Abstract
Background and aim: Leptin, a hormone mainly produced by fat cells, acts primarily on the hypothalamus regulating energy expenditure and food intake. Leptin receptors are expressed in several tissues and the possible physiological role of leptin is being extensively investigated, with the result that important peripheral actions of the hormone in the organism are being discovered. Recent studies have demonstrated leptin and leptin receptor expression in gastric epithelial cells. In the present study, we report the presence of the long leptin receptor isoform (OB-Rb) in human, rat, and mouse small intestine, supporting the hypothesis of leptin as a hormone involved in gastrointestinal function.
Methods: The presence of the leptin receptor was determined by immunocytochemical methods using antibodies against the peptide corresponding to the carboxy terminus of the long isoform of the leptin receptor. Human duodenal biopsies from normal individuals undergoing gastrointestinal endoscopy, and intestinal fragments of Wistar rats and Swiss mice were processed for the study.
Results: Immunoreactivity for the long leptin receptor isoform was observed in the three studied species. Staining was located throughout the cytoplasm of the enterocytes, of both villi and crypts, and in the basolateral plasma membrane. Immunolabelling for OB-Rb protein was also found in the brush border of human enterocytes of formol and paraformaldehyde fixed samples.
Conclusion: This report demonstrates the presence of the long leptin receptor isoform in the absorptive cells of rat, mouse, and human small intestine, suggesting that leptin could have a physiological role in the regulation of nutrient absorption.
Keywords: leptin receptor, small intestine, mouse, rat, immunocytochemistry
Leptin is a 16 kDa hormone mainly produced by fat cells1 that regulates food intake and energy expenditure by providing afferent signals to the hypothalamus.2–4 Its expression and secretion are highly correlated with body fat mass and adipocyte size, and regulated by different factors such as hormones, nutritional state, and the sympathetic nervous system.5–7 Leptin has structural similarities to certain cytokines and its receptor, the OB-R protein cloned from brain,8 is a member of the cytokine class I receptor superfamily.
There are several OB-R isoforms which are splicing variants of a single gene transcript.9–11 Identical regions in all receptor isoforms include an extracellular domain, a transmembrane domain, and the first 29 amino acids of the cytoplasmic domain. According to the length of the intracellular domain, there is one long isoform (OB-Rb) and four short isoforms (OB-Ra, c, d, f), which differ in their cytosolic carboxy terminus. Finally, there is one soluble isoform (OB-Re) which lacks the transmembrane domain and may be involved in leptin transport in the blood. When leptin binds to the OB-Rb receptor, it activates JAK/STAT (Janus kinase/signal transducer and activator of transcription) and MAPK (mitogen activated protein kinase) signal transduction pathways in a variety of in vitro systems.12
Apart from the original tissues from which leptin and its receptor were cloned, expression of both proteins has also been found in many other tissues,13–17 which demonstrates possible important peripheral actions in rodents and humans.18–20 Recent data suggest that leptin could also be considered a gastrointestinal hormone. Leptin mRNA and protein have been found in fundic epithelium of rat stomach,14 and in humans not only leptin but also its receptor is expressed in gastric epithelial cells.15,21 In mouse small intestine, the presence of OB-Rb has been reported using reverse transcription-polymerase chain reaction (RT-PCR) and western blot analysis, and intravenous administration of leptin reduces jejunal apolipoprotein AIV mRNA levels.22 Moreover, functional studies from our laboratory have shown that leptin decreases sugar uptake by rat small intestine in vitro.23
Following on from our previous studies, the present report documents the immunocytochemical localisation of the long leptin receptor isoform in human, rat, and mouse enterocytes. This is the first time that the presence of the leptin receptor protein in mammalian enterocytes has been reported.
MATERIAL AND METHODS
Subjects
Patients (n=10) undergoing upper gastrointestinal endoscopy at the Hospital of the University of Navarra were used with the approval of the ethics committee. All patients included in this study had normal duodenal mucosa. For the immunohistochemical study, two or three duodenal biopsy samples were obtained from each patient and fixed in 10% formol, Bouin's, or 4% paraformaldehyde (PAF) fixatives before paraffin embedding. In addition, some PAF fixed samples were frozen and used for cryostat sectioning.
