Abstract
Background: Cirrhotic patients show increased susceptibility to bacterial infections. It is not known whether tuftsin deficiency, which is associated with an increased incidence of infections in many disease states, is present in cirrhosis. Our aims were to determine whether tuftsin activity is deficient in cirrhosis and if so, whether this deficiency is related to splenic function, contributes to altered neutrophil granulocyte function, or influences the occurrence of bacterial infections and patient survival.
Methods: Tuftsin activity and splenic function were assessed in 31 patients with liver cirrhosis and 31 healthy subjects. The phagocytic activity of neutrophil granulocytes from 23 patients was tested in vitro with addition of both autologous and pooled sera from healthy subjects. In 10 patients and eight controls it was also tested with addition of synthetic tuftsin. Patients were followed up until death or liver transplantation.
Results: Patients had reduced tuftsin activity (median 8% (range 3–24.5)) compared with controls (17% (11.5–37)) (p<0.001) and a higher pitted red cell count (p<0.001). Tuftsin activity was correlated with pitted cell count (p=0.02) and the Child-Pugh score (p=0.002). Nineteen of 23 patients showed deficient phagocytic activity of neutrophil granulocytes, which was correlated with tuftsin activity (p<0.001), improved in all cases but one with addition of serum from healthy subjects, and normalised with addition of synthetic tuftsin. Reduced tuftsin activity did not influence patient survival but was associated with a higher incidence of bacterial infections (p=0.029).
Comment: Tuftsin activity was reduced in cirrhosis, and contributed to impaired phagocytic activity of neutrophil granulocytes. Such an abnormality appears to be related to impaired splenic function and severity of cirrhosis, and probably favours the occurrence of bacterial infections.
Keywords: tuftsin, hepatic cirrhosis, splenic function, bacterial infection, granulocytes
Patients with cirrhosis have an increased incidence of bacterial infections which are a major cause of morbidity and mortality in this setting.1 In addition to alterations in the enteric flora2 and the intestinal barrier due to portal hypertension,3 such an abnormality can be attributed to impairment of defence mechanisms against infections. In fact, impairments of the reticuloendothelial system,4 neutrophil granulocyte functions,5,6 and non-specific humoral7 and cell mediated immunity8 have been described in cirrhotic patients.
Among neutrophil granulocyte functions, chemotaxis, phagocytosis, and bacterial killing capacity5, 6, 9 are often defective in cirrhosis. Although substances present in serum may inhibit leucocyte migration,5, 8 the exact nature of the mechanisms leading to impaired function of these cells is still largely unknown.
Tuftsin is a natural tetrapeptide (Thr-Lys-Pro-Arg) known to stimulate phagocytosis by neutrophil granulocytes.10 Tuftsin is part of a specific carrier γG1 cytophilic γ-globulin (leuokinin) which is cleaved by an enzyme, tuftsin endocarboxypeptidase, while circulating through the spleen. The cleaved molecule, leucokinin-S, then binds to specific receptors on neutrophil granulocytes, monocytes-macrophages, and natural killer cells where the enzyme leucokininase cleaves the tetrapeptide off the parent molecule.11 Once activated, tuftsin modulates the biological activities of phagocytic cells.11, 12 Acquired tuftsin deficiency has been found in a number of diseases characterised by increased susceptibility to bacterial infections.13–19 All of these diseases have in common impaired splenic function which has also been described in patients with alcoholic cirrhosis.20, 21
In this study, we determined tuftsin activity in patients with cirrhosis of different aetiologies and its potential contribution to altered phagocytic activity of neutrophil granulocytes. We also assessed splenic function and its relationship with tuftsin activity. Finally, we investigated whether impaired tuftsin activity influences the occurrence of bacterial infections and patient survival.
PATIENTS AND METHODS
Patients
Thirty one patients with liver cirrhosis (19 males, 12 females; median age 55 years (range 35–74)) consecutively admitted to our clinic took part in the study. The diagnosis of cirrhosis was made on histological findings in six cases, and by clinical, endoscopic, and ultrasonographic findings in the remainder. Thirty one healthy volunteers (18 males, 13 females) were recruited as controls. Their alcohol intake was <20 g/day and they tested negative for hepatitis B surface antigen and anti-hepatitis C virus antibody.
This study was performed according to the principles of the declaration of Helsinki and approved by the senior staff of our institution. Informed consent was obtained from each subject participating in the study.
