Abstract
Background: Intraduodenal bile salts exert negative feedback control on postprandial gall bladder emptying.
Aims: We wished to examine whether a similar control mechanism occurs in the fasting state.
Methods: Intraduodenal bile salt depletion was achieved by 12 g of cholestyramine. Thereafter, in study A (seven subjects), the effects on gall bladder volume (by ultrasound) and antroduodenal motility of intraduodenal infusions of taurocholate egg yolk-phosphatidylcholine micelles were assessed. In study B (nine subjects), the effects on gall bladder volume of infusing mixed micelles composed of taurocholate (100 mM) and low (26 mM) or high (68 mM) amounts of egg yolk-phosphatidylcholine, or low amounts of dipalmitoylphosphatidylcholine were determined.
Results: Cholestyramine induced strong and prolonged gall bladder contraction without cholecystokinin release. In study A, micellar infusions increased gall bladder volume without affecting migrating motor complex cycle length. In study B, intraduodenal infusion induced strong increases in gall bladder volume in the case of taurocholate micelles containing low amounts of egg yolk-phosphatidylcholine, moderate increases in micelles containing low amounts of dipalmitoylphosphatidylcholine but no change in micelles containing high amounts of egg yolk-phosphatidylcholine, in all cases without altered plasma cholecystokinin levels. Phosphatidylcholine hydrolysis was significantly higher after infusion of egg yolk-phosphatidylcholine compared with infusion of dipalmitoylphosphatidylcholine containing micelles. Intermixed micellar-vesicular bile salt concentrations (responsible for detergent effects) were higher in egg yolk-phosphatidylcholine than in dipalmitoylphosphatidylcholine containing model biles and if lyso-phosphatidylcholine was included.
Conclusions: Intraduodenal bile salts exert negative feedback on fasting gall bladder volume. The modulating effects of various phospholipids may relate to their effects on intermixed micellar-vesicular bile salt concentrations.
Keywords: bile salts, gall bladder, migrating motor complex, negative feedback, phospholipids
The hormone cholecystokinin (CCK) plays a major role in postprandial gall bladder contraction. From experiments in humans and in various animal models, considerable evidence has emerged supporting negative feedback control by intraduodenal bile and the bile salts it contains, on plasma CCK levels after stimulation of CCK release by intraduodenal infusion of a meal,1–3 amino acids,4,5 triglycerides,5 or by intravenous bombesin infusion.6 CCK release after intraduodenal or intravenous stimuli is strongly enhanced when bile salts within the duodenal lumen are concurrently depleted with the aid of the anion exchange resin cholestyramine5,6 whereas addition of bile salts inhibits release.4,5
Significant, albeit minor (20–30%), periodic gall bladder emptying occurs in the fasting state in association with a rise in plasma motilin levels and with phase III of the intestinal migrating motor complex (MMC).7,8 The MMC, a cyclic contractile activity pattern displayed in the fasting state by the proximal intestine, is characterised by three phases: in phase I, contractile activity is absent; phase II reveals irregular activity; and during phase III there are intense, regular, coordinated contractions. It is not known whether bile salts play a role in the initiation of phase III or exert a negative feedback control on gall bladder emptying in the fasting state. Therefore, in the present study we examined the effects of intraduodenal bile salt depletion by cholestyramine and subsequent intraduodenal infusion of mixed taurocholate (TC) phospholipid (PL) micelles on small bowel and gall bladder motility and plasma CCK concentrations in the fasting state.
SUBJECTS AND METHODS
Subjects
Seven healthy volunteers participated in study A. Mean age was 29 (3) years, and the male/female ratio was 4/3. Nine volunteers participated in study B. Mean age was 27 (2) years, and the male/female ratio was 6/3. Subjects were non-smokers, had no gastrointestinal symptoms, were not on medication, and had no previous abdominal surgery. Ultrasonography revealed no abnormalities of bile ducts, gall bladder, or liver. All subjects gave informed consent. The protocol was approved by the ethics committee of the University Medical Centre Utrecht.
Protocol study A and B
In both studies, intraduodenal bile salt depletion was first achieved by giving 12 g cholestyramine (Questran; Bristol-Myers Squibb, Woerden, the Netherlands; 8 g before breakfast, 4 g before diner) the day before intraduodenal infusion of micelles. In study B, gall bladder volume was assessed by ultrasound and blood samples were drawn for CCK determination in the fasting state and at 15 minute intervals during the first two hours after ingestion of the first dose of cholestyramine (8 g).
Study A
The next day (after an overnight fast of at least 12 hours), a manometry catheter was introduced under fluoroscopic control. Thereafter, antroduodenal and gall bladder motility were recorded during two complete MMC cycles. At 30 minutes after the first and second phase III observed, 15 ml of mixed micelles (93 mM TC, 32 mM egg yolk-phosphatidylcholine (EYPC), 6.6 mM cholesterol, CSI 0.7) or 15 ml NaCl 0.9% were infused, in random order, over 15 minutes through the second duodenal side hole (that is, at the level of the papilla), with continuous recording of intestinal motility. Gall bladder volume was then assessed by ultrasound every five minutes and blood samples for CCK measurements were drawn every 10 minutes during the two MMC cycles. Investigator and volunteers were blinded to the type of infusion.