Wistar rats (200–250 g; n=4) and Swiss mice (100–110 g; n=2), after a 24 or 12 hour fast, respectively, were killed by cervical dislocation and a jejunum segment of 20 cm and 10 cm, respectively, was quickly excised and rinsed in ice cold saline solution. The segment was cut into small pieces (1–2 cm) which were fixed in Bouin's or formol fixatives for immunohistochemical investigation. After 24 hours of fixation at room temperature, samples were dehydrated and embedded in paraffin.
Rat jejunum was also processed for resin embedding using a perfusion/fixation method.24 The fixative composition was: 4% PAF, 0.1% glutaraldehyde, and 0.2% saturated picric acid in 0.125 M phosphate buffer, pH 7.4. After fixation, the jejunum was cut into 1 mm pieces, dehydrated, embedded in epon, and sliced into 1 μm thick sections.
Immunohistochemical procedure
Sections (3 μm thick) were cut and mounted on glass slides. After dewaxing with xylene, immunocytochemical staining was performed using the streptavidin-biotin method. Sections were hydrated through alcohols, and endogenous peroxidase was blocked by treatment with 3% H2O2 for 10 minutes in the dark and afterwards placed in phosphate buffered saline (65 mM NaCl, 1.05 mM KCl, 4.05 mM Na2HPO4, 0.5 mM KH2PO4, pH 7.4).
Non-specific background was blocked with 2% normal swine or rabbit serum for 30 minutes and sections were incubated overnight at 4°C with the primary antibody. After incubation with biotinylated link antibody (swine antirabbit Dako E0353, rabbit antigoat Dako E466) for one hour and peroxidase labelled streptavidin for another hour, both at a working dilution of 1:200, bound antibodies were visualised by immersion treatment of the sections with 0.25% 3`,3`- diaminobenzidine tetrahydrochloride used as chromogen in 0.05 M Tris-HCl buffer, pH 7.36, with 0.5% H2O2 for 30 seconds. Sections were then rinsed in tap water, counterstained with haematoxylin, dehydrated, and mounted with DPX. For semithin (1 μm) section immunostaining, epon was removed with aged saturated sodium hydroxide in ethanol for one hour. The incubation time for the primary antibody was prolonged to 48 hours, at 4°C, and the biotinylated antibody and streptavidine peroxidase complexes were used at a 1:100 working dilution. In some cases, the reaction was intensified by means of 0.03% diaminobenzidine tetrahydrochloride in 0.1 M sodium acetate/acetic acid, pH 5.6, containing 2.5% ammonium nickel sulphate, 0.2% β-d-glucose, 0.04% ammonium chloride, and 0.01% glucose oxidase.
Two different antibodies were used: human leptin receptor goat polyclonal antibody (C-20; Santa Cruz Biotechnology Inc., Santa Cruz, California, USA) at a working dilution of 1:50 was used for human intestine samples, and rat leptin receptor rabbit polyclonal antibody (OBR13; Alpha Diagnostics International, Inc. San Antonio, Texas, USA) at a dilution of 1:200, was used for both the rat and mouse samples. Antibodies were raised against the peptides corresponding to amino acids 1146–1165 (C-20) and 1145–1162 (OBR13) mapping the carboxy terminus of the full length leptin receptor, and therefore were specific for the long receptor isoform (OB-Rb). Antibody OBR13, specific for rat and mouse receptor, did not cross react with human tissue samples. Microwave pretreatment improved immunolabelling for the two fixatives in the three species but did not change the distribution pattern. Pretreated sections were unmasked by heat in 10 mM sodium citrate buffer (pH 6) for two periods of 15 minutes.
Absorption controls were performed to test the specificity of immunostaining. Antisera to leptin receptor were preincubated with 10-fold excess of homologous peptide at 4°C overnight before application to tissue sections. For all antibodies, preabsorption of the antiserum with the peptide abolished immunoreaction. Immunolabelling was also absent when the primary antibody was omitted.
To check if the human antibody showed cross reactivity with any other similar peptide antigen, a computer search was performed using the Protein BLAST database. The results showed that the peptide recognised by the human C-20 antibody only aligns with the carboxy terminus of the human OB-Rb receptor (100% identity) and with the rat and mouse OB-Rb receptor (95% identity). The alignment with cytokine receptors showed 0% homology. Likewise, the peptide recognised by the rat OBR13 antibody also aligns with the mouse (99% identity) and human (94% identity) OB-Rb receptor. These results indicate that both antibodies are specific for the long leptin receptor isoform. Furthermore, the C-20 antibody has been extensively used in other papers to show immunolocalisation of the long leptin receptor isoform in different tissues.21
In initial experiments, two other polyclonal antibodies were used and found to be positive in rat and mouse tissue samples. Both antibodies were goat antileptin receptor raised, respectively, against the carboxy terminal fragment 877–894 and the amino terminal fragment 32–51 of the leptin receptor of mouse origin (Research Diagnostics Inc. Flanders, New Jersey, USA). However, such antibodies recognise all receptor isofoms and therefore did not discriminate between the short and long receptor isoforms.
