Abstract
Background: Helicobacter pylori infection and non-steroidal anti-inflammatory drugs are two major causes of gastric ulceration but interactions between H pylori and these drugs in gastric mucosal injury are unclear.
Aims: We studied the influence of experimental H pylori infection on gastric mucosal injury induced by aspirin.
Subjects: Male Mongolian gerbils free of specific pathogens were used.
Methods: H pylori ATCC43504 culture broth was administered by oral gavage at seven weeks of age. After three weeks, acidified aspirin (400 mg/kg) was administered orally, and three hours later the total area of gastric erosions, myeloperoxidase (MPO) activity (an index of neutrophil accumulation), thiobarbituric acid reactive substances (TBARS, an index of lipid peroxidation), and KC/GRO (a chemoattractive cytokine in rodents) were measured in gastric mucosa. To determine the role of neutrophils in these circumstances, antigerbil neutrophil rabbit serum (ANS) was administered to some animals 18 hours before aspirin.
Results: Aspirin caused more extensive haemorrhagic erosions (33.1 (12.3) mm2) associated with greater MPO activity (1887.7 (598.5) μU/mg protein) and TBARS (0.33 (0.14) nmol/mg protein) and KC/GRO concentrations (28.3 (9.5) pg/mg protein) in infected than in uninfected gerbils (13.7 (2.3); 204.0 (68.9); 0.12 (0.06); 3.1 (0.8), respectively) Pretreatment with ANS inhibited the increases in gastric erosions, MPO activity, and TBARS but not KC/GRO concentration. The reduction in aspirin induced mucosal injury by administration of ANS was much greater in H pylori infected animals (65%) than in uninfected animals (31%).
Conclusions: H pylori infection potentiates aspirin induced gastric mucosal injury by mechanisms that include accumulation of activated neutrophils.
Keywords: Helicobacter pylori, non-steroidal anti-inflammatory drugs, aspirin, gastric mucosal injury, neutrophils, gerbils
Both Helicobacter pylori infection and non-steroidal anti-inflammatory drug (NSAID) use are well established risk factors for gastrointestinal mucosal injury. Several clinical studies have explored the relationships between these two factors. Very few experimental data have been reported however, leaving many questions unanswered.
Among the clinical studies, Santucci and colleagues1 found that gastroduodenal mucosal lesions caused by four weeks of NSAID administration were more severe if patients had H pylori infection. In addition, Publig and colleagues2 reported that the prevalence of H pylori infection in patients taking NSAIDs who developed gastric ulcers was 83%, significantly greater than in patients without gastric ulcers (45%). However, other studies found no evidence of potentiated injury when H pylori infection coexisted with NSAID use. Among patients with rheumatoid arthritis taking NSAIDs, Graham and colleagues3 reported gastric erosions in 34% and bleeding in 32% of patients who also had H pylori infection, representing lower incidences than in patients without H pylori infection (57% and 61%, respectively). Lipscomb and colleagues4 found no difference in the prevalence of H pylori infection on severity of gastric mucosal injury in healthy volunteers who received NSAIDs for up to four weeks. These disagreements may be related to differences in severity of gastric mucosal atrophy between patients with and without H pylori infection as well as differences in the basal level of mucosal inflammation.
Recent experimental studies have indicated that neutrophils adherent to the endothelium via various adhesion molecules are involved in the development of gastric mucosal injury induced by H pylori infection or NSAID use.5–12 We previously reported that H pylori and NSAIDs cause neutrophils to express adhesion molecules followed by accumulation of neutrophils into the gastric mucosa.7,8 Activated neutrophils have been suggested to injure endothelial and epithelial cells by producing active oxygen species and proteases.9,10,13–15 Additionally, in a randomised controlled trial, Taha et al demonstrated that gastric neutrophils associated with H pylori infection increased the incidence of ulceration in long term NSAID users.16 These findings suggest that H pylori and NSAIDs can elicit an acute inflammatory response in the gastric mucosa leading to neutrophil mediated tissue injury.