Study B
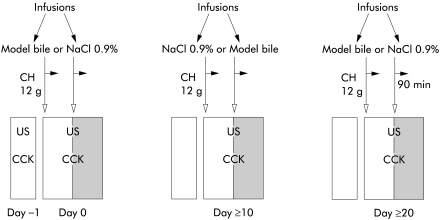
Figure 1 ▶ shows the design of the randomised blinded experiments, performed to examine the potential effects on gall bladder volume of varying PL contents within mixed micelles that were infused after cholestyramine induced bile salt depletion. In each subject, the effects of three mixed micellar solutions (that is, mixed TC-PL micelles containing high or low amounts of EYPC or low amounts of dipalmitoylphosphatidylcholine (DPPC), see below) were tested on three separate days, with intervals of at least 10 days. The day after cholestyramine induced intraduodenal bile salt depletion (after an overnight fast of at least 12 hours), a 12 Ch 110 cm duodenal feeding tube (Intersil; International Medical Produkts, Zutphen, the Netherlands) was positioned under fluoroscopic control in the duodenum with the side hole at the level of the papilla. Subsequently, 15 ml of mixed micelles or NaCl 0.9% (in random order) were infused over 15 minutes through the duodenal sidehole. Gall bladder volume was then assessed by ultrasound every five minutes and blood samples for CCK measurements were drawn every 10 minutes. Investigator and volunteers were blinded to the type of infusion. After the first 90 minutes, the second infusion (mixed micelles or NaCl 0.9%) was started, following the same procedures. Before, 15, and 30 minutes after each infusion, duodenal contents were aspirated for analyses.
Figure 1.
Study protocol. Day −1, ingestion of cholestyramine (CH). Days 0, +10, +20, infusion of NaCl 0.9% (saline) and mixed micelles (model bile) in random order. US, gall bladder ultrasonography; CCK, blood samples for CCK measurements.
Materials
TC was obtained from Sigma Chemical Co. (St Louis, Missouri, USA) and yielded a single spot on thin layer chromatography (butanol-acetic acid-water, 10:1:1 (vol/vol/vol), application of 200 μg bile salt). Phosphatidylcholine from egg yolk (EYPC; Sigma) and DPPC (Sigma) yielded a single spot on thin layer chromatography (chloroform-methanol-water 65:25:4 (vol/vol/vol), application of 200 μg lipid). 3α-Hydroxysteroid dehydrogenase for the enzymatic measurement of bile salt concentrations9 was purchased from Sigma.
Preparation of mixed micelles
Mixed micelles in study A contained 93 mM TC, 32 mM EYPC, and 6.6 mM cholesterol (CSI 0.7), and mixed micelles in study B contained 100 mM TC and either 26 or 68 mM PL (PL/bile salt+PL) ratio=0.2 or 0.4). Mixtures were vortex mixed and dried at 45°C under a mild stream of nitrogen, and subsequently lyophilised over 24 hours, before being dissolved in aqueous 0.15 M NaCl. Tubes were sealed with Teflon lined screw caps under a blanket of nitrogen to prevent lipid oxidation and vortex mixed for five minutes followed by incubation at 37°C in the dark. All solutions were warmed up to 45°C for 10 minutes before use. Final molar percentages of PLs and bile salts did not differ by more than 1% from the intended molar percentages.
Analysis of duodenal aspirates
Each sample (1 ml) was immediately (on site) extracted according to the procedure of Bligh and Dyer.10 Extracted and original samples were stored at −20°C before being analysed.
Lipid analysis
PL concentrations were assayed by determining inorganic phosphate according to Rouser and colleagues.11 Bile salt concentrations were determined with the 3α-hydroxysteroid dehydrogenase method.9 Thin layer chromatography was used for separation of lysophosphatidylcholine (lyso-PC) from PC, with chloroform-methanol-water 65:25:4 (vol/vol/vol) as the mobile phase. Separated PC and lyso-PC spots were then quantified by determining inorganic phosphate.
Measurement of intermixed micellar-vesicular bile salt concentration (IMC)
Apart from mixed (that is, PL-bile salt) micelles, bile systems also contain non-PL associated bile salts, either as monomers or above their critical micellar concentration associated in “simple” micelles. The monomeric plus simple micellar bile salt concentration (thought to be responsible for detergent effects of micelles12,13) is referred to as “intermixed micellar-vesicular (non-PL associated) bile salt concentration”, usually abbreviated as IMC.14 We determined IMC in various model systems using the rapid centrifugal ultrafiltration technique with correction for Gibbs-Donnan effects.13,15,16 This method allows IMC determination in model biles containing TC at concentrations ≤30 mM. At higher TC concentrations, IMC measurements are unreliable as the filter is not completely permeable for simple micelles under these conditions (Moschetta A, unpublished data, and Donovan and Jackson15). We therefore determined IMC values in model biles (after 12 hours of incubation at 37°C) containing 30 mM TC and low or high amounts of EYPC or low amounts of DPPC (PL/(bile salt+PL) ratio 0.2 and 0.4: mol% lipids identical to model biles that were infused intraduodenally). We also evaluated potential effects on IMC values of incorporating lyso-PC in micelles.