RT-PCR procedure
Total RNA was isolated from 24 human duodenal mucosa samples using TriPure Isolation Reagent (Boehringer Mannheim, Germany). First strand cDNA was generated using 4 μg RNA and M-MLV reverse transcriptase (Gibco BRL, Life Technologies, Barcelona, Spain). To detect OB-Rb mRNA expression, we used the primers and PCR conditions previously described for human fundic biopsy samples.21 In addition, the identity of the PCR product was confirmed by sequencing.
RESULTS
Immunoreactivity for the long isoform of the leptin receptor was found in the enterocytes of the three studied species (human, rat, and mouse). In all cases, immunostaining was located in the cytoplasm and basolateral plasma membrane (BLPM) of the absorptive cells. In addition, human enterocytes also displayed immunolabelling in the brush border. Several immunohistochemical processing methods were needed to demonstrate the different locations of the immunolabelling.
Immunostaining for the leptin receptor was present within the cytoplasm of human (fig 1A ▶), mouse (fig 1C–F ▶), and rat enterocytes. Labelling was usually observed extended throughout the cell, both in the apical and basal cytoplasm. Staining was often more intense in the apical cytoplasmic region underlying the brush border (fig 1E, F ▶). Intracytoplasmic staining was observed in enterocytes lining both the intestinal villi (fig 1A, C, E ▶) and crypts (fig 1D ▶). Although villi staining was always found, in some samples crypt staining was weaker or absent. Preabsorption tests demonstrated the specificity of the staining (fig 1B, G ▶).
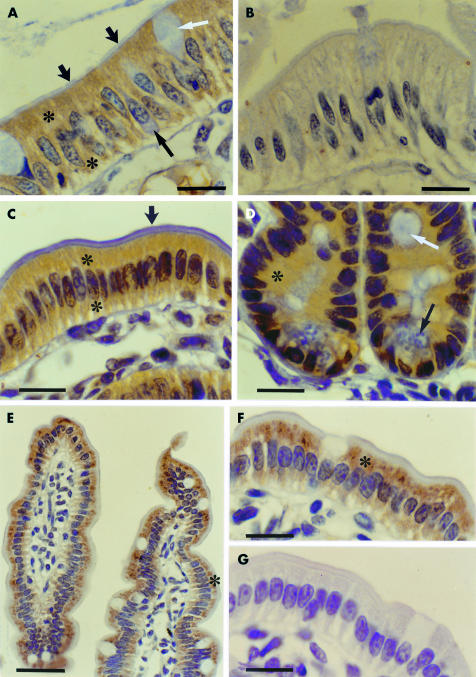
Figure 1.
Intracytoplasmic immunolabelling for the long leptin receptor isoform (OB-Rb) (A, B: human duodenum, Bouin's fixation; C-G: mouse jejunum, formol fixation). (A) Immunostaining for OB-Rb (*) was found throughout the cytoplasm of human enterocytes. Labelling was absent from the brush border (short arrows). White arrow, Goblet cell. Occasional unstained—perhaps endocrine—cells (black arrow) may also occur. (B) Absorption test in human tissue: immunolabelling was abolished using preabsorbed antiserum. (C, D) Immunolabelling for the leptin receptor (*) was present in both the basal and apical cytoplasm of mouse enterocytes. In contrast, the brush border was not immunostained (short arrow). Labelling was observed in both villi (C) and crypts (D). Immunostaining was observed in all enterocytes lining intestinal villi and crypts. White arrow, Goblet cell; black arrow, Paneth cell. (E, F) Panoramic micrograph of two intestinal villi (E) and detailed enterocytes (F). Intracytoplasmic immunolabelling for OB-Rb, although extended throughout the cell, was stronger in the apical cytoplasm underlying the brush border (*). Nickel enhancement method. (G) Absorption test in mouse tissue: no immunostaining was obtained. A–D, F, G: bars=14 μm; E: bar=35 μm.