The objective of the present study was to determine the influences on and consequences of neutrophil associated inflammatory reactions in gastric mucosa exposed to both experimental H pylori infection and NSAIDs. In 1995, Hirayama and colleagues17 described a model in which Mongolian gerbils infected with H pylori developed pathological changes in the stomach that mimicked those seen in humans who harbour the bacteria. These changes included a high incidence of gastritis after six weeks of infection and gastric ulceration after six months of infection.18 In addition, Watanabe and colleagues19 found that inoculation of H pylori isolated from a patient with a gastric ulcer could result in the occurrence of gastric cancer in Mongolian gerbils. Hence the gerbil model can be used to clarify the pathophysiology of H pylori induced gastric mucosal lesions. We chose these animals for our present experiments.
MATERIALS AND METHODS
Animals and bacteria
Seven week old male Mongolian gerbils (MGS/Sea) free of specific pathogens were purchased from Seiwa Experimental Animals (Fukuoka, Japan). H pylori (ATCC43504) was obtained from the American Type Culture Collection (Rockville, Maryland, USA). All experimental procedures were approved by the Animal Care Committee of the Kyoto Prefectural University of Medicine.
Bacterial inoculation
H pylori was grown in 200 ml Erlenmeyer flasks containing Brucella broth (BBL, Cockeysville, Maryland, USA) supplemented with 10% heat inactivated horse serum (Nakarai Tesque, Kyoto, Japan). The flasks were incubated at 37°C for 24 hours in an 8% CO2 atmosphere on a rotary shaker. Broth culture (500 μl) containing H pylori (4×108 CFU/ml) was given to gerbils by oral gavage after a 24 hour fast.
Aspirin induced gastric mucosal injury
Thirty six gerbils were divided into four groups of nine animals. Three weeks after inoculation with H pylori, aspirin (400 mg/kg; Sigma Chemical, St Louis, Missouri, USA) suspended in 0.8 ml of 0.25% carboxymethylcellulose (CMC) and 0.1 N HCl were administered orally to gerbils fasted for 18 hours. In the control group, gerbils received 0.8 ml CMC alone. At three hours after aspirin administration, gerbils were killed by exsanguination during urethane anaesthesia. The stomach was removed and opened along the greater curvature. Macroscopic gastric damage was examined under a dissecting microscope using a square grid, and was quantified as the total area of haemorrhagic erosions (erosion index).
A small piece of gastric mucosa from each animal was plated on Skirrow agar medium containing 7% horse blood. After incubation in an 8% CO2 atmosphere, the organisms were identified as H pylori on the basis of colony morphology, Gram stain, and urease, oxidase, and catalase production. In three gerbils from each group, the remainder of the mucosa was fixed in 10% neutral buffered formalin for histopathological examination. In the other six gerbils from each group, the remainder of the mucosa was scraped off with two glass slides and homogenised in 1.5 ml of 10 mM potassium phosphate buffer (pH 7.8) using a Teflon Potter-Elvehjem homogeniser. The homogenate was used to measure the concentration of thiobarbituric acid reactive substances (TBARS) and also myeloperoxidase (MPO) activity. As an index of lipid peroxidation, the TBARS concentration in gastric mucosa was measured by the method of Ohkawa and colleagues,20 and expressed as nanomoles of malondialdehyde produced. TBA (BDH Chemicals, Poole, UK) and 1,1,3,3-tetramethoxypropane (Tokyo Kasei, Tokyo) were used for the assay. The protein concentration in gastric mucosal homogenates was measured by the method of Lowry and colleagues.21 MPO activity in the gastric mucosa, an index of neutrophil infiltration, was determined by a modification of the method of Krawisz and colleagues.22 Briefly, homogenised gastric mucosal samples were sonicated on ice for 10 seconds and centrifuged at 40 000 g for 15 minutes. The supernatant was used for measurement of MPO activity. One unit of MPO activity was defined as the amount of activity degrading 1 μmol of peroxide per minute at 25°C. The content of KC/GRO, a chemoattractive cytokine in rodents, was measured in gastric mucosa by an enzyme immunoassay using a mouse KC assay kit (IBL, Gunma, Japan).23 After homogenisation, the gastric mucosal samples were centrifuged at 40 000 g for 15 minutes, and the supernatant was used for this enzyme immunoassay.