Tuftsin activity
γ-Globulins were isolated from the serum of patients and controls by saturated ammonium sulphate precipitation. Separation of tuftsin from γ-globulins and assay of its ability to stimulate phagocytosis of latex particles by neutrophil granulocytes from healthy donors were performed according to Najjar and Constantopoulos.22 In particular, the precipitate was dialysed overnight in 0.1 M phosphate buffer (pH 8.1) at 4°C. To cleave tuftsin, 10 mg of γ-globulins were digested at 37°C for one hour by 0.5 mg trypsin (Sigma, St Louis, Missouri, USA) in a final volume of 2.5 ml of phosphate buffer, pH 8.1. The trypsin reaction was stopped by adding 10 ml of 95% ethyl alcohol; the solution was then incubated at 80°C for 15 minutes. Tuftsin was separated from γ-globulins by centrifugation (3000 g, 4°C, 15 minutes) and the alcoholic extract containing tuftsin was evaporated by a vacuum pump evaporator for at least four hours. The residue was then dissolved in 0.25 ml Krebs-Ringer solution. Tuftsin activity was assayed by measuring its ability to stimulate phagocytosis of latex (Sigma) by polymorphonuclear cells (PMNs) extracted from the blood of healthy subjects freshly collected at the blood bank of our hospital. PMNs were isolated from 20 ml of heparinised blood by Histopaque gradient sedimentation (Sigma). To remove residual erythrocytes, a lysis solution was added to the supernatant at 4°C for eight minutes and centrifuged at 1000 g, 4°C for eight minutes. Cells were collected in 1 ml Krebs-Ringer solution. Krebs-Ringer solution (0.1 ml), 2×106 neutrophils, 0.05 ml of extractive tuftsin solution, and 0.05 ml of latex were mixed in a glass tube and incubated in a vertical rotator (Cole Palmer Multipurpose rotator) at 37°C for 30 minutes. Slides preparations were stained by the May-Grünwald-Giemsa method. The percentage of PMNs containing latex was calculated by counting 200 cells per subject by two observers who were unaware of the origin of the samples (interobserver correlation index 0.96) using a direct interference contrast microscope (Leitz “Dialux 20”, equipped with Nomarski optics). The same count was made by preparing slides from PMNs in Krebs-Ringer solution (blank). Tuftsin activity for each sample was calculated by subtracting the percentage of latex positive cells in the blank. The intra-assay variability of the test is 2–5%.
Splenic function
Splenic function was assessed by counting red cells with indentations (or pits), as previously described in detail.18, 23 The normal range for our laboratory is less than two pitted erythrocytes per cent.
Phagocytic activity
Phagocytic activity of neutrophil granulocytes was tested by a chemiluminescence method using a Phagolux kit (Bouty SpA, Milan, Italy) in the last 23 patients and 20 healthy control subjects enrolled in the study. To induce opsonisation, 200 μl of plasma were added to 20 μl of Zymolite (fractions of plasma membrane of Saccaromyces cerevisiae), incubated at 37°C for 20 minutes, and stored at 2–8°C. Blood diluted 1:100 (50 μl) was added to 1 ml of Phagolite (solution of luminol and Krebs-Ringer phosphate) and incubated at 37°C for five minutes. Blood luminol solution and 20 μl of opsonised plasma were mixed in a luminol cuvette. As activated neutrophil granulocytes produce O−2 and H2O−2, which emit photons by oxidising luminol, phagocytic activity was determined using a photoluminometer. Phagocytic activity was expressed as counts per minute (cpm). The normal range in our laboratory is 220–430 cpm.
In each case, phagocytic activity was tested by incubating neutrophil granulocytes with both autologous serum and pooled sera from healthy subjects. In a further set of experiments performed in the last 10 patients and eight healthy control subjects enrolled in the study, phagocytic activity of neutrophil granulocytes was also assessed by incubating cells with autologous serum and after addition of either saline or synthetic tuftsin (Sigma Chemical Co.) at increasing concentrations (1.25, 2.5, and 5 μg/ml). Concentrations of synthetic tuftsin used in our experiments were chosen as it has been shown that these concentrations ensure the best conditions under which to observe the effects of tuftsin in the phagocytic assay.24
Other determinations
The severity of cirrhosis was evaluated by calculating the Child-Pugh score.25 Serum concentrations of albumin, γ-globulins and bilirubin, prothrombin activity, and whole blood examination were assessed using standard methods. Functional liver mass was estimated in 23 patients by determining the galactose elimination capacity according to Tygstrup.26 The lower limit of the normal range for our laboratory is 6 mg/kg/min.
Follow up
Patients were grouped according to preserved (≥11.5%) or reduced (<11.5%) tuftsin activity, and followed after the initial study to death or liver transplantation. The occurrence of bacterial infections during follow up was recorded. The cut off value of 11.5% was chosen according to the lowest value found in the healthy control group.