Cytoplasmic labelling of human enterocytes was always achieved in samples fixed in Bouin's but not in the other fixatives (figs 2, 3 ▶ ▶). Mouse and rat enterocytes showed the same intracytoplasmic labelling pattern using either fixative as well as two other antibodies apart from OBR13. While confirming the presence of the leptin receptor, these antibodies (as indicated in the methods section) cannot distinguish between the short and long receptor isoforms.
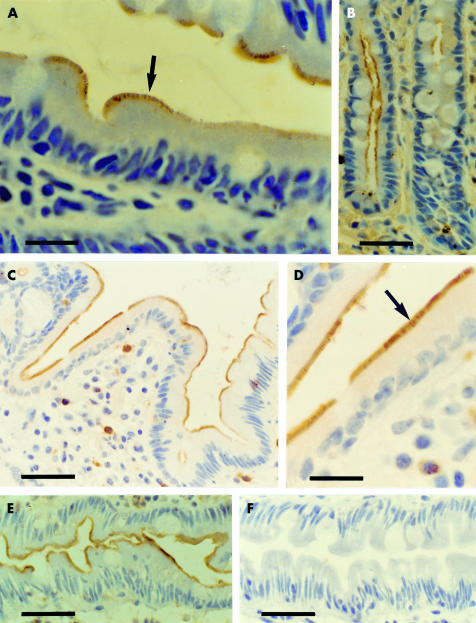
Figure 2.
Immunostaining for the long leptin receptor isoform in the brush border of human enterocytes: formol fixed, paraffin embedded samples (A, B); paraformaldehyde (PAF) fixed, paraffin embedded samples (C, D); PAF fixed, cryostat sections (E, F). The brush border displayed immunostaining in the enterocytes of both intestinal villi (A, C–E) and crypts (B). The striated appearance of the brush border was evident (arrows). Immunostaining for the leptin receptor (E) was absent using preabsorbed antiserum (F). A, D: bars=14 μm; B, C, E, F: bars=35 μm.
Figure 3.
Preabsorption tests demonstrated the specificity of immunostaining for the long leptin receptor in the brush border plasma membrane of formol fixed human intestine. Incubation of antiserum with the antigen (B) abolished immunostaining of the brush border (A). Note that brush border plasma membrane immunolabelling occurred in all enterocytes. Bars=40 μm.
Immunostaining for the leptin receptor was also found in the brush border plasma membrane (BBPM) of human enterocytes in formol (figs 2A, B, 3 ▶ ▶) and PAF (fig 2C–F ▶) fixed samples. As with intracytoplasmic labelling, stained brush border was observed in both intestinal villi (figs 2A, C, E, 3A ▶ ▶) and crypts (fig 2B ▶). Preabsorption tests also demonstrated the specificity of the staining (figs 2E, F, 3 ▶ ▶).
In the BLPM, brown staining was often observed in Bouin's fixed human biopsies and rat and mouse intestine, especially when the nickel enhancement revealing method was used (fig 4A, B ▶) but disappeared when preabsorbed antiserum was used (fig 4C ▶). Given the difficulty in ascertaining immunolabelling of BLPM staining, a third processing method was also used in rodents. When rat specific antibody, OBR13, was applied to 1 μm sections of epon embedded rat intestine, immunolabelling of BLPM was clearly found (fig 4D, E ▶).
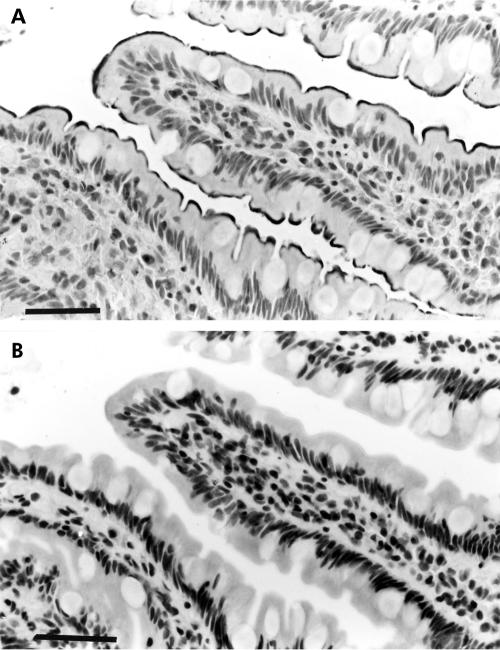
Figure 4.