Effect of antigerbil neutrophil serum
To investigate the role of neutrophils, rabbit antiserum against gerbil neutrophils was administered to gerbils. Gerbil neutrophils were collected by peritoneal lavage with sterile saline six hours after intraperitoneal injection of 0.12% oyster glycogen in saline. Antineutrophil serum (ANS) was obtained by immunising rabbits with these gerbil neutrophils (approximately 2×107 neutrophils/rabbit) in Freund's complete adjuvant (Difco Laboratories, Detroit, Michigan, USA) using the modified method of Ward and Cochrane24 and Yoshida.25 Thirty gerbils were divided into five groups of six animals. Eighteen hours before administration of aspirin to gerbils with or without H pylori infection, 15 ml/kg ANS were injected intraperitoneally. Control gerbils were injected intraperitoneally with normal rabbit serum.
Statistical analysis
All values are expressed as mean (SEM). Results were compared using analysis of variance (ANOVA) followed by Scheffe's test. A value of p<0.05 was considered to indicate statistical significance.
RESULTS
Detection of H pylori infection
At three weeks, H pylori was cultured and identified on the basis of colony morphology, Gram stain, and production of urease, oxidase, and catalase in all gerbils inoculated with the organism.
Macroscopic findings
Mild oedema and erythema were observed macroscopically in the gastric mucosa of gerbils inoculated with H pylori. Oral administration of aspirin produced mild haemorrhagic erosions except in H pylori infected gerbils where severe haemorrhagic erosions resulted.
Erosion index
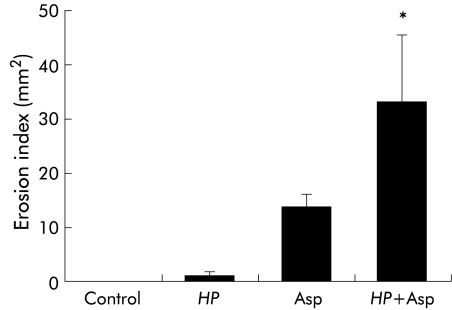
Figure 1 ▶ shows the index of gastric erosion for gerbils exposed to H pylori and/or aspirin. The erosion index in gerbils receiving aspirin was significantly greater than that in untreated control gerbils. In addition, oral administration of aspirin to H pylori infected gerbils resulted in a mean erosion index 2.5 times greater than in aspirin treated gerbils without H pylori infection.
Figure 1.
Erosion index for the gastric mucosa of Helicobacter pylori (HP) and/or aspirin (Asp) treated gerbils. Three weeks after inoculation of HP, gerbils were given Asp. Three hours later, the stomach was removed and gastric damage was quantified as the total area of haemorrhagic erosions (erosion index). Control gerbils received only carboxymethylcellulose without HP. Values are mean (SEM) for six gerbils. *p<0.05 compared with gerbils who received Asp alone.
Microscopic findings
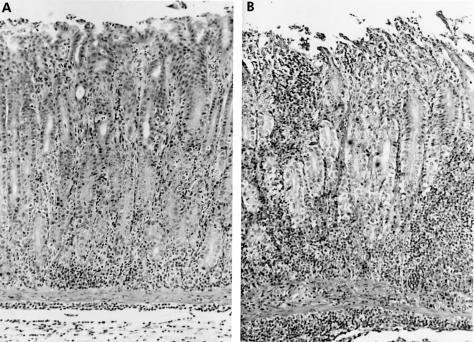
Microscopic examination revealed neutrophils and mononuclear cells in the gastric lamina propria in gerbils infected with H pylori. In gerbils who received only aspirin, occasional neutrophils and mild erosions were observed. In H pylori infected gerbils, administration of aspirin resulted in intense infiltration by neutrophils and severe erosions of the gastric mucosa (fig 2 ▶).
Figure 2.
Microscopic features of the pyloric mucosa in gerbils. (A) Helicobacter pylori group. Moderate amounts of neutrophils and mononuclear cells were seen three weeks after inoculation of H pylori. (B) H pylori and aspirin group. Numerous neutrophils and mononuclear cells as well as deep erosions were evident (haematoxylin and eosin stain, ×100).