Gall bladder motility
Gall bladder volume was measured by means of real time ultrasonography (Scanner 250, 3.5/5 MHz convex transducer; Pie Medical, Maastricht, the Netherlands). Subcostal sonographic images were obtained with subjects in the supine position. Longitudinal and transverse images of the gall bladder were obtained at its largest dimensions. Gall bladder volume was calculated automatically from frozen images by means of built-in electronic calipers and software using the sum of cylinders method.17 Gall bladder volume was assessed at 15 minute intervals over two hours after ingestion of 8 g cholestyramine and at five minute intervals during intraduodenal infusions. The following variables of gall bladder motor function were determined: fasting volume (Vo), minimal volume after maximal contraction (Vmin), and maximal change in volume in ml and % (ΔVmax ml and ΔVmax %).
Antroduodenal manometry
Manometric recordings were obtained with a water perfused polyvinylchloride catheter containing eight side holes located at 5, 15, 25, 35, 40, 41, 42, and 43 cm from the tip. After introduction through the nose, the catheter was positioned under fluoroscopic control with four side holes in the duodenum and four in the gastric antrum. The catheter was plugged to a portable recorder (Medical Measurement Systems, Enschede, the Netherlands). Further details on manometric procedure and analysis of pressure recordings in the interdigestive state have been published previously.18 In addition, the motility index of the 15 minute periods before and after infusion were compared using the formula: ln (n kPa+1).19
Measurement of plasma CCK and bile salt concentrations
An indwelling cannula was placed in the antecubital vein. Blood samples in the fasting state for measurement of CCK and bile salt concentrations were collected at 15 minute intervals before and during the two hours after ingestion of cholestyramine and at 10 minute intervals over 90 minutes after intraduodenal infusion of mixed TC/PL micelles or NaCl 0.9%. Blood samples (3.5 ml) were collected in ice chilled tubes and plasma was stored at −20°C until analysis. Plasma CCK concentrations were determined by a sensitive and highly specific radioimmunoassay that quantitates the bioactive forms of CCK (CCK-58, -33, -22, and -8) with equimolar potency.20 Total plasma bile salt concentrations were determined using a fluorimetric assay.21
Statistical analysis
Results are expressed as means (SEM). Differences in gall bladder volume, plasma bile salt and CCK levels, and intraduodenal lipid concentrations were tested for statistical significance by repeated measures analysis of variance (ANOVA) and the post hoc Student's t tests. Total duration of MMC cycles, time from infusion until start of the next phase III, and motility index in the 15 minute periods before and after infusion were tested by paired Student's t tests with Bonferoni correction. Statistical significance was defined as a two tailed p value <0.05.
RESULTS
Study A
Intraduodenal infusion of mixed micelles at 30 minutes after phase III induced strong increases in gall bladder volume (% of starting volume after infusions of mixed micelles and NaCl 0.9%: 150 (5)% and 103 (4)%, respectively; p<0.05) but did not influence intestinal MMC cycle length (table 1 ▶). Infusion of mixed micelles also did not affect time to occurrence of the next phase III or origin of the next phase III (table 1 ▶). Nevertheless, in the 15 minute period after infusion of mixed micelles compared with the 15 minutes before infusion, a higher frequency of contractions (1.1 (0.7)/minute v 0.5 (0.8)/minute; p=0.012) and a higher motility index (6.8 (1.0) v 4.4 (1.3); p=0.007) were observed 10 cm distal to the infusion port. This effect was not present 20 cm distal to the infusion port. No increase in motor activity occurred after infusion of NaCl 0.9%.
Table 1.
No effects of intraduodenal infusion of mixed micelles on cycle length of the antroduodenal migrating motor complex (MMC)
| Mixed micelles | NaCl 0.9% | |
| MMC cycle length (min) | 115 (9) | 104 (7) |
| Phase I | ||
| Length (min) | 18 (3) | 17 (5) |
| % of cycle | 25 (2) | 20 (3) |
| Phase II | ||
| Length (min) | 83 (4) | 74 (2) |
| % of cycle | 70 (4) | 71 (3) |
| Phase III | ||
| Length (min) | 10 (4) | 5 (2) |
| % of cycle | 7 (2) | 5 (1) |
| Time to next phase III (min) | 91 (9) | 76 (7) |
| Antral/duodenal phase III | 4/3 | 5/2 |
Differences between infusions of mixed micelles and NaCl 0.9% were not significant.
Study B
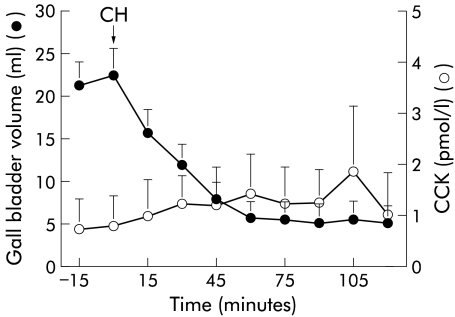
Cholestyramine ingestion induced strong gall bladder contraction (fig 2 ▶). Starting gall bladder volume was 21 (3) ml, and residual volume 4 (2) ml. There was no significant increase in plasma CCK levels (fig 2 ▶). Fasting gall bladder volume remained reduced 24 hours later (11 (2) ml).
Figure 2.
Gall bladder volume and plasma cholecystokinin (CCK) levels over two hours after ingestion of 8 g of cholestyramine (CH).