Statistical analysis
The distribution of variables was tested by the Komolgorov-Smirnov test. As most variables showed a non-parametric distribution, results were expressed as median and range. The Mann-Whitney test, contingency tables with Fisher's exact test, the Wilcoxon test, and Friedman two way ANOVA were used to evaluate the statistical significance of the differences between and within groups, respectively. Correlations were derived by Spearman's rho test. Multiple regression analysis was performed to check simultaneously for the Child-Pugh score and pitted cell count in determining tuftsin activity, after log transformation of the variables showing a non-parametric distribution. Life table estimates were calculated according to the Kaplan-Meier method, and survival curves were compared by the log rank test. Multiple Cox regression analysis was performed to check simultaneously for Child-Pugh score and tuftsin activity in determining infection free survival. A two tailed p value <0.05 was considered to be statistically significant. Statistical evaluation was performed using SPSS for Windows 8.0 statistical software (SPCC Inc, Chicago, Illinois, USA).
RESULTS
The main clinical and laboratory features of the patients are reported in table 1 ▶. All patients had an increased serum γ-globulin concentration, and 29 of 31 had haematological hypersplenism, assessed by low platelet count with or without neutropenia (20 patients) and anaemia (19 patients).
Table 1.
Main clinical and laboratory features of the 31 patients in the study
| Aetiology of cirrhosis (n) | HCV related—12 | Serum bilirubin concn (mg/dl) | 2.7 (0.3–14.9) |
| HBV related—4 | Prothrombin activity (% of control) | 60 (24–100) | |
| HBV and HDV related—2 | Serum IgG concn (g/dl) | 1.96 (0.78–3.66) | |
| HCV and HBV related—3 | Haemoglobin concn (g/dl) | 12.1 (7.9–15.4) | |
| Alcohol related—7 | Neutrophil granulocyte count (/mm3) | 2.4×103 (0.93–7.3×103) | |
| Primary biliary cirrhosis—2 | Platelet count (/mm3) | 61×103 (30–171×103) | |
| Cryptogenic—1 | Splenomegaly (n)* | 31 | |
| Child-Pugh class (n) | A:10; B:14; C:7 | Hepatic encephalopathy (grade:n)† | I:1; II: 1 |
| Child-Pugh score | 8 (5–12) | Ascites (n) | 20 |
| Serum albumin concn (g/dl) | 3.4 (2.6–4.5) | GEC (mg/kg/min; n=23) | 4 (2.6–5.9) |
Continuous variables are expressed as median (range).
HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis delta virus; GEC, galactose elimination capacity.
*Spleen size was assessed by ultrasonography.
†Hepatic encephalopathy was graded from I (mild) to IV (severe).
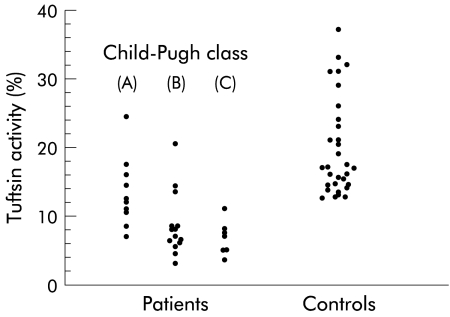
Median tuftsin activity measured in healthy controls was 17.8% (range 11.5–37) (fig 1 ▶). Patients with cirrhosis showed a reduced median tuftsin activity (8% (3–24.5); p<0.001). Tuftsin activity was below the lower limit of the normal range in 5/10 patients belonging to Child-Pugh class A, in 11/14 patients belonging to Child-Pugh class B, and in all seven patients belonging to Child-Pugh class C (fig 1 ▶). No difference in tuftsin activity was found between patients with alcoholic and non-alcoholic cirrhosis (7% (3–24.5) v 8% (3.5–14.5)).
Figure 1.
Individual values for serum tuftsin activity in patients with cirrhosis and in healthy controls. Tuftsin activity was significantly depressed in patients belonging to all Child-Pugh classes (class A: median 12.2% (range 7–24.5) (p<0.001); class B: 6.7% (3–20.5) (p<0.001); and class C: 7% (3.5–11) (p=0.001)).
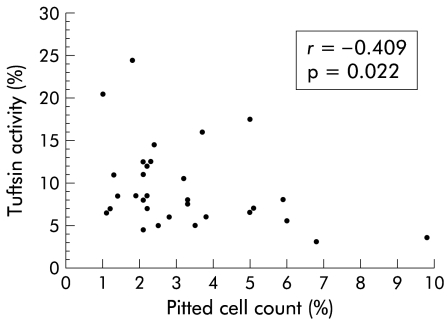
The pitted red cell count was higher in patients (2.4% (1.0–9.8)) than in healthy controls (0.6% (0.2–1.8); p<0.001). There was an inverse correlation between pitted red cell count and tuftsin activity (r=−0.41; p=0.02) (fig 2 ▶).