MPO activity in the gastric mucosa
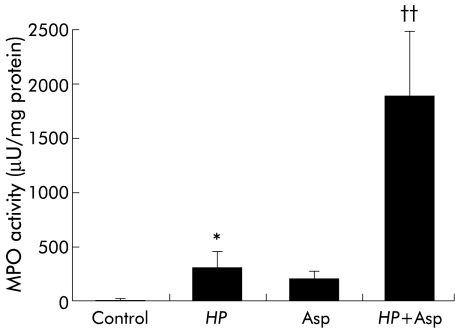
As shown in fig 3 ▶, MPO activity in the gastric mucosa of H pylori infected gerbils was significantly greater than in controls. However, mucosal MPO activity in gerbils treated with aspirin alone was unchanged. MPO activity in gerbils exposed to both H pylori and aspirin was significantly greater than in gerbils who received either H pylori or aspirin alone.
Figure 3.
Myeloperoxidase (MPO) activity in gastric mucosa in Helicobacter pylori (HP) and/or aspirin (Asp) exposed gerbils. HP and/or Asp induced gastric mucosal injury was produced as described for fig 1 ▶. MPO activity in the gastric mucosa was determined by a modification of the method of Krawisz and colleagues.22 Values are mean (SEM) for six gerbils. *p<0.05 compared with control gerbils with neither HP infection nor Asp administration; ††p<0.01 compared with gerbils with HP infection or treatment with Asp alone.
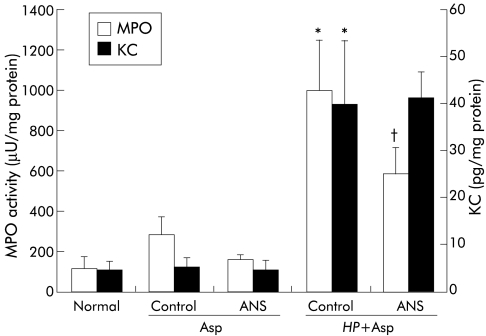
KC content in the gastric mucosa
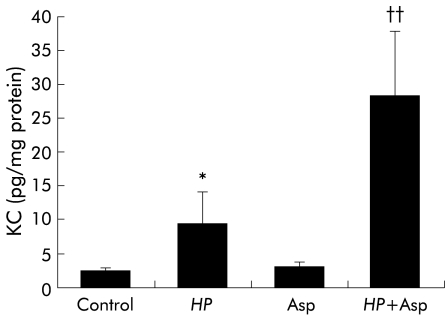
As shown in fig 4 ▶, KC content in the gastric mucosa of gerbils inoculated with H pylori was significantly greater than that in controls. Administration of aspirin alone to gerbils had no significant effect on KC content in the gastric mucosa. Mucosal KC content in gerbils receiving both H pylori and aspirin was significantly greater than that in gerbils receiving either H pylori or aspirin alone.
Figure 4.
KC concentration in gastric mucosa in Helicobacter pylori (HP) and/or aspirin (Asp) exposed gerbils. HP and/or Asp related gastric mucosal injury was induced as described for fig 1 ▶. KC in gastric mucosa was measured by an enzyme immunoassay. Values are mean (SEM) for six gerbils. *p<0.05 compared with control gerbils with neither HP infection nor Asp administration; ††p<0.01 compared with gerbils with HP infection or treatment with Asp alone.
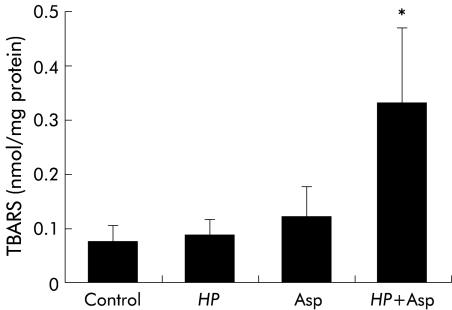
TBARS content in the gastric mucosa
The TBARS content in the gastric mucosa of gerbils treated with either H pylori or aspirin alone was not increased relative to TBARS in untreated control gerbils. However, TBARS content in the gastric mucosa of gerbils treated with both H pylori and aspirin was significantly greater than in gerbils treated with either H pylori or aspirin alone (fig 5 ▶).