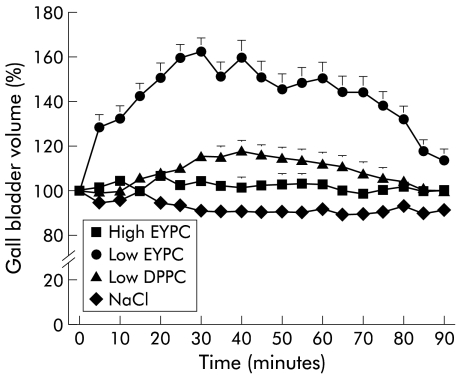
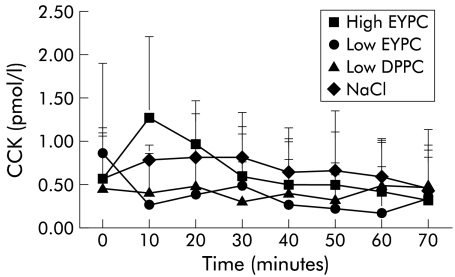
The three mixed micellar solutions exerted different effects on gall bladder volume, depending on their PL content: mixed micelles containing low amounts of EYPC (PL/(BS+PL) ratio=0.2) induced strong and significant temporary increases in gall bladder volume to a maximum of 202 (7)% of the starting volume (table 2 ▶, fig 3 ▶). If mixed micelles with low contents of DPPC (PL/(BS+PL) ratio=0.2) were infused, there was a smaller but still significant increase in gall bladder volume. Infusion of mixed micelles containing high amounts of EYPC (PL/(BS+PL) ratio=0.4), or infusion of NaCl 0.9% had no significant effects on gall bladder volume. There were no significant changes in plasma CCK levels during any infusion (fig 4 ▶).
Table 2.
Gall bladder volume in healthy subjects after intraduodenal infusion of mixed micelles of various compositions. (Starting volume (ml) = 11 (2); % of FV day −1 = 54 (10))
| Intraduodenal infusions of mixed micelles | ||||
| Low EYPC | High EYPC | Low DPPC | NaCl 0.9% | |
| Max volume | ||||
| ml | 18 (2)† | 12 (3) | 14 (2)* | 11 (2) |
| % of starting vol | 202 (7)† | 113 (4) | 126 (5)* | 108 (2) |
| Max increase | ||||
| ml | 9 (2)† | 3 (2) | 3 (1)* | 1 (1) |
| % of starting vol | 102 (7)† | 13 (4) | 26 (5)* | 8 (2) |
EYPC, egg yolk-phosphatidylcholine; DPPC, dipalmitoylphosphatidylcholine.
*p<0.05 compared with NaCl 0.9% and low EYPC; †compared with NaCl 0.9% and DPPC.
All mixed micelles contained 100 mM taurocholate and low (26 mM) or high (68 mM) amounts of phospholipids.
Figure 3.
Gall bladder volume over 90 minutes after intraduodenal infusion of mixed taurocholate (TC) micelles containing high amounts of egg yolk-phosphatidylcholine (EYPC) (EYPC/(TC+EYPC)=0.4), low amounts of EYPC (EYPC/(TC+EYPC)=0.2), low amounts of dipalmitoylphosphatidylcholine (DPPC) (DPPC/(TC+DPPC)=0.2), or NaCl 0.9%. For NaCl 0.9%, SEM bars are within the symbols.
Figure 4.
Plasma cholecystokinin (CCK) concentrations after intraduodenal infusion of mixed taurocholate (TC) micelles containing high amounts of egg yolk-phosphatidylcholine (EYPC) (EYPC/(TC+EYPC)=0.4), low amounts of EYPC (EYPC/(TC+EYPC)=0.2), low amounts of dipalmitoylphosphatidylcholine (DPPC) (DPPC/(TC+DPPC)=0.2), or NaCl 0.9%.
Plasma bile salt concentrations
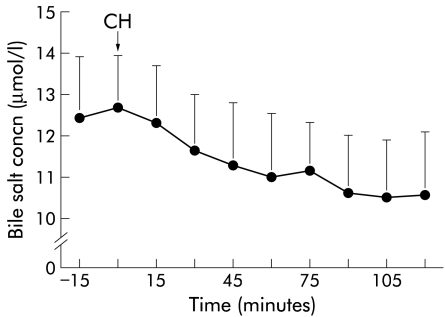
There was a significant reduction in plasma bile salt concentrations after ingestion of cholestyramine (from 12.7 (1.3) to 10.6 (1.5) μmol/l) (fig 5 ▶). During intraduodenal infusion of mixed micelles of various compositions, no significant changes in plasma bile salt concentrations were detected.
Figure 5.
Plasma bile salt concentrations after oral ingestion of 8 g of cholestyramine (CH). There was a significant decrease with time.
Intraduodenal PL hydrolysis
Intraduodenal bile salt concentrations did not vary significantly between the various mixed micellar infusions at comparable time points. In contrast, intraduodenal PL concentrations after infusion of EYPC enriched micelles (EYPC/(bile salt+EYPC) ratio=0.4) at 15 minutes were significantly higher than after infusion of EYPC or DPPC poor micelles (16 (3) mM, 5 (1) mM, and 9 (2) mM; p<0.05). Amounts of lyso-PC were high after infusion of EYPC enriched mixed micelles, moderate with EYPC poor mixed micelles, but low with DPPC poor mixed micelles (table 3 ▶).
