We have noticed the frequent publication of important advances in the serological screening of coeliac disease (CD), such as the interesting and useful technique described by Baldas et al (Gut 2000;47:628–31). Humoral screening of CD is coming closer than ever towards representing an affordable population wide strategy (Gut 2000;47:628–31), largely due to the identification of tissue transglutaminase (tTG) as the main—if not only—autoantigen for antiendomysial antibodies (EMA).1 This finding hightlights the possibility of antigen specific testing and, today, determination of anti-tTG is a valid alternative to EMA.2
However, we believe that the recent advances in the cellular component of the diagnosis of CD have been somewhat overlooked. The study of intestinal intraepithelial lymphocytes (IEL) by flow cytometry3 has added specificity to mere histological study of the small bowel biopsy. It has been shown that CD is characterised by an important increase in the TcR-γδ+ IEL subset (or γδ IEL), a decrease in the natural killer (NK)-like subset and, depending on gluten intake, a considerable increase in the TcR-αβ+ IEL (αβ IEL) subset which constitute the majority of IEL.4 The increase in γδ IEL (average 4% in controls v 25% in coeliacs, with respect to total IEL)4 is not per se diagnostic of CD as it has been observed, although to a lower extent, in food allergy5 and occasionally in other conditions. But CD is the only entity in which γδ IEL have been described as systematically, permanently, and markedly raised.4 The combined study of total, γδ, and NK-like IELs, that could be termed “IEL lymphogram”, allows for nearly 94% specificity and sensitivity in the diagnosis of CD after clinical suspicion.4 This technique, complementary to the diagnosis of symptomatic and silent CD, shows its real value in latent and potential presentations of the disease, and offers important data for the differential diagnosis from other enteropathies. It is noteworthy that the increase in IEL is the earliest detectable alteration in the mucosa,6 prior to the increase in lamina propria lymphocytes or architectural changes.
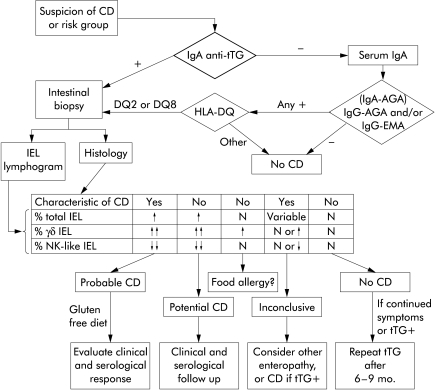
Many recent reviews6,7 have commented on these characteristic serological and cellular findings of CD but their incorporation into clinical practice is very different. While tTG testing is spreading, IEL phenotyping—particularly by flow cytometry—is still regarded as a research tool rather than a diagnostic test. We consider that the easy procedure of IEL procurement and phenotyping4,8 could be routinely performed in many medium sized hospitals, and we propose an initial screening algorithm that takes this “IEL lymphogram” into account (fig 1 ▶).
Figure 1.
Proposal of an initial diagnostic algorithm for coeliac disease (CD). After screening with anti-transglutaminase (tTG), and taking into account the high negative predictive value of HLA typing, study of mandatory intestinal biopsy would include phenotyping of intraepithelial lymphocytes (IEL). The proportion of “total IEL” is calculated with respect to the cellularity of the epithelium while the proportions of “γδ IEL” and “natural killer (NK)-like IEL” are relative to the total IEL. The combined analysis of the pathology and the “IEL lymphogram” allows for a correct classification of >95% of patients after the first biopsy, reducing the need for subsequent invasive procedures. N, normal values.
Screening would be based on tTG IgA determination, and seric IgA quantification if anti-tTG was negative. If there was an IgA deficiency, only IgG tests would then be performed. If serum and blood were obtained at the first visit and temporarily cryopreserved, many tests (serum IgA, AGA, EMA, HLA, IgE, other autoantibodies, etc.) could be performed without the patient attending the clinic again.
The establishment of the putative diagnosis would be achieved by mandatory small bowel biopsy. But the IEL lymphogram would allow for serological and clinical evaluation of gluten withdrawal (and challenge) if it fitted into the coeliac pattern and histology showed a typical coeliac enteropathy. If the lymphogram shows normal values for γδ and NK-like IEL, it has a high negative predictive value of 95% against the existence of CD.4 If the interpretation of the immunohistological study is not straightforward, the classical ESPGAN criteria can be followed.9 We believe that this algorithm, which can be conveniently adapted to the needs of each centre, can correctly classify the vast majority of patients, saving time and money, and avoiding morbidity.
Acknowledgments
Our work was financed by the Spanish Fondo de Investigaciones Sanitarias (FIS), grants Nos 00/0196 (G Roy) and 01/9417 (F León).
References
- 1.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of coeliac disease. Nat Med 1997;3:797–801. [DOI] [PubMed] [Google Scholar]
- 2.León F, Camarero C, Pena R, et al. Anti-transglutaminase IgA ELISA: Clinical potential and drawbacks in celiac disease diagnosis. Scand J Gastroenterol 2001;36:849–53. [DOI] [PubMed] [Google Scholar]
- 3.Eiras P, Roldán E, Camarero C, et al. Flow cytometry description of a novel CD3- CD7+ intraepithelial subset in human duodenal biopsies: potential diagnostic value in coeliac disease. Cytometry 1998;34:95–102. [DOI] [PubMed] [Google Scholar]
- 4.Camarero C, Eiras P, Asensio A, et al. Intraepithelial lymphocytes and coeliac disease: Permanent changes in CD3-/CD7+ and T cell receptor γδ subsets studied by flow cytometry. Acta Paediatr 2000;89:285–90. [PubMed] [Google Scholar]
- 5.Kokkonen J, Holm K, Karttunen TJ, et al. Children with untreated food allergy express a relative increment in the density of duodenal gammadelta+ T cells. Scand J Gastroenterol 2000;35:1137–42. [DOI] [PubMed] [Google Scholar]
- 6.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology 2000;119:234–42. [DOI] [PubMed] [Google Scholar]
- 7.Ciclitira PJ. AGA technical review on celiac sprue. Gastroenterology 2001;20:1526–40. [DOI] [PubMed] [Google Scholar]
- 8.Madrigal L, Lynch S, Feighery C, et al. Flow cytometry analysis of surface major histocompatibility complex class II expression on human epithelial cells prepared from small intestinal biopsies. J Immunol Methods 1993;158:207–14. [DOI] [PubMed] [Google Scholar]
- 9.Report of Working Group of ESPGAN. Revised criteria for diagnosis of celiac disease. Arch Dis Child 1990;65:909–11. [DOI] [PMC free article] [PubMed] [Google Scholar]