Figure 2.
Correlation between patient pitted cell count and tuftsin activity.
Tuftsin activity was also significantly correlated with the severity of cirrhosis, as assessed by the Child-Pugh score (r=−0.54; p=0.002), serum bilirubin concentration (r=−0.56; p=0.001), prothrombin activity (r=0.46; p=0.01), platelet count (r=0.38; p=0.036), and galactose elimination capacity (r=0.56; p=0.005; n=23). In order to weigh the contribution of either severity of cirrhosis or hyposplenism on tuftsin deficiency, we performed multiple regression analysis by plotting Child-Pugh score and pitted cell count (independent variables) against tuftsin activity (dependent variable). Such a correlation proved to be highly significant (F=7,57; p=0.002); both independent variables significantly contributed to the variability in tuftsin activity (Child-Pugh score: T=−2.39; p=0.024; pitted cell count: T=−2.38; p=0.025).
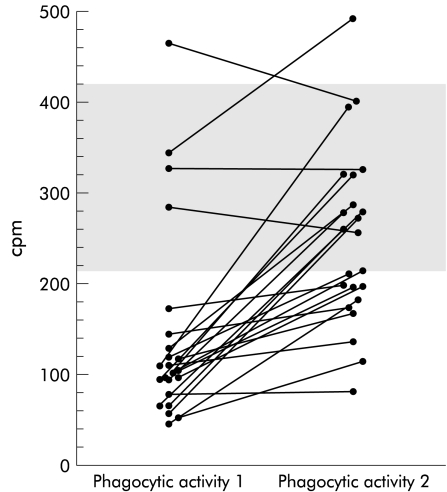
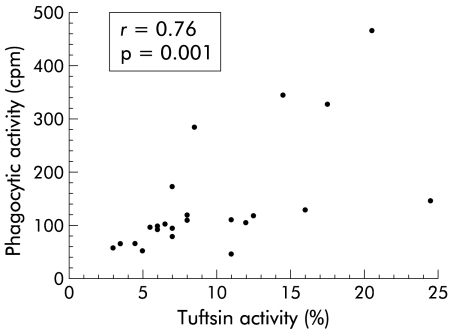
The phagocytic activity of neutrophil granulocytes was below the normal limit (220 cpm) in 19 of 23 patients (104 cpm (45–464)) (fig 3 ▶), and closely correlated with tuftsin activity (r=0.76; p<0.001; n=23) (fig 4 ▶). All 20 healthy control subjects tested in this study showed phagocytic activity within the normal range (325 cpm (220–426); p<0.001 v patients).
Figure 3.
Patient neutrophil granulocyte phagocytic activity (expressed as counts per minute (cpm)) assayed by testing neutrophil granulocytes with autologous serum (phagocytic activity 1) and pooled sera from normal subjects (phagocytic activity 2). The shaded area represents the normal interval of values in our laboratory.
Figure 4.
Correlation between patient tuftsin activity and neutrophil granulocyte phagocytic activity assayed by testing neutrophil granulocytes with autologous serum.
After incubation with pooled sera from healthy subjects, phagocytic activity improved in all patients with a reduced baseline activity except for one (from 96 cpm (45–172) to 209 cpm (80–393); p<0.001), and normalised in eight cases. In patients with normal baseline phagocytic activity, this was virtually unchanged by incubation with serum from healthy subjects (from 335.5 cpm (284–464) to 362.5 cpm (255–495)) (fig 3 ▶). Incubation with pooled sera from healthy subjects had no significant effect on phagocytic activity of neutrophil granulocytes from the 20 healthy control subjects tested (from 325 cpm (220–426) to 333 cpm (225–418)).
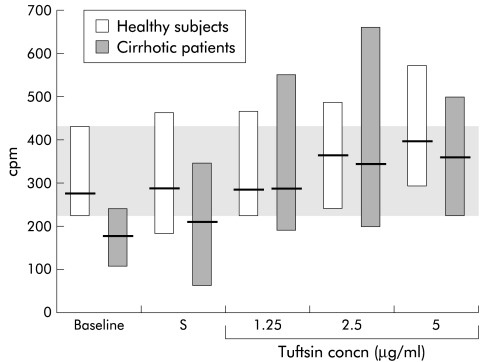
Addition of synthetic tuftsin to the phagocytic activity assay did not produce significant changes in eight healthy subjects but significantly improved phagocytic activity in 10 patients, irrespective of the tuftsin concentration used. As a result, patient phagocytic activity, which was significantly lower than in healthy subjects with autologous serum (189.7 cpm (107–254) v 276 cpm (220–426); p=0.014), fully normalised (fig 5 ▶).
Figure 5.