Table 3.
Percentage of lyso-phosphatidylcholine (lyso-PC) in aspirated duodenal contents after intraduodenal infusion of various mixed micelles
| Lyso-PC (% of total PC) | |||
| 0 min | 15 min | 30 min | |
| Micelles EYPC/(TC+EYPC) ratio=0.4 | nd | 20 (4) | 60 (6) |
| Micelles EYPC/(TC+EYPC) ratio=0.2 | nd | 9 (2)* | 25 (4)* |
| Micelles DPPC/(TC+DPPC) ratio=0.2 | nd | 5 (2)* | 8 (2)† |
EYPC, egg yolk-phosphatidylcholine; TC, taurocholate; DPPC, dipalmitoylphosphatidylcholine; nd, not detectable.
*p<0.05 compared with EYPC/(TC+EYPC)= 0.4; †p<0.05 compared with EYPC/(TC+EYPC)=0.4 and EYPC/(TC+EYPC)= 0.2.
Intermixed micellar-vesicular bile salt concentration
Apart from mixed (that is, PL-bile salt) micelles, bile systems also contain non-PL associated bile salts, either as monomers or, above their critical micellar concentration, associated in “simple” micelles. The monomeric plus simple micellar bile salt concentration is referred to as “intermixed micellar-vesicular (non-PL associated) bile salt concentration”, usually abbreviated as IMC.14 Bile salts in the IMC are thought to be responsible for the detergent effects of micelles.12,13 We determined IMC values in various model systems that contained TC and low or high amounts of EYPC or low amounts of DPPC (PL/(BS+PL) ratio 0.2 or 0.4: mol% lipids identical to model biles that were infused intraduodenally). IMC values were higher when less PL was incorporated in the micelles, in the case of EYPC compared with DPPC containing micelles, and in the case of appreciable amounts of micellar lyso-PC (table 4 ▶).
Table 4.
Effects of phospholipid (PL) content, acyl chain composition, and lyso-phosphatidylcholine (lyso-PC) on intermixed micellar-vesicular bile salt concentration (IMC) in model biles
| IMC value (mM) | |
| Micelles EYPC/(TC+EYPC) ratio=0.4 | 5.9 (0.1) |
| Micelles PL/(TC+PL) ratio=0.4 (60% of PL were lyso-PC and 40% of PL were EYPC) | 7.6 (0.1) |
| Micelles EYPC/(TC+EYPC) ratio=0.2 | 11.4 (0.2) |
| Micelles DPPC/(TC+DPPC) ratio=0.2 | 8.6 (0.1) |
All micelles contained 30 mM taurocholate and were incubated for 12 hours at 37°C.
Differences between all IMC values were significant.
EYPC, egg yolk-phosphatidylcholine; TC, taurocholate; DPPC, dipalmitoylphosphatidylcholine.
DISCUSSION
Negative feedback control by intraduodenal bile salts on postprandial gall bladder emptying and CCK release is well established.1–6 In the present study we found evidence for a similar negative feedback control during the fasting state: intraduodenal bile salt depletion by the bile salt binding resin cholestyramine induced strong gall bladder contraction while intraduodenal infusion of mixed TC micelles induced gall bladder dilatation. Similar increases in gall bladder volume have been reported within minutes after administration of the hydrophilic bile salt ursodeoxycholic acid (often used in patients with cholestatic liver diseases and to dissolve gall stones).22,23 Negative feedback could be involved in the regulation of bile salt antibacterial activity in the fasting state24 or in the regulation of the intestinal MMC by limiting amounts of intraduodenal bile salts. Periodic gall bladder emptying in the fasting state is associated with phase III of the intestinal MMC,7,8,25 possibly mediated by an increase in plasma motilin levels.26–29 It has therefore been suggested that high levels of intraduodenal bile salts may initiate phase III activity. Nevertheless, controversial results have been reported on the relationship of phase III and gall bladder contraction in the interdigestive state.30 Also, in the present study, no effects of intraduodenal bile salt infusions on MMC cycle length were found (table 1 ▶).
The mechanism of the negative feedback control in the fasting state deserves further comment. CCK is considered the most important hormone when gall bladder contraction is stimulated by intraduodenal nutrients or meal ingestion, and there is ample evidence for negative feedback control by intraduodenal bile salts on stimulated CCK release as well as on resulting gall bladder contraction and pancreatic secretion under these circumstances.1–6 In contrast, despite the strong gall bladder contraction observed in this study after acute intraduodenal bile salt depletion by ingestion of cholestyramine alone, there was no clear increase in plasma CCK concentrations (fig 2 ▶). These findings are in agreement with previous studies.2,5,6,31 Furthermore, we demonstrated significant gall bladder dilatation on intraduodenal infusion of various mixed micelles, again without change in CCK concentrations in plasma (figs 3, 4 ▶ ▶). Taken together, these findings argue against a role for CCK in mediating negative feedback of intraduodenal bile salts on gall bladder motility in the fasting state.