Figure 5.
Thiobarbituric acid reactive substances (TBARS) in gastric mucosa in Helicobacter pylori (HP) and/or aspirin (Asp) exposed gerbils. HP and/or Asp induced gastric mucosal injury was produced as described for fig 1 ▶. TBARS in the gastric mucosa were measured by the method of Ohkawa and colleagues.20 Values are mean (SEM) for six gerbils. *p<0.05 compared with gerbils with only HP infection or Asp treatment.
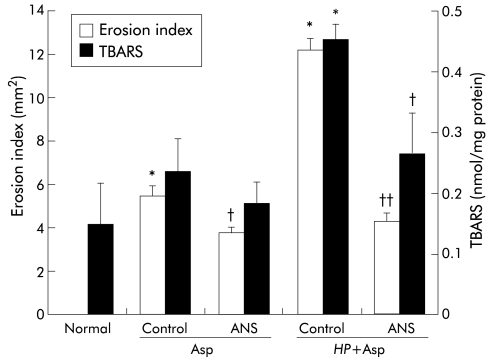
Effect of ANS
The circulating neutrophil count showed a decrease of 77.2%, 18 hours after administration of ANS (1660 (260) v 7300 (840)/mm3 in gerbils treated with normal rabbit serum; p<0.01). Platelet count was not influenced by ANS administration. Erosion index, MPO activity, and TBARS were significantly less in neutrophil depleted gerbils exposed to H pylori plus aspirin than in similarly exposed animals treated with normal rabbit serum (figs 6, 7 ▶ ▶). However, administration of ANS had no influence on the increase in KC content. In contrast, aspirin induced mucosal injury in uninfected animals was also reduced after administration of ANS. The inhibitory effect on mucosal damage by ANS administration was much greater in H pylori infected gerbils (65%) than in uninfected gerbils (31%).
Figure 6.
Effect of antineutrophil serum (ANS) on erosion index and thiobarbituric acid reactive substances (TBARS) in the gastric mucosa after administration of aspirin (Asp) to uninfected or Helicobacter pylori (HP) infected gerbils. Asp (400 mg/kg) and 0.1 N HCl were administered orally to gerbils three weeks after inoculation with HP. ANS (15 ml/kg) was injected intraperitoneally 18 hours before administration of Asp. Control gerbils received intraperitoneal injection of normal rabbit serum. Values are mean (SEM) for six gerbils. *p<0.01 compared with normal gerbils given neither HP nor Asp; †p<0.05, ††p<0.01 compared with control gerbils treated with normal rabbit serum.
Figure 7.
Effect of antineutrophil serum (ANS) on myeloperoxidase (MPO) activity and KC content in the gastric mucosa after administration of aspirin (Asp) to uninfected or Helicobacter pylori (HP) infected gerbils. ANS and Asp were administered to gerbils three weeks after inoculation with HP, as described for fig 6 ▶. Values are mean (SEM) for six gerbils. *p<0.01 compared with normal gerbils with neither HP infection nor Asp administration; †p<0.05 compared with control gerbils treated with normal rabbit serum.
DISCUSSION
In the present study, haemorrhagic erosions in the gastric mucosa caused by aspirin were much more severe in gerbils with H pylori infection than in uninfected gerbils. Recently, neutrophils have been implicated in the pathogenesis of NSAID induced gastric mucosal injury.5–7,9–11 In the present study we therefore evaluated neutrophil infiltration and neutrophil chemoattractant in aspirin and/or H pylori induced gastric mucosal injury. While minimal neutrophil infiltration resulted when only aspirin was administered, neutrophil infiltration was more prominent when H pylori infection was also present. In addition, aggravation of gastric mucosal lesions induced by the combination of aspirin and H pylori infection was significantly inhibited in neutrophil depleted gerbils. The reduction in mucosal injury by administration of ANS was much greater in H pylori infected animals than in uninfected animals. These findings indicate that the effects of aspirin on the gastric mucosa may be potentiated by H pylori infection via neutrophil dependent mechanisms. Our previous in vitro studies showed that aspirin caused adherence of neutrophils to endothelial cells by increasing surface expression of CD11b/CD18 on neutrophils; adherence was followed by neutrophil mediated endothelial cell injury.7,9 Asako and colleagues26,27 reported that superfusion of aspirin or indomethacin promoted neutrophil adhesion to postcapillary venules of rat mesenteric vessels but did not result in transendothelial migration of neutrophils. In addition, Wallace and colleagues5,6 as well as our own investigational group10,11 have demonstrated that NSAID induced gastric mucosal injury in rats can be prevented by inhibiting neutrophil-endothelial cell adhesion using monoclonal antibodies directed against adhesion molecules. Our recent experiments implicated neutrophils adhering to blood vessels, but not neutrophils migrating into the interstitium, in uncomplicated aspirin induced gastric mucosal injury.28 Thus neutrophil-endothelial cell adhesion appears to be an early event in the pathogenesis of NSAID induced gastric mucosal injury.