Immunostaining of the basolateral plasma membrane (BLPM) of enterocytes for the long leptin receptor isoform. (A-C: human duodenum, Bouin's fixation; D, E: rat jejunum, epon embedded). BLPM immunostaining for the leptin receptor (arrows) was observed in human enterocytes in both longitudinal (A) and cross/oblique (B) sections of the epithelium. (C) Immunolabelling of the BLPM disappeared with preabsorption of the antiserum. (D, E) In (D), immunolabelling of the BLPM was evident (arrows). Note that labelling was not present in the most apical lateral regions of cells where junctional complexes are known to exist in the plasma membrane. For this reason a “loop” can be observed systematically in the labelling (curved arrows). In preabsorbed sections (*, E), immunolabelling of the BLPM disappeared. Staining of the lamina propria (*, D) was not specific as it did not disappear after absorption of antiserum (E). Stars, widened intercellular basolateral spaces. Bars=14 μm.
Expression of mRNA encoding for OB-Rb protein was detected in human duodenal mucosa, further supporting the presence of the long leptin receptor isoform in human enterocytes (fig 5 ▶)
Figure 5.
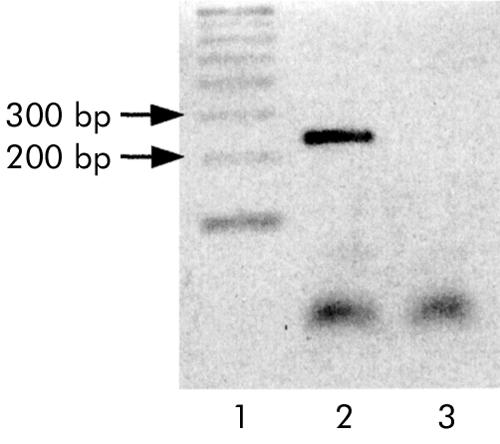
Reverse transcription-polymerase chain reaction detection of long leptin receptor isoform (Ob-Rb) mRNA from human duodenum. Lane 1, 100 base pair DNA ladder; lane 2, OB-Rb (expected size 238 bp); lane 3, reverse transcriptase omission.
DISCUSSION
In this study, we demonstrated the presence of the long leptin receptor isoform (OB-Rb) in human and murine small intestine using immunocytochemical techniques. In the case of human tissue, RT-PCR confirmed the presence of OB-Rb.
Given that achievement of immunolabelling in immunocytochemical studies frequently depends on the histological processing of the samples, we used different processing methods. As a result, immunolabelling was obtained in the brush border, cytoplasm, and basolateral membrane of enterocytes.
While many studies have examined the effect of leptin on hypothalamic neurones involved in regulating food intake and energy metabolism, not much is known about the action of leptin in the small intestine. Recent reports have demonstrated a possible role for leptin in intestinal lipid handling22 and sugar absorption23,25 and the presence of leptin receptors in mouse intestine and Caco-2 cells by biochemical methods.22 Moreover, studies on 125I-leptin tissue distribution have shown that the small intestine of the rat and mouse contains the highest concentration of the hormone.26,27 Also, OB-R mRNA expression has been found in the intestine of juvenile and sexually matured hens, and oestrogen treatment was found to enhance OB-R mRNA expression in the intestine.28
Intracellular immunostaining of OB-Rb found in enterocytes is in agreement with the subcellular localisation of leptin receptors in other tissues. For example, using antibodies which do not discriminate between the different receptor isoforms, the immunostaining pattern in human brain showed a granular appearance within the cytoplasm, with an intense peripheral cytoplasmic rim.29 In mouse brain, ultrastructural immunocytochemistry shows that the staining concentrates at the level of the neuronal Golgi complex.30 Ultrastructural localisation of OB-Rb in human white adipose tissue again reveals the strongest expression in the cytoplasm rim31 and finally, confocal immunofluorescence microscopy in endothelial cells showed a strong signal for OB-Rb characterised by scattered punctuate intracellular staining.32 In COS-7 cells transfected with the cDNA of the human long and short receptor isoforms, immunofluorescence showed intense perinuclear staining in the cytoplasm.33
In the case of enterocytes, we do not know whether intracellular immunostaining corresponds to internalised receptor after leptin binding, recycling of the receptor, or the biosynthetic pathway compartments, including trafficking of newly formed vesicles containing the receptor, as has been shown in COS-7 cells transfected with the leptin receptor.34 Nevertheless, all of the immunocytochemical studies reported that the majority of the receptor, short or long isoform, was located in the intracellular compartments29–34 and with some degree of polarity (that is, more intense immunostaining) at the apical surface, as in brain ependymal cells29 and our enterocytes.