Effect of addition of saline (S) and different synthetic tuftsin concentrations to the phagocytic activity assay in eight healthy subjects and 10 cirrhotic patients. Friedman's two way ANOVA showed that phagocytic activity following addition of synthetic tuftsin to the assay system was significantly higher than baseline values in cirrhotic patients (p<0.001) but not in healthy subjects. Baseline=phagocytic activity with autologous serum. Values are reported as median (range). The shaded area represents the normal interval. Significant difference between groups: baseline: p=0.016; saline: p=0.018.
Follow up lasted from one week to 48 months (median 10 months), and did not differ between patients with preserved or reduced tuftsin activity (16 months (range 2–47) v 8 months (1 week–48 months), respectively; p=0.34). During this period, one patient with preserved and three patients with reduced tuftsin activity dropped out of follow up. Survival analysis did not show a statistically significant difference between the seven patients with preserved (two died and three underwent liver transplantation) and the 20 patients with reduced tuftsin activity (six died and 11 had liver transplantation). The causes of death were liver failure and haemoperitoneum in the first group of patients, and liver failure (three cases), bleeding from oesophageal varices, pulmonary oedema, and stroke in the second.
During follow up, four patients underwent liver transplantation within a week of baseline assessment and were therefore excluded from the analysis of the incidence of bacterial infections. The main characteristics of the remaining patients with preserved or reduced tuftsin activity are reported in table 2 ▶: they did not differ in terms of age, sex, serum IgG concentration, or duration of the follow up period while Child-Pugh score was higher in those with reduced tuftsin activity. Thirty five bacterial infections occurred in 16 patients with a low baseline tuftsin activity (12 urinary tract infections, 12 upper respiratory airway infections, six spontaneous bacterial peritonitis (SBP), three pneumonia, one otitis media, one cholecystitis) while only four infections (two upper respiratory airway infections, one pneumonia, one gluteus abscess) were recorded in the seven patients with preserved tuftsin activity. As a result, the incidence of infections per person per month was 0.18 (range 0–1) in patients with low tuftsin activity and 0.035 (range 0–0.33) in those with normal tuftsin activity (p=0.029). As all episodes of SBP occurred in patients with reduced tuftsin activity, we assessed whether this complication was related to the extent of tuftsin deficiency. Tuftsin activity was 6% (5–7) in the five patients who developed six episodes of SBP and 8% (3.5–24) in the 11 who did not (p=0.05). Interestingly, Child-Pugh class did not differ between the two groups (SBP: class B, four cases; class C, one case; no SBP: class B, seven cases; class C, four cases).
Table 2.
Demographic features, Child-Pugh score, serum IgG concentration, and duration of follow up in patients analysed for incidence of bacterial infections during the follow up period
| Tuftsin activity ≥11.5% | Tuftsin activity <11.5% | p Value | |
| n | 7 | 16 | |
| Age (y) | 55 (35–68) | 55 (43–74) | NS |
| Sex (M/F) | 6/1 | 10/6 | NS |
| Child-Pugh score | 6 (5–8) | 9 (5–12) | 0.027 |
| Serum IgG concn (g/dl) | 1.81 (1.41–2.19) | 2.20 (1.31–3.66) | NS |
| Duration of follow up (months) | 16 (2–47) | 10 (1–48) | NS |
Continuous variables are expressed as median (range).
DISCUSSION
Acquired tuftsin deficiency has been found in several conditions, such as splenectomy,13 myelocytic leukaemia or myelofibrosis,14 idiopathic thrombocytopenic purpura,15 sickle cell disease,16 acquired immunodeficiency syndrome and acquired immunodeficiency syndrome related complex,17 short bowel syndrome,18 and coeliac disease.19 The first novel finding of the present study was that cirrhosis should be added to this list.
