal biopsies against gp160, four weeks after infection, later against gp148, while an intestinal IgG response to p27 and nef could not be detected in the intestinal mucosa. As secretory IgA plays a crucial role in the defence against pathogens in the intestinal mucosa7,23 and is also important in the control of mucosal HIV infection,8,9 rapid spread and high replication of HIV and SIV in the intestinal mucosa2–6 may be due to a missing or insufficient secretory immune response.
An explanation for the complete lack of SIV specific intestinal IgA response may be the early and nearly complete loss of intestinal CD4 T cells in HIV24–26 and SIV5,6 infection because mucosal CD4 T cells are necessary for an intestinal antigen specific IgA response.27 The fact that oral immunisation in healthy uninfected non-human primates with recombinant SIV gag p27 fused to yeast proteins28 or with microencapsulated formalin treated SIV29 also failed to induce a significant secretory IgA response in the rectal or genital tract may be due to induction of oral tolerance and therefore does not necessary argue against the above hypothesis. Augmenting oral or nasal immunisation with rectal or vaginal immunisation and more effectively by direct targeting of the iliac lymph node with recombinant SIV proteins elicits consistent mucosal antibody responses in healthy non-human primates,30 which is in agreement with a T cell dependent mucosal secretory IgA response against SIV and HIV. Moreover, rhesus macaques vaccinated intramuscularly with formalin inactivated SIV and challenged intravenously with SIV do not develop a decline in CD4 T cells, can produce mucosal SIV specific IgA, and are protected against an intrarectal challenge with SIV,31 demonstrating that an IgA response against SIV in the intestine can be induced in healthy animals. In contrast with secretory IgA, IgG is not capable of neutralising intracellular HIV synthesis,9 so it is not known if SIV/HIV specific mucosal IgG plays a protective role or is harmful by inducing immune complexes and causing further deterioration in intestinal inflammation.3,32,33
There was no difference in intestinal immunoglobulin production between animals infected intrarectally or intravenously. This is in accordance with our previous observation that intestinal infection with detectable SIV antigens occurs as early as one week after infection, independent of the route of infection.6 This early spread of SIV into the different parts of the intestinal tract was also reported by other groups.34 Furthermore, a preferential homing of SIV/HIV infected CD4 T cells into the intestinal mucosa was reported.35 Thus the route of infection with pathogenic SIV does not seem to influence local mucosal SIV specific antibody production.
In saliva, IgA concentrations in uninfected animals were about six times higher than IgG and seven times higher than IgM concentrations.
A reduction in IgA concentration early after infection was seen in saliva while IgG and IgM concentrations were not altered during SIV infection. Similar results were obtained in HIV infected patients.12,21,36 The decrease in salivary and intestinal IgA was not reflected by a decrease in serum IgA, indicating that there is a dichotomy between systemic and mucosal immunoglobulin production.
In contrast with the intestine, SIV specific IgA was detected in saliva which was mainly restricted to glycoproteins. However, SC was only present in SIV specific IgA at one time point in 1/8 animals, indicating not only a restricted SIV specific IgA response also found by others13,37 but more importantly a disturbed secretory antibody reaction. This finding further emphasises the fact that studies of saliva do not correctly mirror the situation in the intestine, although both compartments belong to mucosa associated lymphoid tissue. The reaction pattern of SIV specific IgG in saliva was similar to that of IgA and also restricted to glycoproteins. The role of salivary antibodies in the pathogenesis of HIV/SIV infection is poorly characterised.
In the serum of uninfected animals, IgG was the predominant immunoglobulin. In spite of substantial interindividual variability in serum IgG, IgA, and IgM concentration, IgG concentration seemed to be reduced in the first four weeks after infection while IgA was not altered and IgM showed only an intermittent decrease.
In contrast with the intestinal and salivary IgA immune responses against SIV antigens in serum, a strong and broad IgA reaction against almost all SIV proteins was detected. Thus there seems to be an abnormal and paradoxical IgA response in SIV infected animals.
Our results demonstrate a profound alteration of an important effector site of the mucosal immune system which is represented by the production of antigen specific secretory IgA. Despite the predominance of total IgA synthesis in the intestine, there was a complete lack of autochton production of SIV specific IgA in the intestinal mucosa at any time after infection. In contrast, in serum, where there is a predominance of total IgG, SIV specific IgA was found early after infection. Thus SIV specific IgA found in intestinal secretions10–13 may originate from serum. In contrast with peripheral blood where CD4 T cells are preserved for a long period, there is an early and complete loss of intestinal CD4 T cells following infection with pathogenic SIV.5,6,38 This may explain the different SIV specific IgA responses in peripheral blood and intestinal mucosa. Lack of antigen specific secretory IgA production against other enteropathogens may explain the preferential spread of opportunistic agents along mucosal surfaces in SIV/HIV infection.