Another hormone of potential relevance is motilin. In the fasting state, gall bladder contraction is associated with phase III and increased plasma motilin levels. Also, exogenous motilin can elicit gall bladder contraction.32 In a previous study from our group, cholestyramine ingestion at a fixed time point in the MMC cycle (30 minutes after previous phase III) significantly increased plasma motilin levels, accompanied by strong gall bladder contraction.31 However, motilin could play, at best, a minor role in cholestyramine induced gall bladder contraction or bile salt induced gall bladder dilatation: intravenous infusion of motilin induces no more than 20% gall bladder emptying, far below the 80% emptying found in the present study after cholestyramine ingestion.32
One may also hypothesise that cholestyramine or intraduodenal bile salt infusions modulate gall bladder volume by, respectively, reducing or enhancing hepatic bile flow as a result of their opposite effects on the amount of intestinal bile salts available for absorption. The consequence would be that considerable amounts of bile salts would be absorbed in the proximal small intestine: the effects of cholestyramine and bile salt infusions on gall bladder volume occurred within minutes after administration in the proximal small intestine. It appears unlikely that cholestyramine and intraduodenal bile salts modulate gall bladder volume by modulating hepatic bile flow for the following reasons. Firstly, although recent data suggest appreciable absorption of bile salts in the proximal intestine,33 they are generally believed to be absorbed mainly in the ileum34,35 by the recently discovered intestinal bile salt transporter.36 Secondly, although in the present study plasma bile salt concentrations decreased significantly (±20%) on cholestyramine ingestion, coinciding with a strong and prolonged (>24 hours) decrease in gall bladder volume, intraduodenal infusions of mixed TC micelles did not significantly increase plasma bile salt concentrations despite considerable increases in gall bladder volume. In contrast, after postprandial gall bladder contraction there is a marked increase in plasma levels of conjugated bile salts (peak levels at 1–2 hours after meal ingestion).37
Other mechanisms for the negative feedback control in the fasting state could be local interactions of bile salts with the duodenal wall, with the result that cholinergic pathways are affected38,39 or, alternatively, somatostatin40–42 or nitric oxide40,43,44 are released. It is also possible that intraduodenal bile salts exert an effect on the sphincter of Oddi, with its complex mode of innervation, by means of adrenergic excitatory and inhibitory nerves, cholinergic nerves of uncertain function, as well as non-adrenergic non-cholinergic nerves. Sphincter of Oddi contraction induced by intraduodenal bile salts may lead to gall bladder distension through a local reflex pathway between the gall bladder and sphincter.45
We found that gall bladder dilatation occurred on intraduodenal infusion of mixed micelles, regardless of the presence (study A) or absence (study B) of cholesterol within these micelles. In contrast, the effects of infused TC micelles depended to a large extent on the amounts and acyl chain composition of the phosphatidylcholines that were incorporated in these micelles. Bile salts are amphiphilic compounds that act as detergents above their critical micellar concentration (±9 mM for TC). Under physiological conditions—in bile and within the intestinal lumen—bile salts are associated with PLs in mixed micellar structures. However, significant amounts of bile salts are also present under these conditions as monomers and as “simple” micelles (that is, without incorporated PLs). This so-called IMC (bile salt monomers+simple micelles) is thought to be responsible for detergent effects and cytotoxicity.12,13 We found in the present study that mixed bile salt micelles containing low amounts of EYPC exhibited more pronounced effects on gall bladder volume than bile salt micelles containing high amounts of EYPC or low amounts of DPPC. These findings may relate to the fact that IMC values decrease progressively with increasing micellar PL content whereas DPPC containing micelles exhibit lower IMC values than EYPC containing micelles (table 4 ▶). The higher efficacy of DPPC compared with EYPC in mitigating TC induced gall bladder dilatation may also relate to lower intraduodenal hydrolysis of DPPC: as lyso-PC molecules are more hydrophilic than PC molecules, lyso-PC is contained less in bile salt micelles,46 with the result that IMC values (table 4 ▶) and detergent effects are increased.
In summary, in the fasting state, intraduodenal bile salts exert negative feedback control on gall bladder volume by a CCK independent mechanism. Although this effect is diminished by phosphatidylcholines, the exact mechanism of the negative feedback remains to be elucidated.
Acknowledgments
The authors would like to thank Renze Boverhof (Department of Paediatrics, University Hospital Groningen, the Netherlands) for performing the plasma bile salt analyses, and Willem Renooy, Martin de Smet, and Karin Nibbering (Gastrointestinal Research Laboratory, Utrecht, the Netherlands) for their assistance and fruitful discussions.