Deficient tuftsin activity may theoretically originate from several abnormalities, including hypo-γ-globulinaemia, deficient intrasplenic cleavage of neutrophilic IgGs, altered leucokininase activity, and molecular abnormalities of tuftsin or parent molecules.11, 12 Lack of IgG deficiency, and the significant improvement in neutrophil granulocyte phagocytic activity induced by addition of either normal serum or synthetic tufstin to the assay system, focus attention on the splenic step of the tuftsin metabolic pathway, although structural alterations in the tuftsin molecule cannot be ruled out. Our patients, as expected, had splenomegaly and most had haematological hypersplenism—that is, granulocytopenia and/or thrombocytopenia. However, splenic size relates poorly to function,27, 28 and functional hyposplenism—that is, reduced splenic function in the presence of a spleen—29 can also occur in patients with haematological hypersplenism and/or splenomegaly.30 We indirectly assessed splenic function by counting the percentage of pitted erythrocytes normally removed by the spleen. This technique, which has been shown to correlate well with assessment of splenic function by other methods,31 revealed that most of our patients had impaired splenic function. This finding has previously been described in chronic alcoholic liver disease20, 21 and attributed to either the toxic effect of alcohol itself, which is known to impair reticuloendothelial phagocytic capacity,32 or alcohol induced malnutrition.21 Such an interpretation, based mainly on the finding that a drop in pitted cell counts followed abstinence from alcohol,21 is controversial. Firstly, only a minority of patients investigated in the study had proven cirrhosis. Secondly, the degree of hyposplenism found in alcohol users without chronic liver disease was less pronounced than that reported in alcoholic cirrhosis, and did not correlate with either the quantity or duration of alcohol intake.33 Our data showed that hyposplenism occurred irrespective of the aetiology of cirrhosis, confirming the report of Levi and colleagues.34 The causes of hyposplenism in this setting remain undefined although it is commonly believed that portal hypertension, which leads to blood engorgement in the spleen reticuloendothelial meshwork, may interfere with the depitting process. Whether this also interferes with leucokinin-S cleavage from leucophylic IgG molecules, the first step leading to tuftsin release, is conjectural and has not been demonstrated. Beyond these uncertainties, the fact remains that we found an inverse correlation between splenic function and tuftsin activity. This finding, which has also been reported in other clinical settings,17, 18, 23 strengthens the hypothesis that deficient tuftsin activity found in our patients was largely due to functional hyposplenism. However, the correlation we found between tuftsin activity and tests exploring liver function suggests that factors related to the severity of cirrhosis are also involved. The mechanism(s) by which the severity of cirrhosis leads to impaired tuftsin activity is unknown.
We confirmed that cirrhotic patients have severely reduced neutrophil granulocyte phagocytic activity, a finding previously reported by others.5 Together with other defects in defence mechanisms,4–7 this abnormality participates in making patients with cirrhosis more susceptible to bacterial infection than the general population,1 although a clearcut relationship between neutrophil granulocyte dysfunction and occurrence of bacterial infection has not been established.35
Our study demonstrated that the cause of impaired neutrophil granulocyte phagocytic activity was largely due to deficiency of a humoral factor rather than to an intrinsic neutrophil granulocyte defect. In fact, with the addition of serum from healthy controls to the assay system, neutrophil granulocyte phagocytic activity improved in all but one patient with impaired phagocytic activity, and normalised in almost half of the cases. We also showed that tuftsin deficiency was likely a major determinant of neutrophil granulocyte dysfunction. In fact, phagocytic activity correlated directly with tuftsin activity (fig 4 ▶), and was normalised by adding tuftsin to the assay system. The ultimate definition of the role of tuftsin in this setting could be obtained by adding normal serum deprived of tuftsin to the assay system. Unfortunately, this experiment was not performed as a selective inhibitor of tuftsin was not available and the method used to extract tuftsin from serum can alter other factors potentially influencing neutrophil phagocytic activity. In any case, as four patients with normal tuftsin activity exhibited reduced neutrophil granulocyte phagocytic activity (fig 3 ▶), defective tuftsin activity cannot be the only factor impairing neutrophil function in cirrhosis.
Tuftsin is known to stimulate phagocytosis by neutrophil granulocytes although its exact mechanism of action is not fully defined. Current knowledge suggests that tuftsin, after binding to a specific cell surface receptor, undergoes internalisation and influences intracellular cyclic nucleotide levels via a mechanism based on Ca++ association with cellular components or another undefined route.36 In any case, phagocytosis is enhanced through both Fc receptor and non-specific receptors.37 The same mechanisms mediate the effects of tuftsin on phagocytic cells other than neutrophil granulocytes.11 Thus impaired function of macrophage Fcγ receptor described in advanced alcoholic cirrhosis38 may also be attributed to tuftsin deficiency, which can therefore be expected to determine a complex functional defect in all phagocytic cells.
Both congenital39 and acquired tuftsin deficiency are characterised by increased susceptibility to bacterial infections.13–19 A further important finding of the present study was that such an association was also true in cirrhosis. In fact, patients with reduced tuftsin activity developed a significantly higher number of bacterial infections during follow up. Interestingly, patients who developed SBP tended to have lower tuftsin activity than those who did not, irrespective of the severity of cirrhosis, as assessed by Child-Pugh class. Despite these findings, reduced tuftsin activity was not a predictor of death. This can be explained by the fact that our series was rather small and deaths were unrelated to infection. Moreover, patients with deficient tuftsin activity, although showing a greater Child-Pugh score than their counterparts, included subjects belonging to A class who possibly contributed to the improved survival of this group.