In contrast, H pylori associated gastric mucosal injury has been attributed to activated neutrophils that adhere to postcapillary venules and subsequently migrate into the interstitium.8,15,27 Accumulation of neutrophils in H pylori infected human gastric mucosa is related to increased concentrations of interleukin (IL)-8,29 a potent neutrophil chemoattractant that is released by gastric epithelial cells.30 In the present study, we measured KC, an IL-8-like neutrophil chamoattractant in gastric mucosa. KC is considered to be both a potent chemoattractant and an upregulator of CD11b/CD18 cell surface expression in rodent neutrophils.31 Bozic and colleagues31 reported that KC mRNA is constitutively expressed in various tissues. We found that gastric mucosal KC content in gerbils exposed to H pylori alone was somewhat greater than in mucosa exposed to aspirin alone. Furthermore, in gerbils treated with aspirin after inoculation with H pylori, the KC content in gastric mucosa was significantly greater than in gastric mucosa of gerbils that received only aspirin. These findings suggest that increased KC content may be involved in accumulation of neutrophils in the gastric mucosa. In the present study, administration of antiserum against neutrophils remarkably reduced the increase in mucosal MPO activity but not the increase in KC content. Neutrophils therefore were not the main source of KC. While the source of KC was not identified in this study, KC may have been produced by gastric epithelial cells and/or macrophages. In addition, the mechanism by which KC content was particularly increased in gerbil gastric mucosa exposed to both aspirin and H pylori remains undetermined. Further studies are required to investigate regulation of KC in the gerbil gastric mucosa. Overall, we hypothesised that administration of aspirin to H pylori infected mucosa caused adherent neutrophils to easily migrate into the extravascular space by chemoattractants such as KC.
Oxygen derived free radicals are known to cause peroxidation of polyunsaturated fatty acids in cell membranes. The resulting increase in lipid peroxides can lead to changes in membrane fluidity and permeability, and finally to cell lysis.32 We previously reported that lipid peroxidation plays an important role in the pathogenesis of gastric mucosal lesions induced by burn stress,33 platelet activating factor,34 and ischaemia-reperfusion35 or NSAIDs.36 In the present study, TBARS in gastric mucosa, an index of lipid peroxidation, were more abundant in gerbils with both H pylori infection and aspirin administration than in gerbils treated with either H pylori or aspirin alone. These findings indicate that lipid peroxidation, most likely caused by neutrophil derived oxygen free radicals, was easily induced in the gastric mucosa of gerbils exposed to aspirin following inoculation of H pylori. Additional experiments using antioxidants such as free radical scavengers10,36 are needed to determine the involvement of oxygen derived free radicals in gastric mucosal injury induced by concomitant H pylori and NSAIDs.
In summary, the present study indicated that administration of aspirin to gerbils three weeks after H pylori inoculation produced severe gastric mucosal injury via marked infiltration of neutrophils. Hence eradication of H pylori could help prevent NSAID induced mucosal injury, as also indicated by a recent clinical trial where eradication of H pylori before NSAID use reduced the occurrence of NSAID induced ulcers.37,38 In the present study, three weeks after H pylori inoculation, gerbils exhibited typical gastritis with neutrophil and mononuclear cell infiltration in the lamina propria and a few superficial erosions. The Mongolian gerbil model subsequently showed characteristic changes of chronic gastritis, including formation of lymphoid follicles six weeks after H pylori infection and gastric ulcers at more than six months after infection.17,18 The effect of NSAID administration on chronic gastritis or ulcers, showing such mononuclear cell mediated chronic inflammation, is of considerable importance. The effects of an NSAID in gerbils at six weeks and six months following inoculation of H pylori need to be investigated.