Specific immunoreaction was also found in the BLPM of enterocytes. This location of the receptor is what we would expect, considering that leptin reaches the intestinal epithelium from the blood. The fact that this label was not apparent in all preparations could be due to the intensity of the intracellular labelling that would mask basolateral staining, or to a cyclical absence of the receptor in the BLPM, reflecting the physiological status of the individual.
Surprisingly, labelling was also found in the BBPM of human enterocytes. Immunostaining seems certain as it was found with three different histological processing methods and control absorption demonstrated its specificity.
Why is the leptin receptor present in the BBPM if leptin must reach the enterocytes from blood? It has been demonstrated that rat and human stomach mucosa stores and secretes leptin.14,21 More accurately, apart from endocrine cells, secretory granules of chief cells of the human gastric mucosa contain leptin.15 Furthermore, infusion of secretin or pentagastrin in normal individuals increases leptin output both in gastric juice and in plasma.21 As these authors have also found OB-Rb protein in the BLPM of gastric cells, they suggest a paracrine/autocrine role of leptin in the stomach, probably related to control of feeding behaviour.14,15 Given this finding, we may also think of leptin reaching not only the basolateral side of the enterocytes but also the brush border, binding to its receptor and regulating sugar transport and lipid handling.22,23,25 In fact, intracytoplasmic staining is frequently stronger in the apical cytoplasm of enterocytes, which could reflect more intense trafficking of leptin receptor bearing vesicles.
In summary, we have demonstrated the presence of the long leptin receptor isoform in human, rat, and mouse enterocytes which supports consideration of leptin as a new hormone involved in the regulation of gastrointestinal tract function.
Acknowledgments
We thank I Ordoqui, B Irigoyen, and JL Lanciego for technical assistance. This study was supported by grant PM98-0034 (Ministerio de Educación y Cultura, Spanish Government) and Consejería de Educación y Cultura (Navarra Government). JB was a recipient of a fellowship from “Asociación de Amigos”, Universidad de Navarra.
Abbreviations
OB-Rb, long leptin receptor isoform
BBPM, brush border plasma membrane
BLPM, basolateral plasma membrane
RT-PCR, reverse transcription-polymerase chain reaction
PAF, paraformaldehyde
REFERENCES
- 1.Zhang YR, Proenca M, Maffei M, et al. Positional cloning of the mouse ob gene and its human homologue. Nature 1994;372:425–32. [DOI] [PubMed] [Google Scholar]
- 2.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995;269:540–3. [DOI] [PubMed] [Google Scholar]
- 3.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995;269:543–6. [DOI] [PubMed] [Google Scholar]
- 4.Campfield LA, Smith FJ, Guisez Y, et al. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 1995;269:546–9. [DOI] [PubMed] [Google Scholar]
- 5.Gómez JM, Molina A, Fernández-Castañer M, et al. Insulin regulation of leptin synthesis and secretion in humans: the model of myotonic dystrophy. Clin Endocrinol 1999;50:569–75. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Liu R, Hawkins M, et al. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 1998;393:684–8. [DOI] [PubMed] [Google Scholar]
- 7.Maffei M, Fei H, Lee GH, et al. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci USA 1995;92:6957–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tartaglia LM, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995;83:1263–71. [DOI] [PubMed] [Google Scholar]
- 9.Lee G-H, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996;379:632–5. [DOI] [PubMed] [Google Scholar]
- 10.Cioffi JA, Shafer AW, Zupancic TJ, et al. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med 1996;2:585–9. [DOI] [PubMed] [Google Scholar]