In conclusion, we demonstrated that deficient tuftsin activity is common in cirrhosis, and always present in patients with advanced disease. Such an abnormality is likely to be a major cause of impaired phagocytic activity of neutrophil granulocytes, is related to both impaired splenic function and severity of cirrhosis, and favours the occurrence of bacterial infections. These findings, in addition to contributing to our understanding of the mechanisms underlying impairment of the defence mechanism in cirrhosis, may have clinical implications. In fact, tuftsin administration to experimental animals with bacterial, fungal, or protozoan infections had a potential therapeutic role.11 In humans, tuftsin has also been employed without toxic effects, leading to a significant increase in neutrophil granulocytes and CD4 lymphocytes in peripheral blood,40 enhancement in monocyte phagocytosis and bactericidal activity,41 and clinical improvement.42 Thus studies evaluating the effect of tuftsin administration to critically ill patients with cirrhosis and infection, particularly in those awaiting or shortly after liver transplantation, are warranted.
Abbreviations
cpm, counts per minute
SBP, spontaneous bacterial peritonitis
PMNs, polymorphonuclear cells
REFERENCES
- 1.Rimola A, Navasa M. Infections in liver disease. In: McIntyre N, Benhamou JP, Bircher J, et al, eds. Oxford textbook of clinical hepatology, 2nd edn. Oxford: Oxford University Press, 1999:1861–6.
- 2.Morencos FC, de las Heras-Castaño G, Martin-Ramos L, et al. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci 1995;40:1252–6. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G, Lee FY, Barden GE, et al. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology 1995;108:1835–41. [DOI] [PubMed] [Google Scholar]
- 4.Bolognesi M, Merkel C, Bianco S, et al. Clinical significance of the evaluation of hepatic reticuloendothelial removal capacity in patients with cirrhosis. Hepatology 1994;19:628–34. [DOI] [PubMed] [Google Scholar]
- 5.Feliu E, Gougerot MA, Hakim J, et al. Blood polymorphonuclear dysfunction in patients with alcoholic cirrhosis. Eur J Clin Invest 1977;7:571–7. [DOI] [PubMed] [Google Scholar]
- 6.Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology 1986;6:252–62. [DOI] [PubMed] [Google Scholar]
- 7.Fierer J, Finley F. Deficient serum bactericidal activity against Escherichia coli in patients with cirrhosis of the liver. J Clin Invest 1979;63:912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nouri-Aria KT, Alexander GJ, Portmann BC, et al. T and B cell function in alcoholic liver disease. J Hepatol 1986;2:195–207. [DOI] [PubMed] [Google Scholar]
- 9.Altin M, Rajkovic IA, Hughes RD, et al. Neutrophil adherence in chronic liver disease and fulminant hepatic failure. Gut 1983;24:746–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Najjar VA, Nishioka K. “Tuftsin”: a natural phagocytosis stimulating peptide. Nature 1970;228:672–3. [DOI] [PubMed] [Google Scholar]
- 11.Siemion IZ, Kluczyk A. Tuftsin: on the 30-year anniversary of Victor Najjar's discovery. Peptides 1999;20:645–74. [DOI] [PubMed] [Google Scholar]
- 12.Nishioka K, Amoscato AA, Babcock GF, et al. Tuftsin: an immunomodulating peptide hormone and its clinical potential as a natural biological response modifier. Cancer Invest 1984;2:39–49. [DOI] [PubMed] [Google Scholar]
- 13.Constantopoulos A, Najjar VA, Wish JB, et al. Defective phagocytosis due to tuftsin deficiency in splenectomized subjects. Am J Dis Child 1973;125:663–5. [DOI] [PubMed] [Google Scholar]
- 14.Constantopoulos A, Likhite V, Crosby WH, et al. Phagocytic activity of the leukemic cell and its response to the phagocytosis-stimulating tetrapeptide, tuftsin. Cancer Res 1973;33:1230–4. [PubMed] [Google Scholar]
- 15.Spirer Z, Zakuth V, Diamant S, et al. Decreased tuftsin concentrations in patients who have undergone splenectomy. BMJ 1977;2:1574–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spirer Z, Weisman Y, Zakuth V, et al. Decreased serum tuftsin concentrations in sickle cell disease. Arch Dis Child 1980;55:566–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corazza GR, Zoli G, Ginaldi L, et al. Tuftsin deficiency in AIDS. Lancet 1991;337:12–13. [DOI] [PubMed] [Google Scholar]