Abbreviations
CCK, cholecystokinin
DPPC, dipalmitoylphosphatidylcholine
EYPC
egg yolk-phosphatidylcholine
IMC, intermixed micellar-vesicular concentration
MMC, migrating motor complex
PL, phospholipid
TC, taurocholate
lyso-PC, lysophosphatidylcholine
REFERENCES
- 1.Koop I, Koop H, Gerhardt C, et al. Do bile acids exert a negative feedback control of cholecystokinin release? Scand J Gastroenterol 1989;24:315–20. [DOI] [PubMed] [Google Scholar]
- 2.Koop I, Dorn S, Koop H, et al. Dissociation of cholecystokinin and pancreaticobiliary response to intraduodenal bile acids and cholestyramine in humans. Dig Dis Sci 1991;36:1625–32. [DOI] [PubMed] [Google Scholar]
- 3.Koop I, Fellgiebel A, Koop H, et al. Effect of cholestyramine on plasma cholecystokinin and pancreatic polypeptide levels and exocrine pancreatic secretion. Eur J Clin Invest 1988;18:517–23. [DOI] [PubMed] [Google Scholar]
- 4.Malagelada JR, Go VLW, DiMagno EP, et al. Interactions between intraluminal bile acids and digestive products on pancreatic and gallbladder function. J Clin Invest 1973;52:2160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez G, Upp JR, Lluis F, et al. Regulation of the release of cholecystokinin by bile salts in dogs and humans. Gastroenterology 1988;94:1036–46. [DOI] [PubMed] [Google Scholar]
- 6.Thimister PWL, Hopman WPM, Sloots CEJ, et al. Effect of bile salt binding or protease inactivation on plasma cholecystokinin and gallbladder responses to bombesin. Gastroenterology 1994;107:1627–35. [DOI] [PubMed] [Google Scholar]
- 7.Ozeki K, Sarna SK, Condon RE, et al. Enterohepatic circulation is essential for regular cycling of duodenal migrating motor complexes in dogs. Gastroenterology 1992;103:759–67. [DOI] [PubMed] [Google Scholar]
- 8.Stolk MFJ, van Erpecum KJ, Smout AJPM, et al. Motor cycles with phase III in antrum are associated with high motilin levels and prolonged gallbladder emptying. Am J Physiol 1993;264:G596–600. [DOI] [PubMed] [Google Scholar]
- 9.Turley SD, Dietschy JM. Reevaluation of the 3α-hydroxy-steroid dehydrogenase assay for total bile acids in bile. J Lipid Res 1978;19:924–8. [PubMed] [Google Scholar]
- 10.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–17. [DOI] [PubMed] [Google Scholar]
- 11.Rouser G, Fleischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970;5:494–6. [DOI] [PubMed] [Google Scholar]
- 12.Donovan JM, Jackson AA, Carey MC. Molecular species composition of inter-mixed micellar/vesicular bile salt concentrations in model bile: dependence upon hydrophilic-hydrophobic balance. J Lipid Res 1993;34:131–40. [PubMed] [Google Scholar]
- 13.Moschetta A, van Berge-Henegouwen GP, Portincasa P, et al. Sphingomyelin exibits greatly enhanced protection compared with egg yolk phosphatidylcholine against detergent bile salts. J Lipid Res 2000;41:916–24. [PubMed] [Google Scholar]
- 14.Donovan JM, Timofeyeva N, Carey MC. Influence of total lipid concentration, bile salt:lecithin ratio, and cholesterol content on intermixed micellar-vesicular (non-lecithin-associated) bile salt concentrations in model bile. J Lipid Res 1991;32:1501–12. [PubMed] [Google Scholar]
- 15.Donovan JM, Jackson AA. Rapid determination by centrifugal ultrafiltration of inter-mixed micellar/vesicular (non-lecithin-associated) bile salt concentrations in model bile: influence of Donnan equilibrium effects. J Lipid Res 1993;34:1121–9. [PubMed] [Google Scholar]
- 16.Eckhardt ERM, Moschetta A, Renooij W, et al. Asymmetric distribution of phosphatidylcholine and sphingomyelin between micellar and vesicular phases: potential implications for canalicular bile formation. J Lipid Res 1999;40:2022–33. [PubMed] [Google Scholar]
- 17.Everson GY, Braverman DZ, Johnson ML, et al. A critical evaluation of real-time ultrasonography for the study of gallbladder volume and contraction. Gastroenterology 1980;79:40–6. [PubMed] [Google Scholar]
- 18.Stolk MFJ, van Erpecum KJ, Koppeschaar HPF, et al. Effects of long-term octreotide treatment on interdigestive gallbladder emptying, antroduodenal motility and motilin release in acromegaly. Gut 1995;36:755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camilleri M, Malagelada JR. Abnormal intestinal motility in diabetics with gastroparesis syndrome. Eur J Clin Invest 1984;14:420–7. [DOI] [PubMed] [Google Scholar]
- 20.Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clin Chem 1998;44:991–1001. [PubMed] [Google Scholar]
- 21.Murphy GM, Billing BH, Baron DN. A fluoremetric and enzymatic method for the estimation of serum total bile acids. J Clin Pathol 1970;23:594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sailer C, Pauletzki J, Klueppelberg U, et al. Acute effect of ursodeoxycholic acid on gallbladder volume in healthy subjects. Scand J Gastroenterol 1996;31:512–16. [DOI] [PubMed] [Google Scholar]