Abbreviations
MPO, myeloperoxidase
TBARS, thiobarbituric acid reactive substances
ANS, antineutrophil serum
NSAID, non-steroidal anti-inflammatory drug
CMC, carboxymethylcellulose
IL, interleukin
REFERENCES
- 1.Santucci L, Fiorucci S, Patoia L, et al. Severe gastric mucosal damage induced by NSAIDs in healthy subjects is associated with Helicobacter pylori infection and high levels of serum pepsinogens. Dig Dis Sci 1995;40:2074–80. [DOI] [PubMed] [Google Scholar]
- 2.Publig W, Wustinger C, Zandl C. Non-steroidal anti-inflammatory drugs (NSAID) cause gastrointestinal ulcers mainly in Helicobacter pylori carriers. Wien Klin Wochenschr 1994;106:276–9. [PubMed] [Google Scholar]
- 3.Graham DY, Lidsky MD, Cox AM, et al. Long-term nonsteroidal antiinflammatory drug use and Helicobacter pylori infection. Gastroenterology 1991;100:1653–7. [DOI] [PubMed] [Google Scholar]
- 4.Lipscomb GR, Wallis N, Armstrong G, et al. Influence of Helicobacter pylori on gastric mucosal adaptation to naproxen in man. Dig Dis Sci 1996;41:1583–8. [DOI] [PubMed] [Google Scholar]
- 5.Wallace JL, Arfors KE, McKnight GW. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology 1991;100:878–83. [DOI] [PubMed] [Google Scholar]
- 6.Wallace JL, McKnight W, Miyasaka M, et al. Role of endothelial adhesion molecules in NSAID-induced gastric mucosal injury. Am J Physiol 1993;265:G993–8. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida N, Takemura T, Granger DN, et al. Molecular determinations of aspirin-induced neutrophil adherence to endothelial cells. Gastroenterology 1993;105:715–24. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida N, Granger DN, Evans DJ Jr, et al. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology 1993;105:1431–40. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida N, Cepinskas G, Granger DN, et al. Aspirin-induced, neutrophil-mediated injury to vascular endothelium. Inflammation 1995;19:297–312. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida N, Yoshikawa T, Nakamura Y, et al. Role of neutrophil-mediated inflammation in aspirin-induced gastric mucosal injury. Dig Dis Sci 1995;40:2300–4. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida N, Yoshikawa T, Nakamura Y, et al. Role of neutrophil-endothelial cell interactions in gastric mucosal injury induced by aspirin. J Clin Gastroenterol 1995;21:S73–7. [PubMed] [Google Scholar]
- 12.Takemura T, Granger DN, Evans DJ, et al. Extract of Helicobacter pylori induces neutrophils to injure endothelial cells and contains antielastase activity. Gastroenterology 1996;110:21–9. [DOI] [PubMed] [Google Scholar]
- 13.Inauen W, Granger DN, Meininger CJ, et al. Anoxia-reoxygenation-induced, neutrophil-mediated endothelial cell injury: role of elastase. Am J Physiol 1990;259:H925–31. [DOI] [PubMed] [Google Scholar]
- 14.Inauen W, Granger DN, Meininger CJ, et al. An in vitro model of ischemia/reperfusion-induced microvascular injury. Am J Physiol 1990;259:G134–9. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M, Miura S, Suematsu M, et al. Helicobacter pylori-associated ammonia production enhances neutrophil-dependent gastric mucosal cell injury. Am J Physiol 1992;263:G719–25. [DOI] [PubMed] [Google Scholar]
- 16.Taha AS, Dahill S, Morran C, et al. Neutrophils, Helicobacter pylori, and nonsteroidal anti-inflammatory drug ulcers. Gastroenterology 1999;116:254–8 [DOI] [PubMed] [Google Scholar]
- 17.Hirayama F, Takagi S, Yokoyama Y, et al. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol 1996;9:24–8. [PubMed] [Google Scholar]