- 18.Zoli G, Corazza GR, Wood S, et al. Impaired splenic function and tuftsin deficiency in patients with intestinal failure on long term intravenous nutrition. Gut 1998;43:759–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corazza GR, Zoli G, Di Sabatino A, et al. A reassessment of splenic hypofunction in celiac disease. Am J Gastroenterol 1999;94:391–7. [DOI] [PubMed] [Google Scholar]
- 20.Muller AF, Toghill PJ. Splenic function in alcoholic liver disease. Gut 1992;33:1386–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller AF, Toghill PJ. Functional hyposplenism in alcoholic liver disease: a toxic effect of alcohol? Gut 1994. ;35:679–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Najjar VA, Constantopoulos A. A new phagocytosis stimulating tetrapeptide hormone, tuftsin, and its role in disease. J Reticuloendothel Soc 1972;12:197–15. [PubMed] [Google Scholar]
- 23.Zoli G, Corazza GR, D'Amato G, et al. Splenic autotransplantation after splenectomy: tuftsin activity correlates with residual splenic function. Br J Surg 1994;81:716–18. [DOI] [PubMed] [Google Scholar]
- 24.Nishioka K, Wagle JR, Rodriguez T, et al. Studies of human granulocyte phagocytosis stimulation by tuftsin. J Surg Res 1994;56:94–101. [DOI] [PubMed] [Google Scholar]
- 25.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–9. [DOI] [PubMed] [Google Scholar]
- 26.Tygstrup N. Determination of the hepatic elimination capacity (Lm) of galactose by single injection. Scand J Clin Lab Invest (Suppl) 1966;18:118–25. [PubMed] [Google Scholar]
- 27.Smart RC, Ryan FP, Holdworth CD, et al. Relationship between splenic size and splenic function. Gut 1978;19:56–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson PJ, Bullen AW, Hall R, et al. Splenic size and function in adult coeliac disease. Br J Radiol 1980;53:532–7. [DOI] [PubMed] [Google Scholar]
- 29.Foster PN, Losowsky MS. Hyposplenism. In: Bowdler AJ, ed. The spleen—Structure, function and clinical significance. London: Chapman and Hall, 1990:232–9.
- 30.Steinberg MH, Gatling RR, Tavassoli M. Evidence of hyposplenism in the presence of splenomegaly. Scand J Haematol 1983;31:437–9. [DOI] [PubMed] [Google Scholar]
- 31.Corazza GR, Bullen AW, Hall R, et al. Simple method of assessing splenic function in coeliac disease. Clin Sci 1981;60:109–13. [DOI] [PubMed] [Google Scholar]
- 32.Lahnborg G, Friman L, Berghem L. Reticuloendothelial function in patients with alcoholic liver cirrhosis. Scand J Gastroenterol 1981;16:481–9. [DOI] [PubMed] [Google Scholar]
- 33.Corazza GR, Addolorato G, Biagi F, et al. Splenic function and alcohol addiction. Alcohol Clin Exp Res 1997;21:197–200. [PubMed] [Google Scholar]
- 34.Levi D, Mauriño E, Abecasis R, et al. Splenic hypofunction in cirrhosis is not associated with increased risk for infections. Eur J Gastroenterol Hepatol 1996;8:257–60. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Gonzalez M, Boixeda D, Herrero D, et al. Effect of granulocyte-macrophage colony-stimulating factor on leukocyte function in cirrhosis. Gastroenterology 1993;105:527–31. [DOI] [PubMed] [Google Scholar]
- 36.Stabinsky Y, Bar-Shavitz Z, Fridkin M, et al. On the mechanism of action of the phagocytosis-stimulating peptide tuftsin. Mol Cell Biochem 1980;30:71–7. [DOI] [PubMed] [Google Scholar]
- 37.Bar-Shavit Z, Stabinsky Y, Fridkin M, et al. Tuftsin-macrophage interaction: specific binding and augmentation of phagocytosis. J Cell Physiol 1979;100:55–62. [DOI] [PubMed] [Google Scholar]
- 38.Gomez F, Ruiz P, Schreiber AD. Impaired function of macrophage Fc gamma receptors and bacterial infection in alcoholic cirrhosis. N Engl J Med 1994;331:1122–8. [DOI] [PubMed] [Google Scholar]
- 39.Constantopoulos A, Najjar VA. Tuftsin deficiency syndrome. Acta Paediatr Scand 1973;62:645–8. [DOI] [PubMed] [Google Scholar]
- 40.Mathé G. Do tuftsin and bestatin constitute a biopharmacological immunoregulatory system? Cancer Detec Prev 1987;1:445–55. [PubMed] [Google Scholar]
- 41.Szkaradkiewicz A. Phagocytosis and microbicidal capacity of human monocytes in the course of HIV infection. Immunol Let 1992;33:145–50. [DOI] [PubMed] [Google Scholar]
- 42.Marilus R, Spirer Z, Michaeli D, et al. First case of AIDS in a homosexual in Israel. Results of different therapeutic regimes. Isr J Med Sci 1984;20:249–51. [PubMed] [Google Scholar]