- 23.Neubrand MW, Kampmann S, Eschmann K, et al. Influence of ursodeoxycholic acid on intestinal motility, pancreatic secretion and serum levels of pancreatic polypeptide and motilin. Gastroenterology 2000;118:A847. [Google Scholar]
- 24.Sung JY, Shaffer EA, Costerton JW. Antibacterial activity of bile salts against common biliary pathogens. Effects of hydrophobicity of the molecule and in the presence of phospholipids. Dig Dis Sci 1993;38:2104–12. [DOI] [PubMed] [Google Scholar]
- 25.Kajiyama Y, Irie M, Enjoji A, et al. Role of bile acids in duodenal migrating motor complexes in dogs. Dig Dis Sci 1998;43:2278–83. [DOI] [PubMed] [Google Scholar]
- 26.Owyang C, Achem-Karam S, Funakoshi A, et al. Pancreato-biliary secretion stimulates motilin release and initiates the interdigestive migrating motor complex. Clin Res 1982;30:287–91. [Google Scholar]
- 27.Owyang C, Funakoshi A, Vinik AI. Evidence for modulation of motilin secretion by pancreato-biliary juice in health and in chronic pancreatitis. J Clin Endocrinol Metab 1983;57:1015–20. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson BI, Svenberg T, Tollstrom T, et al. Relationship between interdigestive gallbladder emptying, plasma motilin and migrating motor complex in man. Acta Physiol Scand 1990;139:55–61. [DOI] [PubMed] [Google Scholar]
- 29.Plourde V, Trudel L, Poitras P. Interdigestive intestinal motility in dogs with chronic exclusion of bile from the digestive tract. Can J Physiol Pharmacol 1987;65:2493–6. [DOI] [PubMed] [Google Scholar]
- 30.Hughes SJ, Behrns KE, Sarr MG. Chronic bile diversion does not alter canine interdigestive myoelectric activity. Dig Dis Sci 1993;38:1055–61. [DOI] [PubMed] [Google Scholar]
- 31.Portincasa P, Peeters TL, van Berge-Henegouwen GP, et al. Acute intraduodenal bile salt depletion leads to strong gallbladder contraction, altered antroduodenal motility and high plasma motilin levels in humans. Neurogastroenterol Mot 2000;12:421–30. [DOI] [PubMed] [Google Scholar]
- 32.Luiking YC, Peeters TL, Stolk MFJ, et al. Motilin induces gallbladder emptying and antral contractions in the fasted state in humans. Gut 1998;42:830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gui X, Carraway RE. Enhancement of jejunal absorption of conjugated bile acid by neurotensin in rats. Gastroenterology 2001;120:151–60. [DOI] [PubMed] [Google Scholar]
- 34.Marcus SN, Schteingart CD, Marquez ML, et al. Active absorption of conjugated bile acids in vivo. Kinetic parameters and molecular specificity of the ileal transport system in the rat. Gastroenterology 1991;100:212–21. [DOI] [PubMed] [Google Scholar]
- 35.Aldini R, Montagnani M, Roda A, et al. Intestinal absorption of bile acids in the rabbit: different transport rates in jejunum and ileum. Gastroenterology 1996;110:459–68. [DOI] [PubMed] [Google Scholar]
- 36.Wong MH, Oelkers P, Craddock AL, et al. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem 1994;269:1–8. [PubMed] [Google Scholar]
- 37.Salemans JM, Nagengast FM, Tangerman A, et al. Effect of ageing on postprandial conjugated and unconjugated serum bile acid levels in healthy subjects. Eur J Clin Invest 1993;23:192–8. [DOI] [PubMed] [Google Scholar]
- 38.Taylor IL, Feldman M, Richardson CT, et al. Gastric and cephalic stimulation of human pancreatic polypeptide release. Gastroenterology 1978;75:432–7. [PubMed] [Google Scholar]
- 39.Adrian TE, Besterman HS, Bloom SR. The importance of cholinergic tone in the release of pancreatic polypeptide by gut hormones in man. Life Sci 1979;24:1989–94. [DOI] [PubMed] [Google Scholar]
- 40.Chayvialle JA, Miyata M, Rayford PL, et al. Effects of test meal, intragastric nutrients, and intraduodenal bile on plasma concentrations of immunoreactive somatostatin and vasoactive intestinal peptide in dogs. Gastroenterology 1980;79:844–52. [PubMed] [Google Scholar]
- 41.Poitras P, Yamada T, Walsh JH. Absence of effect of somatostatin on the guinea pig gallbladder. Can J Physiol Pharmacol 1980;58:179–82. [DOI] [PubMed] [Google Scholar]
- 42.Poitras P, Steinbach JH, VanDeventer G, et al. Motilin-independent ectopic fronts of the interdigestive myoelectric complex in dogs. Am J Physiol 1980; 239:G215–20. [DOI] [PubMed] [Google Scholar]
- 43.Luiking YC, Weusten BLAM, Portincasa P, et al. Effects of long-term oral L-arginine on esophageal motility and gallbladder dynamic in healthy humans. Am J Physiol 1998;274:G984–91. [DOI] [PubMed] [Google Scholar]
- 44.Konturek JW, Kwiecien N, Sito E, et al. Physiological role of nitric oxide in gallbladder contractions in man. Gastroenterology 1995;108:A422. [Google Scholar]
- 45.Thune A, Saccone GTP, Scicchitano JP, et al. Distension of the gallbladder inhibits sphincter of Oddi motility in humans. Gut 1991;32:690–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Homan R, Hamelehle KL. Phospholipase A2 relieves phosphatidylcholine inhibition of micellar cholesterol absorption and transport by human intestinal cell line Caco-2. J Lipid Res 1998;39:1197–209 [PubMed] [Google Scholar]