Acknowledgments
The authors thank C Bernardi for expert technical help, Dr J Geisel for the immunoturbidimetric measurements of rectal swabs, and T Georg, Dipl-Math, for statistical assistance. The work was supported by grant FKZ 01 KI 9768/1 from the Bundesministerium für Bildung und Forschung (BMBF).
Abbreviations
gp, glycoprotein
HIV, human immunodeficiency virus
MID50, 50% monkey infectious dose
PBS, phosphate buffered saline
SC, secretory component
SIV, simian immunodeficiency virus
REFERENCES
- 1.Schneider T, Ullrich R, Zeitz M. Immunopathology of HIV infection in the gastrointestinal tract. Springer Semin Immunopathol 1997;18:515–33. [DOI] [PubMed] [Google Scholar]
- 2.Kotler DP, Scholes JV, Tierney AR. Intestinal plasma cell alterations in the acquired immunodeficiency syndrome. Dig Dis Sci 1987;32:129–38. [DOI] [PubMed] [Google Scholar]
- 3.Kotler DP, Reka S, Borcich A, et al. Detection, localisation and quantitation of HIV-associated antigens in the intestinal biopsies from patients with HIV. Am J Pathol 1991;139:823–30. [PMC free article] [PubMed] [Google Scholar]
- 4.Fackler OT, Schäfer M, Schmidt W, et al. HIV-1 p24 but not proviral load is increased in the intestinal mucosa compared with the peripheral blood in HIV-infected patients. AIDS 1998;12:139–46. [DOI] [PubMed] [Google Scholar]
- 5.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998;280:427–31. [DOI] [PubMed] [Google Scholar]
- 6.Kewenig S, Schneider T, Hohloch K, et al. Rapid mucosal CD4+ T cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology 1999;116:1115–23. [DOI] [PubMed] [Google Scholar]
- 7.Mazanec MB, Nedrud JG, Kaetzel CS, et al. A three-teered view of the role of IgA in mucosal defense. Immunol Today 1993;14:430–5. [DOI] [PubMed] [Google Scholar]
- 8.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med 1997;3:42–7. [DOI] [PubMed] [Google Scholar]
- 9.Bomsel M, Heyman M, Hocini H, et al. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 1998;9:277–87. [DOI] [PubMed] [Google Scholar]
- 10.Black KP, Fultz PN, Girard M, et al. IgA immunity in HIV type 1-infected chimpanzees II. Mucosal immunity. AIDS Res Hum Retroviruses 1997;13:1273–82. [DOI] [PubMed] [Google Scholar]
- 11.Kuller L, Thompson J, Watanabe R, et al. Mucosal antibody expression following rapid SIVMNE dissemination in intrarectally infected Macaca nemestrina. AIDS Res Hum Retroviruses 1998;14:1345–56. [DOI] [PubMed] [Google Scholar]
- 12.Müller F, Froland SS, Hvatum M, et al. Both IgA subclasses are reduced in parotid saliva from patients with AIDS. Clin Exp Immunol 1991;83:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raux M, Finkielsztein L, Salmon-Ceron D, et al. Comparison of the disturbation of IgG and IgA antibodies in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Res Hum Retroviruses 1999;15:1365–76. [DOI] [PubMed] [Google Scholar]
- 14.Schneider T, Zippel T, Schmidt W, et al. Increased immunoglobulin G production by short-term cultured duodenal biopsies from HIV-infected patients. Gut 1998;42:357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stark K, Warnecke C, Brinkmann V, et al. Sensitivity of HIV antibody detection in saliva. Med Microbiol Immunol 1993;182:147–51. [DOI] [PubMed] [Google Scholar]
- 16.Israel ZR, Marx PA. Nonclassical mucosal antibodies predominate in genital secretions of HIV-infected chimpanzees. J Med Primatol 1995;24:53–60. [DOI] [PubMed] [Google Scholar]
- 17.Stallmach A, van Look M, Scheiffele F, et al. IgG, albumin, and sCD44 in whole-gut lavage fluid are useful clinical markers for assessing the presence and activity of pouchitis. Int J Colorect Dis 1999;14:35–40. [DOI] [PubMed] [Google Scholar]
- 18.Schneider T, Zippel T, Schmidt W, et al. Abnormal predominance of IgG in HIV-specific antibodies produced by short-term cultured duodenal biopsies from patients infected with HIV. J Aquir Immunodefic Syndr 1997;16:333–9. [DOI] [PubMed] [Google Scholar]