- 11.Wang MY, Zhou YT, Newgard CB, Unger RH. A novel leptin receptor isoform in rat. FEBS Lett 1996;392:87–90. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita T, Murakami T, Otani S, et al. Leptin receptor signal transduction: ObRa and ObRb of fa type. Biochem Biophys Res Commun 1998;246:752–9. [DOI] [PubMed] [Google Scholar]
- 13.Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med 1997;3:1029–33. [DOI] [PubMed] [Google Scholar]
- 14.Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature 1998;394:790–3. [DOI] [PubMed] [Google Scholar]
- 15.Cinti S, De Matteis R, Picó C, et al. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int J Obes 2000;24:789–93. [DOI] [PubMed] [Google Scholar]
- 16.Hoggard N, Mercer JG, Rayner DV, et al. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochem Biophys Res Commun 1997;232:383–7. [DOI] [PubMed] [Google Scholar]
- 17.Fei H, Okano HJ, Li C, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA 1997;94:7001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gainsford T, Wilson T, Metcalf D, et al. Leptin can induce proliferation, differentiation and functional activation of hematopoietic cells. Proc Natl Acad Sci USA 1996;93:14564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frühbeck G, Aguado M, Martínez JA. In vitro lipolytic effect of leptin on mouse adipocytes: evidence for a possible autocrine/paracrine role of leptin. Biochem Biophys Res Commun 1997;240:590–4. [DOI] [PubMed] [Google Scholar]
- 20.Emilsson V, Liu YL, Cawthorne MA, et al. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion Diabetes 1997;46:313–16. [DOI] [PubMed] [Google Scholar]
- 21.Sobhani I, Bado A, Vissuzaine C, et al. Leptin secretion and leptin receptor in the human stomach. Gut 2000;47:178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton NM, Emilsson V, Liu YL, et al. Leptin action in intestinal cells. J Biol Chem 1998;273:26194–201. [DOI] [PubMed] [Google Scholar]
- 23.Lostao MP, Urdaneta E, Martínez-Ansó E, et al. Presence of leptin receptor in rat small intestine and leptin effect on sugar absorption. FEBS Lett 1998;423:302–6. [DOI] [PubMed] [Google Scholar]
- 24.Lanciego JL, Wouterlood FG. Dual anterograde axonal tracing with Phaseolus vulgaris-leucoagglutinin (PHA-L) and biotinylated dextran amine (BDA). Neurosci Protoc 1994;506:1–13. [Google Scholar]
- 25.Pearson PY, O'Connor DM, Schwartz MZ. Novel effect of leptin on small intestine adaptation. J Surg Res 2001;97:192–5. [DOI] [PubMed] [Google Scholar]
- 26.Hill RA, Margetic S, Pegg GG, Gazzola C. Leptin: its pharmacokinetics and tissue distribution. Int J Obes 1998;22:765–70. [DOI] [PubMed] [Google Scholar]
- 27.Dal Farra C, Zsuger N, Vincent J, et al. Binding of a pure (125)-I-monoiodoleptin analog to mouse tissues: a developmental study. Peptides 2000;21:577–87. [DOI] [PubMed] [Google Scholar]
- 28.Ohkubo T, Tanaka M, Nakashima K. Structure and tissue distribution of chicken leptin receptor (cOb-R) mRNA. Biochim Biophys Acta 2000;1491:303–8. [DOI] [PubMed] [Google Scholar]
- 29.Couce ME, Burguera B, Parisi JE, et al. Localization of leptin receptor in the human brain. Neuroendocrinology 1997;66:145–50. [DOI] [PubMed] [Google Scholar]
- 30.De Matteis R, Cinti S. Ultrastructural immunolocalization of leptin receptor in mouse brain. Neuroendocrinology 1998;68:421–9. [DOI] [PubMed] [Google Scholar]
- 31.Bornstein SR, Abu-Asab M, Glasow A, et al. Immunohistochemical and ultrastructural localization of leptin and leptin receptor in human white adipose tissue and differentiating human adipose cells in primary culture. Diabetes 2000;49:532–8. [DOI] [PubMed] [Google Scholar]
- 32.Sierra-Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science 1998;281:1683–6. [DOI] [PubMed] [Google Scholar]
- 33.Baskin DG, Schwartz MW, Seeley RJ, et al. Leptin receptor long-form splice-variant protein expression in neuron cell bodies of the brain and co-localization with neuropeptide Y mRNA in the arcuate nucleus. J Histochem Cytochem 1999;47:353–62. [DOI] [PubMed] [Google Scholar]
- 34.Barr VA, Lane K, Taylor SI. Subcellular localization and internalization of the four human leptin receptor isoforms. J Biol Chem 1999;274:21416–24. [DOI] [PubMed] [Google Scholar]