- 18.Hirayama F, Takagi S, Kushuhara H, et al. Induction of gastric ulcer and intestinal metaplasia in Mongolian gerbils infected with Helicobacter pylori. J Gastroenterol 1996;31:755–7. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe T, Tada M, Nagai H, et al. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gatstroenterology 1998;115:1–8. [DOI] [PubMed] [Google Scholar]
- 20.Ohkawa H, Ohnishi N, Yagi K. Assay for lipid peroxides for animal tissues by thiobarbituric acid reaction. Anal Biochem 1986;95:351–8. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosenbrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265–75. [PubMed] [Google Scholar]
- 22.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology 1984;87:1344–50. [PubMed] [Google Scholar]
- 23.Watanabe K, Konishi K, Fujioka M, et al. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem 1989;264:19559–63. [PubMed] [Google Scholar]
- 24.Ward PA, Cochrane CG. Bound complement and immunologic injury of blood vessels. J Exp Med 1965;121:215–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida N. Role of oxygen radicals and polymorphonuclear leukocytes in platelet activating factor-induced gastric mucosal injury in rats. J Kyoto Pref Univ Med 1989;98:1141–51. [Google Scholar]
- 26.Asako H, Kubes P, Wallace J, et al. Indomethacin-induced leukocyte adhesion in mesenteric venules: role of lipoxygenase products. Am J Physiol 1992;262:G903–8. [DOI] [PubMed] [Google Scholar]
- 27.Asako H, Kubes P, Wallace J, et al. Modulation of leukocyte adhesion in rat mesenteric venules by aspirin and salicylate. Gastroenterology 1992;103:146–52. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida N, Yoshikawa T. Role of intravascular neutrophils in the pathogenesis of aspirin-induced gastric mucosal injury. In: Asakura H, Aoyagi Y, Nakazawa S, eds. Trends in gastroenterology and hepatology. Tokyo: Springer-Verlag, 2001:219–23.
- 29.Crabtree JE, Lindley IJ. Mucosal interleukin-8 and Helicobacter pylori-associated gastroduodenal disease. Eur J Gastroenterol Hepatol 1994;6:S33–8. [PubMed] [Google Scholar]
- 30.Crabtree JE, Farmery SM, Lindley IJ, et al. CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines. J Clin Pathol 1994;47:945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozic CR, Kolakowski LF, Gerard NP, et al. Expression and biologic characterization of the murine chemokine KC. J Immunol 1995;154:6048–57. [PubMed] [Google Scholar]
- 32.Tappel AL. Lipid peroxidation damage to cell components. Fed Proc 1973;32:1870–4. [PubMed] [Google Scholar]
- 33.Yoshikawa T, Yoshida N, Miyagawa H, et al. Role of lipid peroxidation in gastric mucosal lesions induced by burn shock in rats. J Clin Biochem Nutr 1987;2:163–70. [Google Scholar]
- 34.Yoshida N, Yoshikawa T, Ando T, et al. Pathogenesis of platelet-activating factor-induced gastric mucosal damage in rats. Scand J Gastroenterol 1989;24:210–14. [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa T, Ueda S, Naito Y, et al. Role of oxygen-derived free radicals in gastric mucosal injury induced by ischemia or ischemia-reperfusion in rats. Free Radic Res Commun 1989;7:285–91. [DOI] [PubMed] [Google Scholar]
- 36.Yoshikawa T, Naito Y, Kishi A, et al. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut 1993;34:732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan FK, Sung JJ, Chung SC, et al. Randomised trial of eradication of Helicobacter pylori before non-steroidal anti-inflammatory drug therapy to prevent peptic ulcers. Lancet 1997;350:975–9. [DOI] [PubMed] [Google Scholar]
- 38.Konturek JW, Dembinski A, Konturek SJ, et al. Infection of Helicobacter pylori in gastric adaptation to continued administration of aspirin in humans. Gastroenterology 1998;114:245–55. [DOI] [PubMed] [Google Scholar]