- 19.Janoff EN, Jackson S, Wahl SM, et al. Intestinal mucosa immunoglobulins during human immunodeficiency virus type 1 infection. J Infect Dis 1994;170:299–307. [DOI] [PubMed] [Google Scholar]
- 20.Janoff EN, Wahl SM, Kelly T, et al. Modulation of human immunodeficiency virus type 1 infection of human monocytes by IgA. J Infect Dis 1995;172:855–8. [DOI] [PubMed] [Google Scholar]
- 21.Sweet SP, Rahman D, Challacombe SJ. IgA subclasses in HIV disease: dichotomy between raised levels in serum and decreased secretion rates in saliva. Immunology 1995;86:556–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Stockmann M, Fromm M, Schmitz H, et al. Duodenal biopsies of HIV-infected patients with diarrhoea exhibit epithelial barrier defects but no active secretion. AIDS 1998;12:43–51. [DOI] [PubMed] [Google Scholar]
- 23.Stokes CR, Soothill JF, Turner MW. Immune exclusion is a function of IgA. Nature 1975;255:745–6. [DOI] [PubMed] [Google Scholar]
- 24.Lim SG, Condez A, Lee CA, et al. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin Exp Imunol 1993;92:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider T, Ullrich J, Jahn H-U, et al. Loss of activated CD4-positive T cells and increase in activated cytotoxic CD8-positive T cells in the duodenum of patients infected with human immunodeficiency virus. Adv Exp Med Biol 1995;371B:1019–21. [PubMed] [Google Scholar]
- 26.Schneider T, Ullrich R, Bergs C, et al. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Gut 1995;37:524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hörnquist CE, Ekman L, Grdic KD, et al. Paradoxical IgA immunity in CD4-deficient mice—Lack of cholera toxin-specific protective immunity despite normal gut mucosal IgA differentiation. J Immunol 1995;155:2877–87. [PubMed] [Google Scholar]
- 28.Lehner T, Bergmeier LA, Panagiotidi C, et al. Induction of mucosal and systemic immunity to a recombinant simian immunodeficiency viral protein. Science 1992;258:1365–9. [DOI] [PubMed] [Google Scholar]
- 29.Marx PA, Compans RW, Gettie A, et al. Protection against vaginal SIV transmission with microencapsulated vaccine. Science 1993;260:1323–7. [DOI] [PubMed] [Google Scholar]
- 30.Lehner T, Wang Y, Ping L, et al. The effect of route of immunization on mucosal immunity and protection. J Infect Dis 1999;179(suppl 3):S489–92. [DOI] [PubMed] [Google Scholar]
- 31.Cranage MP, Baskerville A, Ashworth LA, et al. Intrarectal challenge of macaques vaccinated with formalin-inactivated simian immunodeficiency virus. Lancet 1992;339:273–4. [DOI] [PubMed] [Google Scholar]
- 32.Brandtzaeg P, Tolo K. Mucosal penetrability enhanced by serum-derived antibodies. Nature 1977;266:262–3. [DOI] [PubMed] [Google Scholar]
- 33.Kozlowski PA, Chen D, Eldridge JH, et al. Contrasting IgA and IgG neutralization capacities and responses to HIV type gp 120 V3 loop in HIV-infected individuals. AIDS Res Hum Retroviruses 1994;10:813–22. [DOI] [PubMed] [Google Scholar]
- 34.Heise C, Vogel P, Miller CJ, et al. Distribution of SIV infection in the gastrointestinal tract of rhesus macaques at early and terminal stages of AIDS. J Med Primatol 1993;22:187. [PubMed] [Google Scholar]
- 35.Donze HH, Cummins JE, Schwiebert R, et al. HIV-1/simian immunodeficiency virus infection of human and nonhuman primate lymphocytes results in the migration of CD2+ T cells into the intestine of engrafted SCID mice. J Immunol 1998;160:2506–13. [PubMed] [Google Scholar]
- 36.Jackson S. Secretory and serum IgA are inversely altered in AIDS patients. In: Mac Donald TT, Challacombe SJ, Bland PW, et al, eds. Advances in mucosal immunology (Proceedings of the Fifth International Congress of Mucosal Immunology). Dordrecht: Kluwer Academic Press, 1990:665–8.
- 37.Jackson S, Moldoveanu Z, Mestecky J, et al. Decreased IgA-producing cells in the gut of SIV-infected rhesus monkeys. Adv Exp Med Biol 1995;371:1035. [PubMed] [Google Scholar]
- 38.Harouse JM, Gettie A, Tan RCH, et al. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 1999;284:816–19. [DOI] [PubMed] [Google Scholar]